Ultrastructural organization of transmitters in the cat lateralis medialis-suprageniculate nucleus of the thalamus: An immunohistochemical study
Abstract
The lateralis medialis-suprageniculate nuclear (LM-Sg) complex of the cat's posterior thalamus receives a rather wide variety of inputs from diverse cortical and subcortical areas. Previous ultrastructural studies of this nucleus demonstrated the presence of four types of vesicle-containing profiles and characterized some of these as γ-aminobutyric acid (GABA)–containing terminals (Norita and Katoh [1987] J. Comp. Neurol. 263:54–67; Norita and Katoh [1988] Prog. Brain Res. 75:109–118). The present study has extended these observations by examining the immunoreactivity (ir) of LM-Sg, with antibodies raised against aspartate (Asp), glutamate (Glu), GABA, the acetylcholine (ACh) marker, choline acetyltransferase (ChAT), and substance P (SP), by using light and electron microscopy. Neuronal somata immunopositive for the excitatory amino acids (EAAs) Asp and Glu, were of medium size. EAA-ir terminals also were of medium size and contained round synaptic vesicles; they made asymmetrical synaptic contacts with dendritic profiles. Neuronal somata immunopositive for GABA were small. GABA-positive terminals also were small and contained pleomorphic synaptic vesicles; they formed symmetrical synaptic contacts with dendritic profiles. No neurons immunolabeled for ChAT were found. Terminals immunopositive for ChAT were small and contained round synaptic vesicles; these made symmetrical synaptic contacts, asymmetrical synaptic contacts, or both, of the en passant type with dendritic profiles. SP-immunolabeled neuronal somata were not found. Immunolabeled terminals were small, contained round synaptic vesicles, and made asymmetrical synaptic contacts with dendritic profiles. ChAT-ir and SP-ir axon terminals were not expressed evenly within LM-Sg. This difference in distribution suggests that within the LM-Sg, there may be a difference in specific sensory processing functions which correlate with transmitter type. J. Comp. Neurol. 419:257–270, 2000. © 2000 Wiley-Liss, Inc.
Visual processing within the central nervous system has been said to take place within at least two distinct systems of synaptic circuitry, which have been fairly well characterized to date in terms of constituent nuclei or regions (Diamond and Hall, 1969; Schneider, 1969). In functional terms, these two systems have been known traditionally as either “what” and “where” systems (Ungerleider and Mishkin, 1982), “object-” and “spatial-sensitive” systems (Mishkin et al., 1983), or “what” vs. “how” (or perception vs. action) systems (Milner and Goodale, 1993). Other dichotomies within the visual projections exist; in the primate these are the M and P systems on the one hand (Merigan and Maunsell, 1993) and the geniculostriate and extrageniculostriate systems, on the other (Van Essen et al., 1992). Within the latter set of projections, visual information reaches the striate cortex principally by means of the dorsal lateral geniculate nucleus. It has been shown that this geniculostriate visual system is related primarily to objects placed within the central visual field or to the processing of information highly associated with fundamental aspects of visual perception (Orban, 1984). By contrast, the extrageniculostriate system is configured in such a way that visually elicited responses arrive to extrastriate, so-called “higher-order” visual cortices, by means of, for example, the superior colliculus (SC), as relayed through posterior thalamic nuclei (PTN) such as the lateral posterior (LP) complex (lateral division, LPl; medial division, LPm; lateralis medialis nucleus, LM) and the suprageniculate (Sg) nuclei (Hutchins and Updyke, 1988), to name just two routes. This system is known to be related to areas within the peripheral visual field, or to ambient vision and to a number of other roles in visual information processing, as well as in some nonvisual sensory processing (Jones, 1975; Casanova and Molotchnikoff, 1990; Shumikhina, 1993).
In the cat, for example, the lateral suprasylvian cortex (LS) is an important constituent member of this extrageniculostriate system. Descending projections also reveal the distinctiveness of the extrageniculostriate projection from its companion system. Different corticofugal pathways exist amongst the various extrastriate visual areas and may be associated with diverse roles in sensory and sensory-motor integration. The direct corticotectal projection from LS comprises an essential neural pathway for the performance of visually guided behaviors (Stein et al., 1993). Not only is this latter corticotectal pathway of critical importance for visual orientation, but another projection to the substantia nigra from the striatum provides a relatively important indirect route — the striatonigral pathway — for the expression of visual behaviors by way of a loop relaying information from LS through those structures and on to additional output centers (Norita et al., 1991b; McHaffie et al., 1993; Onodera and Hicks, 1995).
The lateralis medialis-suprageniculate nuclear (LM-Sg) complex in the PTN is connected reciprocally with the anterior ectosylvian visual area (AEV), LS, the deep layer of SC, and the substantia nigra (SN) (Graybiel and Berson, 1981; Takada et al., 1984; Norita et al., 1986, 1996). Furthermore, it has been reported that the neurons in this area of the thalamus respond to a considerably greater variety of visual stimuli than do thalamic cells of the dorsal lateral geniculate nucleus (Mason, 1981; Hicks et al., 1984; Hutchins and Updyke, 1989). Thus, LM-Sg may make very significant contributions to certain visually guided behaviors. Moreover, we have found in previous work that there are numerous classes of synaptic transmitters present, such as the EAAs, ACh, SP, and γ-aminobutyric acid (GABA), perhaps indicative of the fact that LM-Sg cells receive afferent fibers from a variety of subcortical structures and from visual cortical areas (Norita and Katoh, 1987; Onodera et al., 1991).
Given the apparent diversity of functional roles played by the cells of these nuclei and the obvious complexity of the network of afferent and efferent connections in place there (Takada et al., 1984; Fitzpatrick et al., 1984; Hoshino et al., 1993), it was considered a worthwhile goal to characterize the chemical identities and relative distributions of LM-Sg cells that contain amino acids, acetylcholine, and peptide transmitters. The eventual aim of this project is to correlate structure and function and to determine whether there might be regional differences in the chemical expression of their transmitters within LM-Sg. A second major impetus for this work derives from our recent observations that the dorsal LM-Sg receives a relatively denser projection of corticothalamic inputs than the ventral LM-Sg (Norita et al., 1996). Patel and Bickford (1997) showed in other visual thalamic regions that ChAT-immunoreactive (-ir) terminals are located in close proximity to corticothalamic terminals. Given the role that ACh is believed to have as a facilitator, or modulator, of synaptic transmission and plasticity (de Lima et al., 1985; Metherate et al., 1988; Dykes, 1997), it was considered useful to examine the relative distributions of types of excitatory and inhibitory amino acid transmitters in relation to ACh and SP.
The EAAs and GABA are the most prevalent synaptic transmitters of sensory pathways in the thalamus (Dykes et al., 1988; Onodera et al., 1991; Vahle-Hinz et al., 1994). ACh is present in many specific nuclei in cat and rat (Hökfelt et al., 1986; Vincent and Reiner, 1987; Onodera et al., 1991) and may be involved in certain aspects of sensory plasticity (Metherate et al., 1988), whereas peptides such as SP (Albus et al., 1992; Hicks et al., 1993) are known to play specific roles in the short-term modification of receptive field properties at certain neuronal types. Therefore, in the present study, we examined the ultrastructural organization of the neuronal elements within LM-Sg that the different transmitters are associated with the use of immunocytochemical techniques, and discuss the likely origins of the cells that project these transmitter-specific fibers. A part of this work has been reported previously in abstract form (Hoshino et al., 1993).
MATERIALS AND METHODS
All experimental procedures with animals and conduct relating to their humane use and treatment were approved by the Animal Care Committee of Niigata University. Four cats were anesthetized deeply with sodium pentobarbital (40 mg/kg, i.p.) and perfused transcardially with a 0.9% solution of saline followed by a fixative containing 4.0% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M sodium phosphate buffer (PBS), pH 7.4. The brains were removed, cut coronally with a microslicer (Dohan EM) into 50-μm-thick serial sections, and collected in the same buffer. Serially cut sections were numbered 1 to 6. For immunohistochemistry, free-floating sections were incubated for 1 hour with 10% normal goat serum at room temperature to reduce the level of nonspecific binding of antibody. Similarly numbered sections were then incubated overnight at 4°C in one of the following primary antisera: rabbit polyclonal anti-Asp and anti-Glu (used at a dilution of 1:5,000 in PBS), anti-SP (diluted 1:20,000) (all three provided courtesy of Dr. P. Petrusz, UNC-Chapel Hill), rabbit polyclonal anti-GABA (diluted 1:5,000, purchased from Boehringer), and rat monoclonal anti-ChAT (diluted 1:30, purchased from Boehringer). The staining with AChE allowed for the easier identification of the nuclear boundaries of LM-Sg (Graybiel and Berson, 1980). Sections were rinsed in PBS and then immersed in biotinylated anti-rabbit IgG or anti-rat IgG for 2 hours at room temperature. After exposure to the secondary antibody, sections were washed with PBS and placed in avidin-biotinylated horseradish peroxidase for 2 hours. They were transferred to a fresh solution of diaminobenzidine containing 0.01% H2O2 for 7 minutes. After completion of the immunohistochemical procedures, alternate groups of six sections (each section of a group reacted with a different antibody) were prepared for light and for electron microscopic observation, respectively. Six groups of six sections per animal were analyzed bilaterally; half were studied at the light and half at the electron microscopic level. For electron microscopy, sections from each of the four cats studied were immersed in 1% osmium tetroxide at room temperature for 1 hour, then dehydrated and flat-embedded in Epon. Areas in LM-Sg were selected and trimmed. Ultra-thin sections were cut and mounted on Formvar-coated, single-slot grids. Sections were examined and photographed with a Hitachi 7000 electron microscope.
For control purposes, some sections were incubated identically as described above, but with the omission of the primary antibodies. In all cases, the omission of these primary antibodies resulted in a complete lack of immunostaining of somata and axon terminals.
Measurements of the sizes of somata were performed by selecting the section in which the nucleolis was present or was most prominent, and taking the diameter of the soma at its greatest width. Different numbers of somata were measured for each antibody studied; these data appear in Figure 2, expressed as means ± SD.

Light photomicrographs illustrating somata and fibers of aspartate-immunoreactive (Asp-ir), glutamate-immunoreactive (Glu-ir), γ-aminobutyric acid–immunoreactive (GABA-ir), choline acetyltransferase–immunoreactive (ChAT-ir), and substance P–immunoreactive (SP-ir) neurons in lateralis medialis-suprageniculate nuclear. a: Asp-ir somata (arrows) are usually quite intensely stained; labeling extended into the primary dendrites and occasionally into secondary dendrites as well. Note the immunoreactive fibers in the neuropil (arrowheads). b: In contrast to the dense reactive products of the Asp-ir neurons, the somata of Glu-ir neurons (arrows) are usually stained only lightly; little staining is seen in the stem dendrites. c: The somata of GABA-ir neurons (arrow) are stained densely, with label occasionally extending into the stem dendrites. d: ChAT-ir labeling is restricted to fibers. e: Detail of SP-ir fiber structure are seen in this photomicrograph, taken from the indicated square shown in Fig. 3f, presented at relatively lower magnification. Scale bars = 20 μm.

Histogram displaying the mean diameters measured along their broadest axis of neuronal somata stained by aspartate (Asp), glutamate (Glu), and γ-aminobutyric acid (GABA) antisera in the lateralis medialis-suprageniculate nuclear complex. Ordinate scale expressed in micrometers. Error bars represent ± 1.0 SD. Difference between mean diameter of GABA- and both of Asp- and Glu-containing cells reaches statistical significance (see Results section).
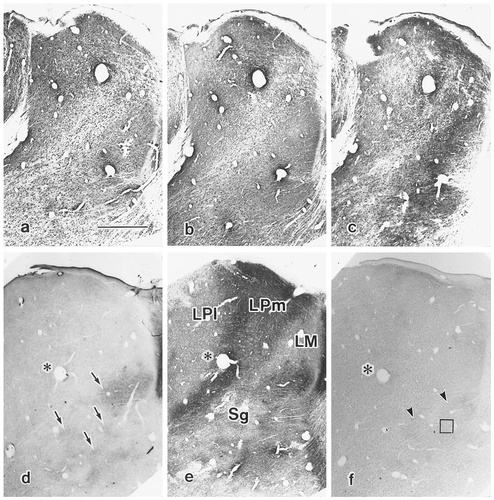
Low-power photomicrographs of the lateralis medialis-suprageniculate nuclear (LM-Sg) complex and surrounding regions showing staining of two sets of transverse serial sections (a–c,d–f) showing labeling for a: aspartate (Asp); b: glutamate (Glu); and c: γ-aminobutyric acid (GABA); and d: choline acetyltransferase (ChAT); e: acetylcholinesterase (AChE); and f: substance P (SP). Label with AChE enables the better delineation of the nuclear boundaries of the LM-Sg complex. Note the co-incidence of relatively more intense ChAT-positive patchy zones in the ventral aspect (arrows) of Sg compared with the sparser label seen dorsally and laterally (d); these patches also correspond with AChE-positive regions. Also note the fiber and puncta terminal-like label seen medially (arrowheads) for SP immunoreactivity (f). The square in f indicates the position from which the higher-power micrograph illustrated in Figure 1e was taken. The asterisks mark the same blood vessel and are shown as reference points in d–f. LPl, lateral posterior complex, lateral division; LPm, medial division; LM, lateralis medialis nucleus. Scale bar = 1.0 mm (applies to a–f).
To quantify the different densities of terminal labeling, composite photomontages of the thalamic posterior complex were constructed from photomicrographs and the sites of individual puncta as detected light-microscopically were plotted. Areas of the LM-Sg complex in which were found densities of 20 puncta or less per 100 μm2 were classified as “sparse,” of 20 to 100 as “intermediate,” and of 100 or more as “dense.” These classifications were used in the construction of schematic illustration of Figure 2.
RESULTS
Light microscopy
The results of the present study are in good agreement with our previous brief report on the localization and number of EAA-, SP-, and ChAT-ir neuronal elements in the cat LM-Sg (Onodera et al., 1991). The light photomicrographs shown in Figure 1 illustrate some typical examples of ir neurons and terminal-like elements as observed when examining LM-Sg. Asp-like-ir (Fig. 1a), Glu-like-ir (Fig. 1b), and GABA-like-ir (Fig. 1c) were found in neurons distributed throughout LM-Sg. No neuronal ChAT-ir somata (Fig. 1d) or SP-ir somata (Fig. 1e) were found. For each different antiserum used, an ir-fiber network also could be observed. This network varied in density, depending on the antibody used (Fig. 1); on the whole, GABA-ir fibers were highest in density, whereas SP-ir fibers were the weakest. The other antisera produced densities of relatively intermediate level.
In LM-Sg, Asp-ir is present both in the neuropil and in somata. Asp-ir somata (Fig. 1a) usually are stained quite intensely. Labeling extends into the primary dendrites and is seen occasionally as far as the secondary dendrites as well. The labeled somata are relatively large (mean, 30.24 ± 3.13 μm; n = 25) and are morphologically multipolar or triangular. The arrowhead in Figure 1a indicates immunoreactive fibers in the neuropil, where a considerably extensive network of such fibers is seen. Glu-ir neurons (Fig. 1b), resemble Asp-ir neurons both in terms of shape and of size and possess mean somata diameters of 29.71 ± 3.65 μm (n = 21; difference with Asp-ir somata not significant). By contrast with the dense labeling seen with the antiserum to Asp, that raised against Glu produced a relatively lighter, although still quite distinct stain, with little Glu-ir evident in the stem dendrites. GABA-ir, on the other hand, labeled neurons that possessed relatively small-sized somata (mean, 17.20 ± 2.28 μm; n = 20; difference with both EAA-ir somata sizes statistically significant, P < 0.001; Tukey-Kramer's test) and that exhibited a variety of morphologies, including oval, round, triangular, and polygonal. The somata of GABA-ir neurons (Fig. 1c) were stained densely, with immunoreactivity occasionally extending into the stem dendrites. The labeling of ChAT-ir and SP-ir was restricted to fibers (Fig. 1d,e). A comparison of the mean soma sizes of Asp-ir, Glu-ir, and GABA-ir neurons is presented in the histogram of Figure. 2.
A large number of labeled cells showing either Asp-ir or Glu-ir to an approximately equal extent are distributed evenly throughout LM-Sg (Fig. 3a,b). However, comparatively fewer GABA-ir somata were seen, and these latter differed somewhat from the distribution of the former EAAs (Fig. 3c). There were no somata observed to contain ChAT-ir or SP-ir in any region of LM-Sg.
With regard to the labeling of terminals and terminal-like elements with the various antisera, a homogeneous distribution throughout LM-Sg was observed with the exception of ChAT-ir and SP-ir. The ventral and ventromedial aspects of Sg showed an especially dense network of ChAT-ir fibers. For SP-ir, a dense network was observed along the medial border of LM-Sg. The density of terminal labeling with anti-GABA antibody always seemed greater than that of fibers labeled with any other antiserum. The pattern of punctal label seen in sections stained with ChAT and SP antisera can be observed in the example shown in Figure 3d,f. ChAT-ir fibers and terminal-like puncta are represented relatively more strongly in AChE-positive regions than in AChE-negative regions (compare Fig. 3d with 3e), and this relationship was more pronounced in the ventral areas of Sg than in LM. Although SP label was very sparse throughout LM-Sg, terminal-like puncta showed an uneven distribution with a denser margin of label running ventromedially along the border with rostral portions of the pretectal nucleus and the nucleus limitans.
Figure 4 schematically summarizes the different distributions of immunoreactive somata and fibers that are seen in LM-Sg. Immunoreactive somata are represented by the large circles, whereas the small dots represent immunoreactive fibers, terminal-like puncta, or both. The relative density of each symbol is proportional to the degree of label that is present in the corresponding area of the sections (see Materials and Methods section for description of quantitation).
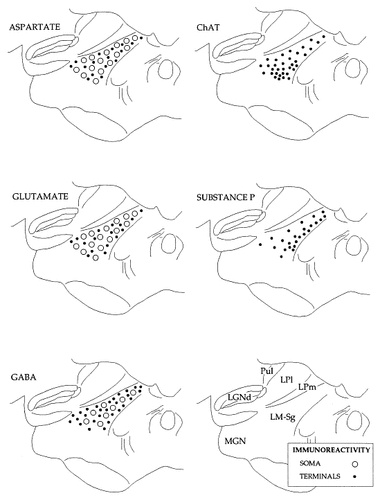
Immunoreactive neurons and fibers are distributed differentially in lateralis medialis-suprageniculate nuclear (LM-Sg) complex. The distribution of immunoreactive somata (large circles) and fibers/terminals (small dots) observed in LM-Sg is shown in this summary line drawing. The relative density of each symbol is proportional to the degree of labeling present. Note that no neurons immunoreactive for choline acetyltransferase (ChAT) or substance P could be detected in any region of LM-Sg. GABA, γ-aminobutyric acid; Pul, pulvinar; LPl, lateral posterior complex, lateral division; LPm, medial division; MGN, medial geniculate nucleus; LGNd, lateral geniculate nucleus, dorsal.
Electron microscopy
Neurons identified as Asp-ir, Glu-ir, and GABA-ir by light microscopy were subsequently re-examined with the electron microscope (Fig. 5). Asp-ir neurons (Fig. 5a) exhibited abundant cytoplasm rich in organelles, including lysosomes, microtubules, mitochondria, and a well-developed rough endoplasmic reticulum (ER) and Golgi apparatus. The immunoreactive products were particularly prominent in the rough ER, mitochondria, and microtubules. The nuclei were indented and consistently devoid of immunoreactive product. Cell bodies received contacts from only a few, if any, axon terminals. Although the synaptic contacts on these cells were difficult to categorize as symmetric or asymmetric because of the presence of immunoperoxidase reaction product associated with the postsynaptic membrane, most of the terminals were thought probably to be GABA-containing because they seemed to contain pleomorphic vesicles (Fig. 6a).
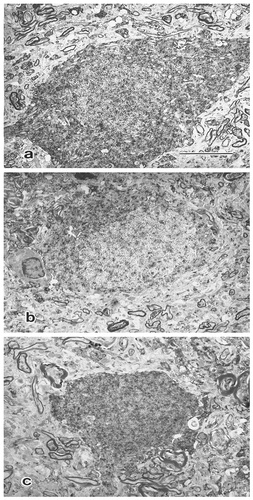
Electron photomicrographs showing the somatic profilesof aspartate-immunoreactive (Asp-ir), glutamate-immunoreactive (Gluir), and γ-aminobutyric acid-immunoreactive (GABA-ir) neurons in lateralis medialis-suprageniculate nuclear. a: Asp-ir neuron exhibits abundant perikaryal cytoplasm and is richly endowed with organelles. b: Glu-ir neuron is comparable to Asp-ir neuron. c: GABA-ir soma is small and has little perikaryal cytoplasm. Scale bar = 5 μm (applies to a–c).
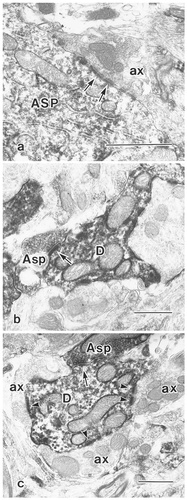
Electron photomicrographs showing the profiles of aspartate-immunoreactive (Asp-ir) neurons. a: Asp-ir soma (ASP) is contacted (arrows) by an unlabeled axon terminal (ax). b: An Asp-ir axon terminal (Asp), containing round synaptic vesicles, forms an asymmetrical synaptic contact (arrow) with an Asp-ir dendritic profile (D). c: An Asp-ir dendrite (D) makes a contact with an asp-ir terminal (Asp) and is contacted by three unlabeled axon terminals (ax). Arrows and arrowheads indicate postsynaptic sites. Scale bars = 0.5 μm in a–c.
Glu-ir somata (Fig. 5b) were comparable to Asp-ir neurons with respect to the abundance of cytoplasmic organelles and indented nuclei. The nuclei displayed deposition of immunoreactive product. Only a few axon terminals could be found making contact with the Glu-ir cell body.
GABA-ir somata (Fig. 5c) had small cytoplasmic compartments with a rich complement of organelles and contained indented nuclei. Immunoreactive products were observed on ER, mitochondria, and microtubules in the cytoplasm and in nuclei. A few axon terminals containing round synaptic vesicles were observed on the GABA-ir neuronal surface.
As described previously for LM-Sg (Norita and Katoh, 1987, 1988), at least four types of vesicle-containing profiles have been identified ultrastructurally. Briefly, these are large axon terminals containing round synaptic vesicles and a number of mitochondria (RL); small axon terminals having round vesicles (RS); small-to-medium axon terminals displaying a rather dark matrix and having pleomorphic vesicles (F1); and various-sized dendritic profiles displaying a rather pale matrix and having pleomorphic vesicles as well as clusters of ribosomes (presynaptic dendrite PSD, or F2). When these axon terminals were densely labeled immunohistochemically, it was sometimes difficult to identify immunoreactive synaptic processes on the basis of internal morphologic features alone. Therefore, distinctions between immunoreactive axonal and dendritic elements were based on (1) the polarity of a synapse, (2) the morphology of unlabeled synaptic processes that were in contact with the immunoreactive processes, and (3) the degree of asymmetry present in a synapse involving an immunoreactive process. Immunoreactive dendrites were identified by the criterion that the dendrites were postsynaptic to axon terminals featuring a variety of membrane specializations at the synaptic zone. Immunoreactive microtubules, running parallel to the long axis of processes, were a common feature of the dendrites.
Asp-ir was found in terminals that usually were of medium size (mean diameter, 0.70 ± 0.18 μm, n = 13), contained round vesicles and made asymmetrical synaptic contacts with neuronal elements having a dendritic profile. In 6 of 13 cases examined, these were observed to be structures that could be unambiguously identified as belonging to a synaptic glomerulus. In Figure 6b,c, Asp-ir axon terminals are observed that contain round synaptic vesicles and form asymmetrical synaptic contacts with Asp-ir dendritic profiles. Asp-ir dendrites frequently were found to be contacted by unlabeled axon terminals having round synaptic vesicles making asymmetrical synaptic contacts (Fig. 6c).
Glu-ir in somata and terminals exhibits features very similar to those seen in elements of the neuropil that possess Asp-ir. An axon terminal exhibiting Glu-ir is seen in Figure 7a. It contains round synaptic vesicles, forms an asymmetrical synaptic contact with a neuronal element having a Glu-ir dendritic profile (Fig. 7a), whereas a Glu-ir dendrite is seen occasionally to receive input from unlabeled axon terminals (Fig. 7a,b). Glu-ir terminals had a mean diameter of 0.69 ± 0.21 μm, (n = 14). Three of 14 cases examined allowed the assignment of a terminal to a glomerulus.

Electron photomicrographs showing typical glutamate-immunoreactive (Glu-ir) profiles. Glu-ir immunoreactive somata and terminals have properties similar to aspartate-ir (Asp-ir) neurons. a: A Glu-ir axon terminal (Glu), containing round synaptic vesicles, forms an asymmetrical synaptic contact (arrow) with a Glu-ir dendritic profile (D). b: A Glu-ir dendrite (D) receives contacts by three unlabeled axon terminals (ax). Arrowheads indicate postsynaptic sites. Scale bars = 0.5 μm in a,b.
GABA-ir found in neuronal profiles lying within the neuropil of LM-Sg was identified as being in either the presynaptic dendrites of interneurons or in axonal fibers and terminals (Fig. 8). Neuronal elements having a GABA-ir dendritic profile contain pleomorphic synaptic vesicles and of 13 cases examined, 5 were found clearly to be participating in a synaptic glomerulus. By contrast, axonal terminals that display GABA immunoreactivity are found mainly in the extra-glomerular neuropil (9 of 16 cases examined showed the terminal to be a glomerular component; see also Ohara et al., 1983; Norita and Katoh, 1987); however, sometimes a few GABA-ir terminals could be found on unlabeled cell bodies. Whereas GABA-ir terminals seemed to be somewhat smaller on average than those staining with anti-Asp or anti-Glu antisera, statistically this apparent difference did not reach significance (mean diameter, 0.59 ± 0.11 μm; n = 16). Figure 6a illustrates a GABA-ir dendrite making synaptic contact with an unlabeled dendritic and unlabeled axonal element. Figure 6b illustrates a GABA-ir dendrite making a symmetrical synaptic contact with a nonreactive dendrite, and this GABA-ir dendrite receives input from an unlabeled axon terminal containing round vesicles that makes a synaptic contact with another unlabeled dendrite. The immunopositive dendrite contained pleomorphic vesicles. These elements resemble those known to take part in glomerular structures; thus, these GABA-ir dendrites may be synonymous with the presynaptic dendrites of interneurons described previously by Norita and Katoh (1987).
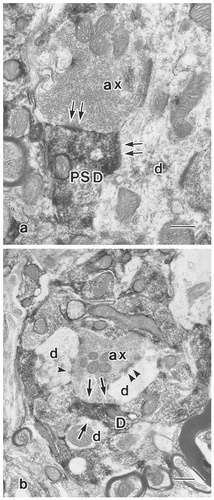
Electron photomicrographs showing γ-aminobutyric acid-immunoreactive (GABA-ir) profiles. a: A GABA-ir dendrite (PSD), containing pleomorphic vesicles, forms symmetrical synaptic contacts (arrows) with an unlabeled dendrite (d) and with an unlabeled axon (ax). b: A GABA-ir dendrite (D), containing microtubules and pleomorphic vesicles, makes symmetrical synaptic contact (single arrow) with an unlabeled dendrite (lower d). This GABA-ir dendrite also receives contact from an unlabeled axon terminal (ax) with an asymmetrical synaptic specialization (double arrows) making synaptic contact (arrowheads) with an another unlabeled dendrite (lower d). Scale bars = 0.5 μm in a,b.
ChAT-ir located in axon terminals revealed that the labeled elements seem to be of small-to-medium size (mean diameter, 0.47 ± 0.07 μm, n = 16; difference with other terminal diameters, however, not statistically significant). They contained rather large, round synaptic vesicles and often formed asymmetrical synaptic contacts with unlabeled elements having dendritic profiles (Fig. 9a). Most seemed not to participate in the synaptic glomerulus (only 5 of 16 examined were clearly glomerular). Occasionally, however, large-sized terminals were seen (there were two cases where the size was >0.8 μm diameter, and another two cases where the size was 0.8 μm diameter) in the glomerular-like synaptic complex where the terminal made synaptic contact with conventional dendrites (Fig. 9b). Sometimes ChAT-ir axons formed en passant terminals and made synaptic contacts with unlabeled dendrites.
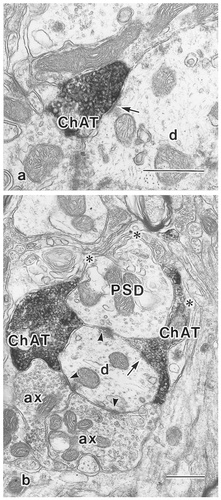
An electron photomicrograph showing a choline acetyltransferase-immunoreactive (ChAT-ir) axon terminal. a: ChAT-ir axon terminals (ChAT) containing large round synaptic vesicles typically are very small and often formed asymmetrical synaptic contact (arrow) with an unlabeled dendritic profile (d). b: In the glomerulus-like synaptic complex, a ChAT-ir terminal (ChAT) forms an asymmetrical synaptic contact (arrow) with unlabeled conventional dendrite (d) receiving contacts from two axon terminals (ax) and a vesicle-containing dendrite (PSD) (arrowheads). Note uncompleted glial enveloping is seen around this synaptic complex (asterisks). Scale bar = 0.5 μm in a,b.
SP-ir found in axon terminals typically also labeled structures of small-to-medium diameter (mean, 0.47 ± 0.07 μm; n = 10) containing round synaptic vesicles (Fig. 10). Between 80 and 90% of these SP-ir–labeled terminals were seen forming asymmetrical contacts with unlabeled elements having dendritic profiles.
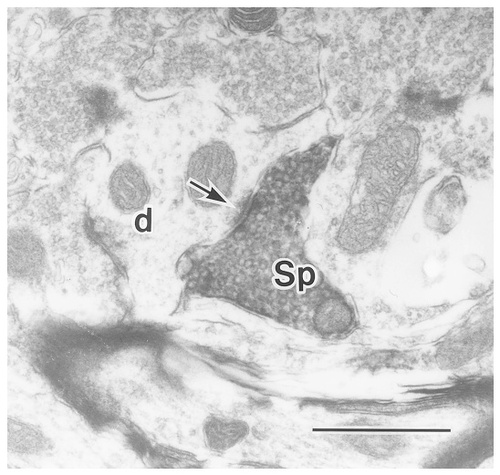
An electron photomicrograph showing a substance P–immunoreactive (SP-ir) axon terminal. The SP-ir axon terminal (SP) typically is small and contains round synaptic vesicles. This type of terminal usually is seen forming asymmetrical contact (arrow) with an unlabeled dendritic profile (d). Scale bar = 0.5 μm.
DISCUSSION
The LM-Sg of the cat is well recognized as belonging to the extrageniculate visual thalamus (Hutchins and Updyke, 1988), which, however, functions not only in the processing of visual information but also in the synaptic mediation of other modalities of sensation (Huang and Lindsley, 1973). These diverse operations accrue through the accessing of sensory information by means of cortical and subcortical routes. Perhaps as a consequence of this diversity, and in keeping with a likely role for LM-Sg as a structure providing polysensory integration (Benedek et al., 1996), we have observed from the present analysis that a broad range of transmitter-specific markers is found in its constituent neurons, including, but undoubtedly not limited to, Asp, Glu, GABA, ChAT, and SP. One of the hypotheses driving the present investigation was the idea that there might be some relationship found between areal distribution of transmitter label or pattern of synaptic connectivity that could be linked to functional differences known to exist within the LM-Sg (Hicks et al., 1984; Benedek et al., 1996).
From evidence gained from previous studies, relay neurons in the thalamus typically are characterized by large somata and, consistent with the proposed role of EAAs as mediators of thalamocortical transmission (Oka et al., 1987; Hagihara et al., 1988; Hicks et al., 1988c, 1991; Tamura et al., 1990; Hicks, 1995), Asp-ir and Glu-ir were seen distributed widely throughout this nucleus, contained for the most part in large somata. GABA is found mostly in neurons with relatively smaller somata, and this also is in line with the conventional view that intrinsic interneurons process inhibitory information in local circuit manner and for the most part do not project cortically (Jones, 1975; Palestini et al., 1993).
At least four types of vesicle-containing profiles have been identified ultrastructurally in the PTN complex, including LM-Sg, and the origin of some of these terminals as well as their immunoreactive characteristics have been described in part previously (Hajdu et al., 1974, 1976; Norita and Katoh, 1987, 1988; Kaneko and Mizuno, 1988). For example, F2 is derivative of the Golgi type II interneuron in the LM-Sg and is GABA-ir (Norita and Katoh, 1987). The types of terminals projecting from visual cortical areas to the PTN are RS and RL (Mathers, 1972; Hadju et al., 1976). In this study, the majority of Asp-ir and Glu-ir terminals were of the RS type, and the remainder was RL. In various immunocytochemically treated tissues (e.g., Figs. 6, 7), it was observed that many RS profiles remain unstained for either Asp-ir or Glu-ir. At least two possibilities can be suggested to account for this lack of label. First, it may be that the unlabeled RS or RL terminals derive from SC or PPT and simply are not EAA-containing. Second, it may be that all RS and RL profiles are EAA-containing, but for any of a myriad of possible reasons, they do not react with the antibody. For example, there may be subtle differences in the cytoplasmic membrane of only some Asp- and Glu-containing cells that prevent access of the antibody to the amino acid; alternatively, the tissue penetration of the antibody may have been uneven and, thus, inadequate to stain all EAA-containing terminals. Interpretation of negative results is, therefore, always problematic.
Vahle-Hinz et al. (1994) presented neuropharmacologic evidence in support of an important role for EAAs in mediating transmission within a thalamic sensory relay nucleus in cats studied in vivo but were unable to block naturally induced synaptic driving from the periphery by using then-available EAA antagonists. Accordingly, it was not possible to determine whether the observed synaptic excitations derived from Asp-releasing or Glu-releasing neurons, if indeed such distinct forms of synaptic relay do exist. However, it is now well known that synaptic excitation from the thalamus to cortex is mediated by EAA receptors of both N-methyl-D-aspartate and non–N-methyl-D-aspartate varieties and, moreover, that in the unanesthetized animal both types of excitation contribute to synaptic transmission at normal resting potentials (Swadlow and Hicks, 1997). Whether this synaptic drive derives from separate populations of thalamic-based Asp- and Glu-releasing cells, or a single population of EAA cell that releases both Asp and Glu simultaneously or “discretionary” according to prevailing conditions, remains undetermined because of the limitations of investigative tools currently at our disposal. Certainly, the anatomic data presented here does little to encourage continuance of the view that separate populations of Asp- and Glu-containing cells exist in the thalamus and act separately, although there is evidence to suggest that such separate populations of cells do exist in visual thalamocortical pathways and that there may in certain systems be a preferential release of Asp and Glu (Hicks et al., 1985). If indeed separate populations of EAA-containing somata exist, it would be a reasonable expectation that there should be differences in distribution of Asp-ir and Glu-ir within a given relay nucleus or nuclei, or that the staining intensity for one EAA over the other would be greater than that seen here, or that soma/terminal size would differ in some systematic way. However, this study uncovered no such suggestive data; a resolution to this problem may await new approaches contingent on technological development or novel, innovative strategies.
Whereas it has been proposed often that Asp, Glu, or both, are used as important transmitters mediating excitatory synaptic transmission between thalamus and cortex, it is not yet established firmly that this is so also for the reverse projection: that is, that the terminal labeling in LM-Sg showing Asp-ir and Glu-ir reflects corticofugal input. However, we have shown that the neurons projecting to cortex (LS, AEV), or to striatum, were Asp-ir, Glu-ir, or both, by means of a double-labeling technique by using wheat germ agglutinin-horseradish peroxidase (WGA-HRP) and immunohistochemistry (Hoshino and Norita, 1997, 1998). In addition, because some thalamic neurons project their axons to the amygdala (LeDoux and Farb, 1991) and to cortex (Norita et al., 1986; Weinberg and Kharazia, 1996), the possibility should be entertained that some proportion of the EAA-labeled terminals seen in LM-Sg derive from thalamoamygdaloid, thalamostriatal, or thalamocortical axon collaterals. There are also known axon collaterals deriving from neurons of the LP-pulvinar complex; these are Asp-ir and Glu-ir (Palestini et al., 1993).
In the present study, GABA-ir profiles were found throughout LM-Sg and were observed in intra- and extra-synaptic glomeruli. In the glomerulus, the majority of these are considered to be dendrites and axons of interneurons, based on related observations made in previous studies (Norita and Katoh, 1987). GABA-ir terminals in extra-glomerular regions are axon terminals projecting from the reticular nucleus of the thalamus (Jones, 1975, Norita and Katoh, 1987) and the substantia nigra, pars reticulata (Takada et al., 1984; Mendez et al., 1993). ChAT-ir terminals are distributed throughout LM-Sg, predominantly in the ventral part of Sg. Fitzpatrick et al. (1989) showed that LM-Sg is also rich in cholinergic fibers in a report describing the projection from the pedunculopontine tegmental nucleus (PPT) to lateral geniculate nucleus of the cat by means of a double-labeling technique by using anterograde tracing and immunohistochemistry. In addition, Hallanger et al. (1987) in the rat and Hoshino et al. (1997) in the cat have demonstrated the existence of ChAT-ir neurons of PPT after WGA-HRP injection in LM-Sg. In the present study, because no ChAT-ir neurons were found in LM-Sg (see Kimura et al., 1981; Armstrong et al., 1983; Mesulam et al., 1984; Vincent and Reiner, 1987), it is highly unlikely that the ChAT-ir terminals we observed are LM-Sg-fugal axon collaterals, although it should be noted in this context that thalamostriatal fibers have been reported as being cholinergic (Saelens et al., 1979).
It has been shown in numerous studies that ChAT-ir terminals participate in the formation of glomeruli (n. medialis dorsalis of the thalamus in monkey, Schwarts and Mrzljak, 1993; dorsal lateral geniculate n. in cat, de Lima et al., 1985; olfactory bulb in rat, Jeune and Jourdan, 1993; pulvinar n. in the cat, Patel and Bickford, 1997). In the present study, although a small number of ChAT-ir terminals seemed to participate in the glomerulus, most of the ChAT-ir terminals were found to be in the extra-glomerular neuropil. Furthermore, Patel and Bickford (1997) showed in the dLGN or pulvinar that ChAT-ir terminals are located in close proximity to corticothalamic terminals. In the LM-Sg, as the terminals from cortex (e.g., LS) project relatively more densely to the dorsal part of this nucleus (Norita et al., 1996), it was suggested that the form of mediation of synaptic transmission in this ChAT-rich area could be different and, thereby, reflect a difference in synaptic processing for the region thought to be primarily involved in visually guided behavior compared with the region concerned with visual sensation.
Hicks et al. (1984) and Benedek et al. (1996) have reported crude visuotopic organization to exist in LM-Sg and other modalities than vision to be represented by subclasses of neurons. However, neither study was able to determine a consistent dorsal-ventral segregation of modality expression, which would be consistent with the different densities of corticothalamic terminals. Thus, the close association of ChAT-positive terminals with putative glutamate-containing cortical afferents in LM-Sg likely subserves some functional role distinct from modality expression. A possible role for this association could be the facilitation of synaptic plasticity. Indirect support for this thesis comes from the report of Salt and Eaton (1996) who presented evidence that sensory corticothalamic projections releasing glutamate have their information processed at least minimally through receptors of the metabotropic subtype and these receptors are well known to participate in processes of expression of synaptic plasticity (Tsumoto, 1990; Haruta et al., 1994; Kamishita et al., 1995; Hicks and Conti, 1996).
Regarding the likely sites of origin of the SP-ir terminals, one possibility is that they derive from neurons of SC. Graybiel and Berson (1981) have reported that many fibers project from the deep layers of SC to LM-Sg, and Hutsler and Chalupa (1991) have showed that SP-ir fibers project from sublayer ll of SC to PTN, especially to LPm. Furthermore, Katoh and Benedek (1995) have described retrograde-labeled neurons in SC after the injection WGA-HRP into Sg. The spinal cord also provides a further likely source of SP input. Battaglia et al. (1992) showed SP-ir fibers projecting from cervical spinal levels to the medial part of LM and Sg. Furthermore, PPT is yet another candidate, because it is known that about one-third of the cells of PPT that are ChAT-ir also contain SP (Vincent et al., 1983).
Casanova and Molotchnikoff (1993) showed in rabbit that the visual activation of about one-third of the cells in LP is modulated by the activity of SC, whereas the remaining two-thirds are little affected by collicular modulation. Corticofugal activity also seemed to be effective in modifying the responses of cells to visual stimuli. Chalupa and Abramson (1988) showed that cells of the cat's LP-pulvinar complex are relatively more turned for orientation than was reported for cells of LM-Sg (Hicks et al., 1984), although both regions contain cells with very large receptive fields.
In the macaque, Bender (1982) showed that cells of the inferior pulvinar have properties very similar to those reported for the striate cortex and dissimilar to those of SC, in contrast to the situation in the rabbit (Casanova and Molotchnokoff, 1993). Indeed, in macaques, lesions of SC do little to modify response properties of inferior pulvinar cells (Bender, 1988). In the cat, however, Hicks and colleagues (Benedek and Hicks, 1988; Hicks et al., 1988a,b) reported that the visual response properties of cells of the insular visual area (IVA), a region of the cortex that forms close anatomic links with the extrageniculate thalamus (Norita et al., 1991a), are remarkably similar to those of LM-Sg.
Niebur et al. (1993) proposed a very close link between cells of the primate pulvinar and extrastriate cortex in processes of attention, a role that may be considered to be consistent with the present findings of synaptic modulating substances such as markers for ChAT and SP in terminals of PTN. Of course, given the existence of interspecific differences in the brain areas responsible for visual processing among those previously mentioned, it is difficult to draw too many general conclusions about the functional roles of the various structures involved in thalamocortical processing, despite a common nomenclature. Nevertheless, the pharmacologic and synaptic effects of the substances studied histochemically here are consistent and predictable across species; therefore, certain generalizations may be considered appropriate. Whether or not these substances actually are released within LM-Sg during sequences of visual activation (Olshausen et al., 1993) cannot of course be shown by using the techniques used in the present investigation; therefore, this possibility must await further study by using different experimental approaches. However, modulating events such as might be needed for the control of attention, incorporating appropriate synaptic transmitter candidates, and glomerular synaptic architectures, are in place within the extrageniculate visual thalamus. It will be for future studies that use pharmacologic and electrophysiological techniques comparing multimodal and specific relay nuclei to determine whether such links in fact do exist, correlating neurochemical identity and functional role subserving sensory processing and synaptic plasticity.
Acknowledgements
The authors thank Mr. Seiji Takahashi for his skillful technical assistance. M.N. received support from the Japanese Ministry of Education, Science, and Culture and the Nissan Science Foundation, and T.P.H. received intramural support from NRC of Canada.




