Distribution of GAD-immunoreactive neurons in the diencephalon of the African lungfish Protopterus annectens: Colocalization of GAD and NPY in the preoptic area
Abstract
The distribution of GABAergic neurons was investigated in the diencephalon of the African lungfish, Protopterus annectens, by using specific antibodies directed against glutamic acid decarboxylase (GAD). A dense population of immunoreactive perikarya was observed in the periventricular preoptic nucleus, whereas the caudal hypothalamus and the dorsal thalamus contained only scattered positive cell bodies. Clusters of GAD-positive cells were found in the intermediate lobe of the pituitary. The diencephalon was richly innervated by GAD-immunoreactive fibers that were particularly abundant in the hypothalamus. In the periventricular nucleus, GAD-positive fibers exhibited a radial orientation, and a few neurons extended processes toward the third ventricle. More caudally, a dense bundle of GAD-immunoreactive fibers coursing along the ventral wall of the hypothalamus terminated into the median eminence and the neural lobe of the pituitary. Double-labeling immunocytochemistry revealed that GAD and neuropeptide tyrosine (NPY)-like immunoreactivity was colocalized in a subpopulation of perikarya in the periventricular preoptic nucleus. The proportion of neurons that coexpressed GAD and NPY was higher in the caudal region of the preoptic nucleus. The distribution of GAD-immunoreactive elements in the diencephalon and pituitary of the African lungfish indicates that GABA may act as a hypophysiotropic neurohormone in Dipnoans. The coexistence of GAD and NPY in a subset of neurons of the periventricular preoptic nucleus suggests that GABA and NPY may interact at the synaptic level. J. Comp. Neurol. 419:223–232, 2000. © 2000 Wiley-Liss, Inc.
γ-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the central nervous system (CNS) of vertebrates. The distribution of GABA-containing neurons has been investigated by immunohistochemistry in the brain of mammals (for review, see Mugnaini and Oertel, 1985), birds (Domenici et al., 1988; Granda and Crossland, 1989), reptiles (Bennis et al., 1991), amphibians (Franzoni and Morino, 1989; Naujoks-Manteuffel et al., 1994), teleosts (Martinoli et al., 1990), elasmobranchs (Nicholson et al., 1994), cyclostomes (Schotland et al., 1996), andprotochordates (Anadón et al., 1998). In all species studied so far, GABA-immunoreactive cell bodies and fibers are widely distributed throughout the brain, from the olfactory bulbs to the caudal rhombencephalon. GABAergic neurons are particularly abundant in the diencephalon, notably in the hypothalamic nuclei (for review, see Tappaz et al., 1982). These neurons project dense bundles of fibers to the neurointermediate lobe of the pituitary in mammals (for review, see Tappaz et al., 1982) and amphibians (Franzoni and Morino, 1989; De Rijk et al., 1990; Tonon et al., 1992), and to the intermediate and distal lobes in fish (Kah et al., 1987b).
Neuropeptide tyrosine (NPY) is one of the most abundant regulatory peptide in the CNS. Prominent populations of NPY-containing neurons are present in the hypothalamus of mammals (Pelletier et al., 1984; Chronwall et al., 1985; De Quidt and Emson, 1986), birds (Aste et al., 1991), reptiles (Reiner and Oliver, 1987), amphibians (Danger et al., 1985; Perroteau et al., 1988), teleosts (Pontet et al., 1989; Danger et al., 1991) and elasmobranchs (Vallarino et al., 1988). Dense networks of NPY-containing nerve fibers are found in the median eminence of most species, as well as in the pars intermedia of the pituitary in amphibians and fish (for review, see Danger et al., 1990).
Coexistence of GABA and NPY has been observed in the CNS of mammals (Hökfelt et al., 1987; Polgár et al., 1999), reptiles (Davila et al., 1991), amphibians (Jansen et al., 1997), and teleosts (Yáñez et al., 1997). In the hypothalamo–pituitary complex, GABA and NPY occur in the same neurons of the arcuate nucleus in the rat (Meister et al., 1989; Horvath et al., 1997), in nerve fibers innervating the pars intermedia in the toad Xenopus laevis (De Rijk et al., 1990, 1992) and the frog Rana ridibunda (Tonon et al., 1992), and in neurons of the saccus vasculosus in the trout Salmon trutta fario and Oncorhynchus mykiss (Yáñez et al., 1997). In amphibians, both GABA and NPY are potent inhibitors of the secretory and electrophysiological activity of melanotrope cells (Adjeroud et al., 1986; Danger et al., 1986, 1987; Verburg van Kemenade et al., 1987a,b; Louiset et al., 1990; Chartrel et al., 1991; Valentijn et al., 1994).
The transition between fish and amphibians during the Devonian period, i.e., some 380 million years ago, was one of the major events in the evolution of vertebrates (for review, see Bemis et al., 1986). Lobe-finned fish, which today comprise lungfish and coelacanth, diverged from the other Osteichthyii at this period. Lungfish thus represent a group of particular interest in which to investigate the evolutionary transition between fish and tetrapods (Romer, 1966; Miles, 1977; Rosen et al., 1981). We have previously described the distribution of NPY-containing neurons in the brain of the African lungfish Protopterus annectens, and we have shown the occurrence of numerous cell bodies and fibers in the hypothalamus (Vallarino et al., 1995b). In contrast, to our knowledge, the mapping of GABA-containing neurons has not yet been determined in the CNS of Dipnoans.
In the present study, we have used an antibody against glutamic acid decarboxylase (GAD) to investigate the distribution of GABAergic neurons in the diencephalon of P. annectens. Since the prominent population of GAD-immunoreactive cell bodies was found in the periventricular preoptic nucleus, which also contains numerous NPY-positive perikarya, we have investigated by double-labeling immunocytochemistry the possible colocalization of GAD and NPY in this hypothalamic region.
MATERIALS AND METHODS
Animals
Four adult and four juvenile specimens of African lungfish, P. annectens, of both sexes were used in the present study. The animals, captured in October, were maintained for 2 weeks in aquaria at constant temperature (18 ± 0.5°C) with a natural light/dark cycle (12 hours light per day), and were fed daily. The animals were killed by decapitation, and the brains with the attached pituitaries were quickly dissected. Animal manipulations and experimental protocols were performed according to the recommendations of the Ethical Committees at our institutions and under the supervision of authorized investigators.
Antisera
The antiserum against rat glutamate decarboxylase was developed in sheep (Oertel et al., 1981). The antiserum against porcine neuropeptide Y was raised in rabbit (Pelletier et al., 1984). Fluorescein isothiocyanate-conjugated donkey anti-sheep γ-globulins (DAS/FITC) was supplied by Jackson Immuno Research Laboratories (Milan, Italy) and Texas Red-conjugated donkey anti-rabbit γ-globulins (DAR/TXR) was purchased from Amersham International (Buckinghamshire, UK).
Immunocytochemical procedure
The tissues were fixed for 12 hours in Bouin solution, dehydrated in ethanol, cleared in xylene, and embedded in paraplast. Serial coronal sections of the diencephalon (7-μm thick) were cut and mounted on chrome-alum gelatin-coated slides. Tissue sections were rehydrated and processed for indirect immunofluorescence microscopy. Briefly, the sections were rinsed in phosphate-buffered saline 0.5 M (PBS, pH 7.4) and incubated overnight at 4°C in a moist chamber with the sheep antiserum against rat GAD (diluted 1:800 in a PBS solution containing 0.3% Triton X-100 and 1% bovine serum albumin). The sections were rinsed three times and incubated for 90 minutes at room temperature with DAS/FITC (1:100). For colocalization studies, the periventricular preoptic nucleus of three specimens was arbitrarily subdivided into four regions of equal length (A–D; 200-μm-long each). Serial sections were incubated simultaneously with the GAD antiserum and the NPY antiserum (1:800 each), and the immunoreactivity was revealed with DAS/FITC and DAR/TXR. Finally, the sections were rinsed three times in PBS and mounted in PBS–glycerol (1:1). The preparations were examined under a Leitz Orthoplan microscope equipped with a Vario-Orthomat photographic system. Selected sections were analyzed using a confocal laser scanning microscope (CLSM) (Leica, Heildelberg, Germany) equipped with a Diaplan optical system and an argon/krypton ion laser (excitation wavelengths, 488/568/647 nm). Dual-channel CLSM analysis was performed using a bandpass filter (λ = 535 ± 7 nm) for detection of FITC and a longpass filter (λ = 610 nm) for detection of TXR. Microphotographs were taken using a Polaroid Freeze Frame video recorder (Leica, Heidelberg, Germany). To check the specificity of the immunoreaction, the following controls were performed: (1) omission of one of the steps of the immunocytochemical procedure; (2) replacement of the primary antiserum by preimmune sheep or rabbit serum, or PBS; and (3) preadsorption of the NPY antiserum with synthetic porcine-NPY (10−6 M).
Nomenclature of diencephalic areas of P. annectens was based on the work of Northcutt (1977) and Reiner and Northcutt (1987).
Statistical analysis
Statistical analysis of the quantification of GAD and NPY-positive cell bodies was performed by using the Student–Newman–Keuls test. Differences were taken to be statistically significant at P < 0.05.
RESULTS
Immunohistochemical localization of GAD
The distribution of GAD-immunoreactive cell bodies and nerve fibers in the diencephalon of P. annectens is schematically illustrated in Figure 1, with additional photographic documentation where appropriate. The relative density of GAD-immunoreactive elements is reported in Table 1. No differences in the distribution and abundance of GAD immunoreactivity were observed between male and female animals. Similarly, no differences in the distribution of GAD-immunoreactive neurons were observed between adult and juvenile animals.

Schematic coronal sections depict the distribution of glutamic acid decarboxylase (GAD)-like immunoreactivity in the diencephalon of Protopterus annectens. GAD-immunoreactive perikarya and fibers are shown as large dots and small dots, respectively. The relative density of the symbols is meant to be proportional to the density of the immunoreactive elements. The letters under the drawings refer to the rostrocaudal levels of the sections, as indicated on the sagittal section. Apr, anterior preoptic area; Apt, pretectal area; Ca, anterior commissure; Dp, dorsal pallium; H, habenula; Hyd, dorsal hypothalamus; Hyv, ventral hypothalamus; I, intercalate nucleus; In, infundibulum; Lfb, lateral forebrain bundle; Lp, lateral pallium; Mfb, medial forebrain bundle; Ncp, posterior commissure nucleus; Nflm, nucleus of the medial longitudinal fascicle; Npp, periventricular preoptic nucleus; Nt, terminal nerve; Pd, pituitary, distal lobe; Pg, periaqueductal gray; Pi, pituitary, intermediate lobe; Pn, pituitary, neural lobe; Sc, central subpallium; Sl, lateral subpallium; Te, tectum of the mesencephalon; Tg, tegmentum of the mesencephalon; Thd, thalamus, dorsal part; Thv, thalamus, ventral part; Vd, diencephalic ventricle.
| Structures | GAD-ir cell bodies | GAD-ir fibers | NPY-ir cell bodies2 | NPY-ir fibers2 |
|---|---|---|---|---|
| Diencephalon | ||||
| Anterior preoptic area (Apr) | − | + | + | − |
| Habenula (H) | − | − | − | + |
| Thalamus, dorsal part (Thd) | ||||
| Rostral–medial region | − | − | − | − |
| Lateral region | + | + | − | − |
| Medial region | − | − | − | + |
| Thalamus, ventral part (Thv) | ||||
| Medial region | − | − | + | ++ |
| Lateral region | − | − | − | + |
| Periventricular preoptic nucleus (Npp) | +++ | +++ | ++ | +++ |
| Habenular commissure | − | − | − | + |
| Dorsal hypothalamus (Hyd) | − | + | ++ | ++ |
| Ventral hypothalamus (Hyv) | + | ++ | +++ | +++ |
| Postoptic decussation | − | +++ | − | + |
| Pretectal area (Apt) | − | − | − | − |
| Median eminence | − | +++ | − | + |
| Pituitary | ||||
| Pituitary, distal lobe (Pd) | − | − | − | − |
| Pituitary, intermediate lobe (Pi) | ++ | − | − | − |
| Pituitary, neural lobe (Pn) | − | +++ | − | + |
- 1 GAD-ir, glutamic acid decarboxylase immunoreactive; NPY-ir, neuropeptide tyrosine immunoreactive; +, low density; ++, moderate density; +++, high density; −, no immunoreactive cell bodies or fibers.
- 2 Vallarino et al., 1995b.
Cell bodies.
A prominent population of GAD-positive neurons was localized in the periventricular preoptic nucleus (levels b–d in Fig. 1). These neurons surrounded the third ventricle and projected long processes toward the lateral region of the brain (Fig. 2a). The cell bodies generally exhibited a round shape and a thin fluorescent cytoplasmic rim (Fig. 2b). Scattered GAD-immunoreactive perikarya were also observed in the lateral part of the dorsal thalamus (level c in Fig. 1) and in the caudal region of the ventral hypothalamus (level g in Fig. 1). In addition, a few clusters of brightly immunofluorescent cells were seen in the intermediate lobe of the pituitary (Fig. 2c; level g in Fig. 1).
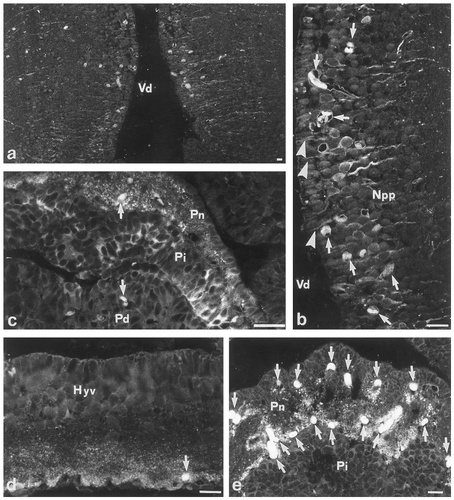
Immunofluorescence microphotographs illustrate the location of glutamic acid decarboxylase (GAD)-like immunoreactivity in the hypothalamus of Protopterus annectens. a: Coronal section through the medial subdivision of the periventricular preoptic nucleus shows numerous immunoreactive perikarya and fibers surrounding the third ventricle (Vd). b: At higher magnification, GAD-containing neurons appear round and exhibit a thin cytoplasmic rim. A few neurons send processes (arrowheads) toward the wall of the third ventricle. Npp, periventricular preoptic nucleus. c: Sagittal section through the rostral region of the pituitary shows the presence of clusters of GAD-immunoreactive cells in the intermediate lobe (Pi) and a dense fiber network in the neural lobe (Pn). In contrast, the distal lobe (Pd) is totally devoid of GAD-immunoreactive elements. d: Coronal section through the ventral hypothalamus (Hyv) shows the presence of a high density of GAD-immunoreactive nerve fibers in the rostral part of the nucleus. e: Coronal section through the pituitary shows a dense accumulation of brightly immunofluorescent fibers in the neural lobe (Pn). The arrows indicate nonspecific labeling of red blood cells. Scale bars = 50 μm.
Fibers.
The diencephalon was richly innervated by GAD-positive fibers, particularly in the hypothalamic area (levels a–g in Fig. 1). In the periventricular preoptic nucleus, GAD-immunoreactive fibers were oriented laterally (Fig. 2a) and a few neurons sent processes toward the third ventricle (Fig. 2b). More caudally, a conspicuous bundle of GAD-immunoreactive fibers exhibiting a rostrocaudal orientation was seen coursing along the ventral wall of the hypothalamus (Fig. 2d; levels e–g in Fig. 1). A dense network of fibers and nerve terminals was also observed in the median eminence (level g in Fig. 1). In contrast, the dorsal diencephalon only contained scattered GAD-positive processes located in the lateral region of the dorsal thalamus (level c in Fig. 1). Finally, numerous GAD-immunoreactive fibers were detected in the neural lobe of the pituitary (Fig. 2e; levels g, h in Fig. 1).
Colocalization of GAD and NPY-like immunoreactivity
Double labeling with the sheep GAD antiserum revealed by a second antibody coupled to FITC and the rabbit NPY antiserum revealed by a second antibody coupled to TXR was performed on coronal sections through the periventricular preoptic nucleus. Several neurons exhibited both GAD- and NPY-like immunoreactivity (Fig. 3a–c). However, many GAD-containing cell bodies were not immunolabeled with the NPY antibodies (Fig. 3a–c). Reciprocally, several NPY-containing perikarya were not immunolabeled with the GAD antibodies (Fig. 3d–f).
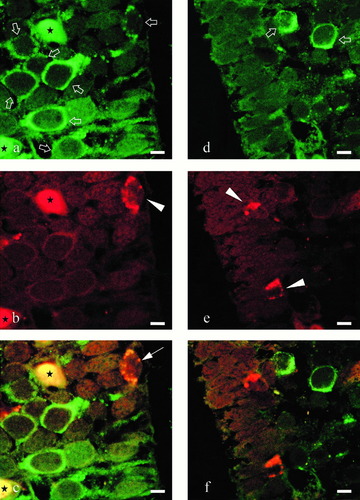
Confocal laser scanning microscope analysis of a coronal section through the periventricular preoptic nucleus of Protopterus annectens. a: Immunolabeling of glutamic acid decarboxylase (GAD)-containing neurons with the sheep GAD antiserum revealed with fluorescein isothiocyanate-conjugated donkey anti sheep γ-globulins (DAS/FITC) (arrows). b: Immunolabeling of neuropeptide tyrosine (NPY)-containing neurons with the rabbit NPY antiserum revealed with Texas Red–conjugated donkey anti-rabbit γ-globulins (DAR/TXR) (arrowhead). c: Combination of the two images acquired in (a) and (b) shows that one of the GAD-positive neurons exhibits NPY-like immunoreactivity (arrow). d: Immunolabeling of GAD-containing neurons with the sheep GAD antiserum revealed with DAS/FITC (arrows). e: Immunolabeling of NPY-containing neurons with the rabbit NPY antiserum revealed with DAR/TXR (arrowheads). f: Combination of the two images acquired in (d) and (e) shows that several NPY-positive neurons do not contain GAD-like immunoreactivity. Black stars in (a), (b), and (c) indicate nonspecific labeling of red blood cells. Scale bars = 10 μm.
Quantification of GAD- and NPY-immunoreactive neurons was performed in four anteroposterior subdivisions of equal length in the periventricular preoptic nucleus in three distinct female specimens (Table 2). Counting revealed that the number of cell bodies labeled with the GAD antiserum was low in the anterior subdivision of the nucleus (1.05 ± 0.02 cell body/7-μm-thick brain section), increased in subdivision B (5.9 ± 0.6 cell bodies/section), reached a maximum in subdivision C (14.3 ± 0.3 cell bodies/section) and then decreased in the most caudal part (subdivision D) of the nucleus (8.2 ± 0.6 neurons/section). The highest percentage of GAD-positive neurons exhibiting NPY-like immunoreactivity was observed in the most caudal subdivision of the periventricular preoptic nucleus (53.8 ± 0.6%) and the lowest percentage in subdivision C (13.5 ± 3.4%). The percentage of GAD-positive neurons that displayed NPY-immunoreactivity was not significantly different in portions A and B (38.3 ± 7.0% and 36.7 ± 0.9%, respectively). The number of NPY-containing cell bodies detected in the periventricular preoptic nucleus was globally lower than the number of GAD-containing cell bodies. The number of neurons labeled with the NPY antibodies raised gradually from the anterior to the posterior subdivisions of the nucleus (A, 1.6 ± 0.2; B, 4.4 ± 0.5; C, 5.1 ± 0.5; D, 5.9 ± 0.2 cell bodies/section). The percentage of NPY-containing cell bodies that were also labeled with the GAD antibodies was much lower in the most rostral subdivision of the nucleus (25.2 ± 0.3%) than in the posterior subdivision (74.9 ± 7.8%). The overall percentage of GAD-positive neurons that displayed NPY-like immunoreactivity on the entire length of the nucleus was 35.6 ± 2.0% while the overall percentage of NPY-positive neurons that displayed GAD-like immunoreactivity was 47.7 ± 2.6%.
| Rostral–caudal subdivisions of the periventricular preoptic nucleus | No. of GAD-positive neurons that exhibited NPY-like immunoreactivity | No. of NPY-positive neurons that exhibited GAD-like immunoreactivity | ||||
|---|---|---|---|---|---|---|
| GAD-positive neurons | GAD-positive neurons that exhibited NPY-like immunoreactivity | % of GAD-positive neurons that exhibited NPY-like immunoreactivity | NPY-positive neurons | NPY-positive neurons that exhibited GAD-like immunoreactivity | % of NPY-positive neurons that exhibited GAD-like immunoreactivity | |
| A2 | 30 ± 0 | 11 ± 2 | 38.3 ± 7 | 45 ± 7 | 11 ± 2 | 25.2 ± 0.3 |
| B2 | 169 ± 16 | 67 ± 9 | 36.7 ± 0.9 | 125 ± 13 | 67 ± 9 | 51.9 ± 1.6 |
| C2 | 408 ± 9 | 60 ± 8 | 13.5 ± 3.4 | 145 ± 14 | 60 ± 8 | 38.7 ± 6.5 |
| D2 | 234 ± 18 | 126 ± 8 | 53.8 ± 0.6 | 169 ± 6 | 126 ± 8 | 74.9 ± 7.8 |
- 1 GAD, glutamic acid decarboxylase; NPY, neuropeptide tyrosine.
- 2 A is the most rostral and D is the most caudal subdivision.
When the GAD antiserum was substituted with either preimmune sheep serum or PBS, no immunoreactivity was observed. Similarly, replacement of the NPY antiserum by PBS, or preincubation of the NPY antiserum with synthetic NPY (10−6 M) resulted in total loss of the immunostaining.
DISCUSSION
The present report describes for the first time the distribution of a GAD-related enzyme and its colocalization with NPY in the diencephalon of a lungfish.
Two isoforms of GAD (GAD65 and GAD67), encoded by distinct genes, have been characterized in the CNS (Erlander et al., 1991). It has been previously reported that, in most brain regions, both GAD isoforms are present in cell bodies as well as axon terminals (Esclapez et al., 1994). The antibody against GAD used in the present work primarily recognized GAD65-related enzyme (Esclapez et al., 1994). This antiserum has been used previously to localize GAD-immunoreactive neurons in the brain of the rat (Oertel et al., 1981; Kawaja et al., 1989; François-Bellan et al., 1990; Fabri and Manzoni, 1996), turtle (Blanton et al., 1987), toad (Barale et al., 1996), and teleosts (Denizot et al., 1987). In fact, the primary structures of GAD have been highly conserved during vertebrate evolution (Bosma et al., 1999). In particular, it has been recently shown that the cDNA encoding the two isoforms of GAD in zebrafish possess more than 84% identity with their human counterparts (Martin et al., 1998).
GAD-like immunoreactivity in the lungfish diencephalon: comparative and functional aspects
The present data have shown that GAD-immunoreactive cell bodies are primarily located in the periventricular preoptic nucleus of P. annectens. A few GAD-positive perikarya were also observed in the ventrocaudal hypothalamus and the dorsal thalamus. The occurrence of a prominent population of GAD-positive cell bodies in the periventricular preoptic nucleus of the lungfish is consistent with previous reports that have described the existence of numerous GABAergic neurons in the preoptic region of teleosts (Martinoli et al., 1990; Médina et al., 1994) and amphibians (Franzoni and Morino, 1989; Naujoks-Manteuffel et al., 1994; Barale et al., 1996). Similarly, GABA-immunopositive perikarya have been described in the periventricular preoptic nucleus of the chameleon (Bennis et al., 1991), chicken (Granda and Crossland, 1989), and pigeon (Domenici et al., 1988), and in the periventricular and paraventricular nuclei of the rat (Mugnaini and Oertel, 1985; Sakaue et al., 1988). The occurrence of GABA-immunoreactive cell bodies in the preoptic region of all groups of vertebrates, including lungfish, indicates that this population of GABAergic neurons was already present in a common ancestor before the divergence of sarcopterygians and actinopterygians.
The periventricular preoptic nucleus of P. annectens contains a set of bipolar GAD-immunoreactive cells extending neuronal processes toward the third ventricle. The presence of cerebrospinal fluid–contacting neurons expressing GABA has already been described in the hypothalamus of amphibians (Roberts et al., 1987; Franzoni and Morino, 1989; Barale et al., 1996) and in the epithalamus of mammals (Harandi et al., 1986). These observations indicate that GABA may act as a neurocrine factor on target cells located at a distance through volume transmission (Zoli et al., 1998; Nicholson, 1999).
The existence of GAD-positive cell bodies in the ventral region of the caudal hypothalamus of the lungfish is in agreement with previous data showing the presence of GABA-immunoreactive neurons in homologous regions of the diencephalon of other vertebrates, i.e., in the nucleus lateralis tuberis of teleosts (Martinoli et al., 1990; Médina et al., 1994), in the ventral hypothalamus of amphibians (Franzoni and Morino, 1989; Naujoks-Manteuffel et al., 1994), and in the arcuate nucleus of mammals (Mugnaini and Oertel, 1985; Sakaue et al., 1988; Thind and Goldsmith, 1995). The distribution of GAD-positive perikarya in the thalamus of lungfish was more restricted than that found in other groups of vertebrates. In P. annectens, GAD-positive cell bodies were confined to the dorsal thalamus. In contrast, in teleosts, GABA-immunoreactive neurons are localized in several nuclei of the thalamus, including the dorsolateral and ventral nuclei, the lateral geniculate nucleus, the dorsal and medial pretectal nuclei, and the nucleus of the posterior commissure (Martinoli et al., 1990; Médina et al., 1994). Similarly, in anuran amphibians and other groups of tetrapods, GABAergic neurons are widely distributed in most thalamic nuclei (Mugnaini and Ortel, 1985; Franzoni and Morino, 1989; Domenici et al., 1988; Bennis et al., 1991). Conversely, in the crested newt as in the lungfish, GABAergic neurons are restricted to the dorsal thalamus (Franzoni and Morino, 1989). The discrete distribution of GABAergic neurons in the thalamus of lungfish and newt indicates the existence of species variations in the organization of the GABAergic system in vertebrates.
In the lungfish, GAD-containing perikarya located in the preoptic nucleus sent projections toward the ventral hypothalamus, and numerous immunoreactive fibers terminated in the median eminence, suggesting that GABA may be released into the hypothalamo–pituitary portal vessels and may regulate the activity of the adenohypophysis. In teleosts, which do not possess a median eminence or a pituitary portal system, a well-organized nerve fiber tract, originating from GABAergic hypothalamic neurons, innervates the pars distalis of the pituitary (Kah et al., 1987a,b; Martinoli et al., 1990; Médina et al., 1994). In addition, it has been shown that, in the goldfish, nerve terminals containing GABA make synaptic contacts with various types of cells in the adenohypophysis, indicating that GABA may control the secretion of several pituitary hormones in fish (Kah et al., 1987a,b; Sloley et al., 1992) as previously demonstrated in mammals (for review, see Tappaz et al., 1982).
The intermediate lobe of the pituitary is generally densely innervated by GABA-containing processes in most vertebrate classes including teleosts (Kah et al., 1987a,b), amphibians (Adjeroud et al., 1986; De Rijk et al., 1990; Tonon et al., 1992), reptiles (Bennis et al., 1991), and mammals (for review, see Tappaz et al., 1982). It has also been shown that GABA is a potent inhibitor of α–melanocyte-stimulating hormone (α-MSH) secretion in amphibians (Adjeroud et al., 1986; Verburg-van Kemenade et al., 1987b) and mammals (for review, see Tappaz et al., 1982). The pars intermedia of P. annectens was devoid of GAD-immunoreactive fibers but contained clusters of GABA-positive cells. The occurrence of GABA-containing cells has been reported previously in the intermediate lobe of the rat pituitary (Sakaue et al., 1988), suggesting that, in the lungfish and in the rat, GABA may be secreted by melanotrope cells. In addition, the neural lobe of the pituitary of P. annectens was richly innervated by GABA-containing fibers. This observation suggests that, in lungfish, GABA may regulate the release of neural pituitary hormones, as previously proposed in amphibians (Franzoni and Morino, 1989) and mammals (Feldberg et al., 1978; Oertel et al., 1982; Rabhi et al., 1987; Brüstle et al., 1988).
Colocalization of GAD and NPY-like immunoreactivity in the periventricular preoptic nucleus of the lungfish
We have previously shown that the preoptic nucleus of P. annectens possesses several populations of neurons containing different neuropeptides, including α-MSH (Vallarino et al., 1992), gonadotropin-releasing hormone (King et al., 1995), FMRFamide (Vallarino et al., 1995a), NPY (Vallarino et al., 1995b), atrial natriuretic factor (Vallarino et al., 1996), somatostatin (Vallarino et al., 1997), and enkephalins (Vallarino et al., 1998). In the course of the present study, we have noticed that the distribution of GAD-positive perikarya in the periventricular preoptic nucleus overlaps that described for NPY-immunoreactive neurons. This observation led us to investigate the possible colocalization of GAD and NPY in these neurons. Our data indicate that GAD and NPY coexist in a subpopulation of neurons of the preoptic nucleus and that the proportion of neurons coexpressing GAD and NPY was higher in the most caudal region of the nucleus. Consistent with these data, it has been shown that, in the rat, GAD- and NPY-like immunoreactivity coexists in a subset of neurons of the arcuate nucleus (Horvath et al., 1997) and cerebral cortex (Hendry et al., 1984). It has also been found that GABA and NPY coexist in some neurons of the nucleus of the saccus vasculosus in the trout hypothalamus (Yáñez et al., 1997). In amphibians, colocalization of GAD or GABA and NPY has been demonstrated in neurons of the suprachiasmatic nucleus (Ubink et al., 1998) and in axon terminals innervating the intermediate lobe of the pituitary (De Rijk et al., 1990, 1992; Tonon et al., 1992; Tuinhof et al., 1994). In lungfish, the suprachiasmatic ridge is continuous with the caudal–dorsal periventricular preoptic nucleus (Northcutt, 1977). Therefore, the coexistence of GAD-and NPY-like immunoreactivity in the caudal part of the periventricular nucleus of P. annectens is consistent with the presence of GAD- and NPY-positive neurons in the suprachiasmatic nucleus of amphibians. The existence of a dense network of GAD- (this study) and NPY-containing fibers (Vallarino et al., 1995b) in the median eminence of P. annectens together with the presence of a high density of NPY receptors in the distal lobe of the pituitary (Vallarino et al., 1998) suggest that, in lungfish, GABA and NPY may interact to regulate the activity of adenohypophysial cells. In support of this hypothesis, it has been reported recently that, in the spinal cord of the lamprey, NPY and GABA, colocalized in the same terminals of the dorsal horn, exert complementary actions in modulating sensory inputs (Parker et al., 1998).
CONCLUSION
The present study has provided the first anatomical distribution of GAD-immunoreactive neurons and fibers in the diencephalon and pituitary of a lungfish. The data show the occurrence of a dense population of GAD-positive neurons in the periventricular preoptic nucleus and the presence of scattered immunoreactive perikarya in the caudal hypothalamus and thalamus. The distribution of GAD-immunoreactive fibers indicates that, in lungfish, GABA may act as a hypothalamic hypophysiotropic neurohormone as well as a neurotransmitter and/or neuromodulator in the brain. In addition, colocalization of GAD and NPY in the periventricular preoptic nucleus suggests that GABA and NPY may interact with each other to modulate the activity of target cells.
Acknowledgements
The authors thank Dr. Marcel Tappaz (INSERM U433, Lyon, France) for his generous gift of GAD antiserum. M.T. was the recipient of a fellowship from the University of Genova (D.R. no. 2307).




