Remodeling of the leg sensory system during metamorphosis of the hawkmoth, Manduca sexta
Abstract
The adult legs of the hawkmoth Manduca sexta are supplied by a diverse array of sensory organs and associated neurons (Kent and Griffin [1990] Cell Tissue Res. 259:209–223) that differ from those in the larval legs. In the present study, a combination of nerve-tracing techniques [biocytin, 1,1`-dioctadecyl-3,3,3`,3`-tetramethyl-indocarbocyanine perchlorate (DiI)], birth date labeling (5-bromodeoxyuridine), confocal microscopy, and electrophysiology were used to describe the remodeling of the prothoracic leg sensory system. Four primary sensory branches carry the axons of all of the sensory neurons in the larval leg. At the onset of metamorphosis, the imaginal leg epidermis develops underneath the larval cuticle and encircles the sensory neurons, thus separating them from their target-organs. Most of the larval neurons degenerate during the larval-to-pupal transition and are replaced by new-adult sensory neurons that are born and differentiate in the pupa. Six sensory neurons that supply hair sensilla in the larval leg, together with 13 femoral and tibial chordotonal organ neurons, persist into the developing adult leg to serve similar functions. Early in the pupal stage, electrical activity can be recorded from these neurons despite the absence of target sensory structures. During the differentiation of the adult sensory system, the axons of the new-adult sensory neurons contact and fasciculate with the axons of the persistent neurons. Thus, five of the primary sensory branches of the adult leg are built on the preexisting larval sensory trajectories. Two sensory branches, however, are established de novo by the axons of specific adult sensory neurons. J. Comp. Neurol. 419:154–174, 2000. © 2000 Wiley-Liss, Inc.
Insect metamorphosis entails a dramatic morphologic transition that involves the loss of larval features followed by the acquisition of adult-specific components. Changes in form are accompanied by changes in behavior, as the postembryonic nervous system underlying feeding and simple locomotor activity in the larva is reorganized to control walking, flight, and reproductive behavior in the adult. This reorganization requires extensive changes in the central nervous system (CNS) and the peripheral nervous system (PNS). The conversion from larval CNS to adult CNS involves the production of new-adult neurons from embryonically derived neuroblasts (Booker and Truman, 1987; Truman and Bate, 1988; Procop and Technau, 1991), the degeneration of larval neurons that are not required further (Truman, 1983; Truman and Schwartz, 1984; Fahrbach and Truman, 1987; Kimura and Truman, 1990; Truman et al., 1992; Robinow et al., 1993; Weeks et al., 1993; Fahrbach and Schwartz, 1994; Fahrbach et al., 1994; Streichert et al., 1997; Hoffman and Weeks, 1998), and remodeling of persistent larval neurons (Casaday and Camhi, 1976; Truman and Reiss, 1976; Levine and Truman, 1982, 1985; Technau and Heisenberg, 1982; Breidbach, 1987a,b; Kent and Levine, 1988, 1993; Thorn and Truman, 1989; Sandstrom and Weeks, 1991; for a review see Levine et al., 1995).
The remodeling of the PNS, although it is less well documented, probably involves the same mechanisms. Cell death and neurogenesis are observed as the embryonically-derived larval sensory organs and their associated neurons (Campos-Ortega and Hartenstein, 1985; Ghysen et al., 1986) degenerate and are replaced by new sensory organs and neurons in the adult (Sanes and Hildebrand, 1976; Jan et al., 1985; Bate, 1978), but some larval sensory neurons persist (Bate, 1973; Jan et al., 1985; Levine et al., 1985; Tix et al., 1989a,b; Lakes-Harlan et al., 1991b; Paspalas et al., 1993; Lewis and Fullard, 1996; Shepherd and Smith, 1996).
The vast majority of the adult sensory neurons develop de novo during metamorphosis. The peripheral cell bodies of these new neurons must extend axons into the CNS, which then must establish specific synaptic contacts with central neurons. Thus, the growing axons of the adult-specific neurons, like the embryonically derived larval sensory neurons before them, must navigate into the CNS. Embryonic sensory axons are guided to the CNS by the axons of pioneer neurons that arise early during embryogenesis and bridge the distance between the periphery and the CNS (Bate, 1976; Keshishian and Bentley, 1983a,b). Similarly, during postembryonic development, sensory axons that are generated at each molt have a strong propensity to fasciculate and to follow previously established nerves (Wigglesworth, 1953; Sanes and Hildebrand, 1976). Axon guidance over long distances may be facilitated in a similar fashion during the establishment of new sensory pathways in the adults of holometabolous insects. Indeed, in flies, specific larval sensory neurons survive metamorphosis, and their axons provide a scaffold for the growing adult sensory neurons (Jan et al., 1985; Tix et al., 1989a,b; Lakes-Harlan et al., 1991b; Shepherd and Smith, 1996).
The leg system of the hawkmoth Manduca sexta provides a useful model for addressing the guidance of adult sensory neurons. Manduca sexta larvae bear three pairs of small thoracic legs that degenerate during metamorphosis and are replaced by new legs in the adult that markedly differ in shape, size, and articulation. The adult legs are equipped with new sensory organs reminiscent of hemimetabolous insect legs (Kent and Griffin, 1990). Most, if not all, of the larval leg motoneurons survive metamorphosis (Kent and Levine, 1988, 1993). After the loss of the larval muscles, the motor axons remain in the periphery to innervate the new muscles of the adult leg (Consoulas et al., 1996). Adult sensory nerves fasciculate with the main motor nerve (Consoulas et al., 1996). Thus, motor nerves may provide a neuronal scaffold for navigation of new sensory axons into the CNS. In addition, some larval sensory neurons may persist through metamorphosis to aid in the formation of new-adult sensory pathways.
Larval legs are capable of restricted flexion and extension movements, whereas the freely articulated adult legs can perform a variety of movements ranging from flexion-extension to protraction-retraction. Both sets of legs are involved in terrestrial locomotion. During crawling, larval legs of the same segment flex and extend in synchrony as the wave of motor activity progresses from abdominal body wall and proleg to thoracic body wall motor systems. In contrast, during waking in the adults, the pattern of leg movements is variable, and segmental pairs of legs rarely show synchronized movements (Johnston and Levine, 1996). Thus, leg movements and motor activity patterns differ significantly between larval and adult stages. Probable differences in the requirements for sensory feedback are reflected in the class, number, and spatial arrangement of the sensory organs (Kent and Griffin, 1990; Kent et al., 1996). Apart from comparing the sensory elements of larval and adult legs, the leg system offers a unique opportunity to study at the level of identified cells the morphologic and functional modification of the sensory-motor circuit that allows the behavioral transition from larval crawling to adult walking. Thus, it is important to know the types of sensory organs and their innervation for both sets of legs, when the larval leg sensory system degenerates and how this is correlated with the dismantling of the crawling circuitry, whether some larval sensory neurons and organs survive metamorphosis, when the adult sensory neurons are born, and when their axons reach the CNS to make new synaptic connections.
This article describes the post-embryonic remodeling of the prothoracic leg sensory system. Specific larval leg sensory neurons, including exteroceptors and proprioceptors, survive metamorphosis to serve a new function in the adult leg. New adult sensory neurons are born within a short time early in the pupal stage and reach the CNS, to a large extent by using preexisting-larval nerve pathways as a scaffold. A preliminary account of portions of this study was published previously (Consoulas and Levine, 1998b).
MATERIALS AND METHODS
Animals
M. sexta (L) were obtained from a laboratory culture reared on an artificial diet (Bell and Joachim, 1976) under a long-day photoperiod regimen (17 hours light and 7 hours dark) at 26°C and at approximately 60% relative humidity. Both chronologic and morphologic criteria were used for the staging of animals (Nijhout and Williams, 1974; Bell and Joachim, 1976; Reinecke et al., 1980; Tolbert et al., 1983; Consoulas et al., 1996). In summary, larval day 0 (L0), L1, L2, etc., represent the days of the last (5th) larval instar. Similarly, wandering day 0 (W0) signifies the first day of wandering, and W1–W4 represent the remaining days before pupation. Stages W2a and W2b are defined as the periods on day W2 before and after ocellar retraction occurs. Stage W4 is subdivided into four substages according to Truman et al. (1980). After pupation, stage pupal 0 (P0) indicates the day of the pupal molt, and P1–P18 are the next stages of pupal development.
Nerve staining techniques
Biocytin staining.
A biocytin protocol was used to reveal the peripheral leg nerves and sensory neurons (Horikawa and Armstrong, 1988; Consoulas et al., 1996). To fill the sensory neurons, the animals were anesthetized first by chilling on ice. After removing the head and abdomen, the thoracic segments were dissected along the dorsal midline and pinned down on a Sylgard-coated Petri dish in saline (in mM: NaCl, 140; KCl, 5; CaCl2, 4; glucose, 28; HEPES, 5; final pH, 7.4; Trimmer and Weeks, 1989). The whole prothoracic ganglion with intact nerves or the cut stumps of nerves were isolated in a Vaseline pool to allow the infusion of a biocytin solution (3% weight/volume biocytin; Sigma, St. Louis, MO) in distilled water. The preparations were stored at 7°C. After biocytin infusion for a maximum of 2 days, the preparations were dissected and fixed in freshly prepared fixative solution containing 4% paraformaldehyde, 0.15% glutaraldehyde, and 0.2% saturated picric acid in 0.1 M phosphate buffer (PB), pH 7.4, overnight (Sun et al., 1993). They were dehydrated subsequently in ethanol, permeabilized in xylol or in propylene, rehydrated in ethanol, and washed in 10 mM phosphate-buffered saline (PBS), pH 7.4, three times for 15 minutes each and in PBS containing 1% Triton X-100 (PBSX), pH 7.4, three times for 15 minutes each. The preparations were then treated with 3% collagenase-dispase (Sigma) for 15 minutes at 37°C. To block nonspecific, staining the preparations were incubated in 10% normal goat serum (NGS; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and 3% bovine serum albumin (BSA; Boehringer Mannheim Biochemicals, Indianapolis, IN) in PBSX for 1 hour. In some cases (n = 75), they were incubated in indocarbocyanine (Cy3)-conjugated streptavidin (Jackson ImmunoResearch Laboratories, Inc.) for 12 hours at 7°C. In other cases (n = 30), the preparations were incubated in avidin-biotin-horseradish peroxidase complex (ABC complex; Vectastain Elite PK-6100; Vector Laboratories, Inc., Burlingame, CA) overnight. After several rinses in PBSX and PBS, the labeling was revealed by incubation in 0.1% 3.3′-diaminobenzidine (DAB; Sigma), 0.015% H2O2, and 0.1% nickel sulfate in 10 mM PBS for up to 10 minutes. The reaction was controlled under a dissecting microscope and was stopped by washing the tissue extensively in PBS. In any case, the preparations then were washed several times with PBS, dehydrated in ethanol, and cleared in methyl salicylate.
1,1′-Dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate staining
Animals (n = 72) from the second day of the last larval instar (L2) were anesthetized on ice and placed ventral side up on plasticine. A small incision along the ventral midline was made without damage of any internal tissue. The prothoracic ganglion was exposed with the aid of a glass hook made from a Pasteur pipette. Small crystals of 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (DiI; Molecular Probes, Eugene, OR) were deposited with the aid of a glass electrode within central areas of the ganglion, avoiding spread of the dye in the hemolymph. After the DiI deposition, the incision was sutured with the aid of surgical thread, and hemolymph leakage was stopped with a drop of adhesive tissue glue (Consoulas and Levine, 1997; Vetbond, St. Paul, MN). Oxidation of hemolymph was prevented by adding a few crystals of phenylthiocarbamide (PTU) to the wound. The rate of animal survival was approximately 80%. After the operation, the animals were allowed to develop under normal conditions and then were dissected in later larval and pupal stages.
Sectioning
The positions of the sensory neurons in the developing leg (stages W3, W4, and P2) were examined in serial longitudinal sections (n = 12). The legs were removed from the animals, fixed in alcoholic Bouin's solution for 2–3 days, embedded in paraffin (paraplast), and serially sectioned (10–12 μm). After deparaffinization and rehydration, the sections were stained with hematoxylin and eosin.
5-Bromodeoxyuridine labeling
To reveal the time course during which the sensory neurons of the adult legs appear as the result of the division of epidermal cells, 50 μg/g body weight of 5-bromodeoxyuridine (BrdU; Sigma) dissolved in distilled water was injected into the animals at specific developmental stages (from W0 to P5). The animals were then allowed to reach pupal stages P10 or P13, when all the axons of adult leg sensory neurons have reached the CNS but the epidermal tissue is still soft and easily sectioned. The prothoracic legs were then dissected out of the animals and fixed for 2 days in alcoholic Bouin's solution, embedded in paraffin, and sectioned. After deparaffinization, rehydration, and extensive washing in PBS and PBSX (0.1%) the DNA of the cells was denatured by treatment with 2 N HCl in PBS for 15 minutes. Nonspecific activity was blocked with 10% NGS in PBS for 30 minutes. The sections were then incubated for 2 hours in the primary antibody against BrdU (Becton Dickinson Immunocytometry Systems, San Jose, CA) diluted 1:100 in PBS with 5% NGS. After washing the sections in PBSX and PBS for 1 hour, they were incubated in goat anti-mouse secondary antibody diluted 1:200 (Cy3-conjugated; Sigma) in PBS for 1 hour (Consoulas et al., 1997; Consoulas and Levine, 1997).
The stained preparations were viewed with a confocal microscope (MRC-600 with a Nikon Optiphot-2 microscope [Nikon, Tokyo, Japan] and a Krypton/Argon laser light source [BioRad, Cambridge, MA]). By using the two shambling channels and dichromatic cubes (BioRad K1 and K2; excitation wave lengths, 488 nm and 568 nm, respectively), optical sections were recorded simultaneously through the depth of wholemount preparations for the one or two dyes used. In cases in which two dyes were used, the images were merged by using different pseudocolors (red for Cy3-conjugated streptavidin or DiI and green for streptavidin-dichlorotriazinylamino fluorescein). Images were prepared by using Confocal Assistant (Bio-Rad) and Corel 8 (Corel Corp., Ottawa, Ontario, Canada).
Electrophysiology
The animals were dissected as described above. The spontaneous activity of leg sensory neurons was recorded with a saline-filled glass suction electrode from the main nerve, IN2a. The extracellular signals were amplified with a differential alternating current amplifier (A-M systems, Everett, WA), stored on a Vetter PCM 8-channel recorder (A.R. Vetter Co. Inc., Rebersburg, PA), and subsequently transferred to a computer (acquisition sample rate, 10 KHz) for analysis using Data-Pac II software (Run Technologies, Laguna Hills, CA).
RESULTS
Larval leg sensory system
The larval prothoracic legs in Manduca sexta are small, segmented, but poorly articulated structures. The trochanter is fused with the femur, and the two coxae are fused with one another. The overall organization of the sensory system of the distal leg segments, as revealed after filling the nerves with biocytin, is shown in Figures 1D and 2A. Two main nerves (IN2a and IN1b; Eaton, 1974) enter the fused trochanter-femur segment. Nerve IN1b is a motor nerve innervating the tibial extensor muscle (Consoulas et al., 1996). The other leg nerve, IN2a, gives off five secondary nerve branches: Branches 2a5, 2a6, 2a8, and 2a9 are purely sensory nerves. Branch 2a7 is a motor nerve carrying the axons of the motoneurons that innervate all of the femoral and tibial muscles (Consoulas et al., 1996). Sensory organs located at the anterior region of the leg are innervated by neurons with axons in nerve branches 2a5 (trochanter and femur) and 2a8 (tibia, tarsus, and pretarsus), whereas sensory organs of the posterior region of the leg are innervated by branches 2a6 (trochanter and femur) and 2a9 (tibia and tarsus; Figs. 1D, 2A).
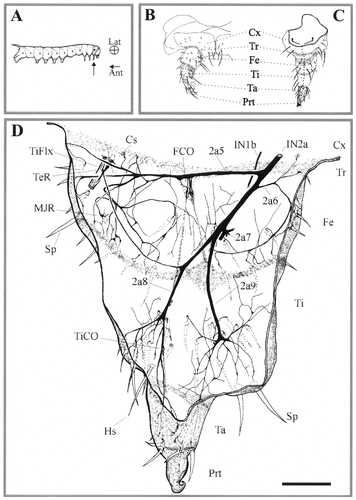
Camera lucida drawing of the innervation pattern in a biocytin-stained preparation of the fifth instar larval prothoracic leg (larval stage 2; L2). A–C: Schematic drawings of a fifth instar larva (arrow points to prothoracic leg) and of prothoracic leg in lateral (B) and medial (C) views. Dotted lines in C shows the incision used for the dissection, and the curved arrows indicate how the leg was pinned down (see D). D: Internal and external sensory organs of the distal leg segments are innervated by five secondary branches of nerve IN2a. Nerve branches 2a5 and 2a6 innervate sensory structures in the trochanter (Tr) and femur (Fe). Nerves 2a7 and 2a8 supply sensory organ in the tibia (Ti), tarsus (Ta), and pretarsus (Prt). Nerve branch 2a7 is a pure motor nerve carrying the axons of the motoneurons innervating all but the tibial extensor muscle and is innervated by nerve IN1b (not shown; see Consoulas et al., 1996). Ant, anterior; Cs, campaniform sensillum; Cx, coxa; FCO, femoral chordotonal organ; Hs, hair sensillum; Lat, lateral; MJR, multiterminal junctional receptor; TeR, tension receptor; TiCO, tibial chordotonal organ; TiFlx, tibial flexor muscle; Sp, spine. Scale bar = 100 μm.
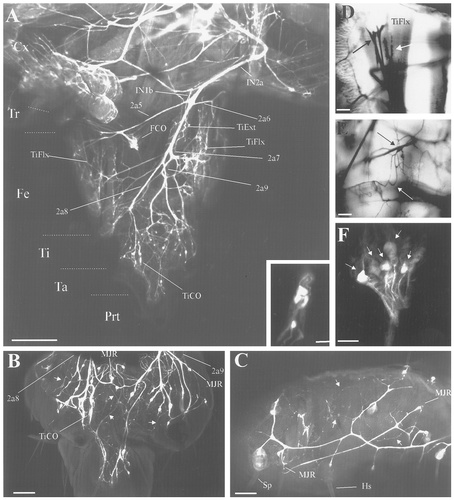
Confocal photomicrographs and photomicrographs of biocytin-filled nerve branches of the fifth instar larval prothoracic leg (stage L2). A: Wholemount preparation at low magnification showing the innervation pattern of the prothoracic leg. The pretarsal flexor muscle has been removed to reveal the four secondary sensory branches (2a5, 2a6, 2a8, and 2a9). Inset: The sensory neurons associated with the tibial chordotonal organ (TiCO). B: Higher magnification of the tibial and tarsal segments of the same preparation shown in A. C: Innervation pattern of the sensory organs in the posterior part of the femur in a different preparation. Note that multiterminal junctional receptor (MJR) neurons ramify extensively (arrows). D: The sensory dendrites (black arrow) of the tension receptor organ can be differentiated clearly from the motor terminals (white arrow). E: The cell body (black arrow) and ramifications (white arrow) of the multipolar joint receptor sensory neuron. F: The sensory neurons (arrows) associated with the femoral chordotonal organ (FCO). For abbreviations, see Figure 1. Scale bars = 500 μm in A; 20 μm in inset; 100 μm in B–E; 50 μm in F.
Femoral, tibial, and tarsal leg segments are covered by a number of proprioceptive sensilla of different size (Kent et al., 1996). Hair-like sensilla and spines are innervated singly by sensory neurons with cell bodies measuring 10–20 μm (Figs. 1D, 2B,C). Single sensory neurons supply the eight campaniform sensilla that are located at the trochanter-femur boundary (n = 3), at the tibia (n = 1), the tarsus (n = 3), and at the pretarsus (n = 1; Kent et al., 1996). Multipolar joint receptors (MJRs; Coillot and Boistel, 1968) were found in the femur (n = 5) and the tibia (n = 3). The fine dendrites of the MJRs arborize extensively not only close to the joints (Fig. 2E) but also in almost every part of the leg segments, thus forming an epidermal plexus (Fig. 2B,C, arrows). A structure resembling a putative tension receptor (Theophilidis and Burns, 1979) attaches to the posteromedial bundle of the tibial flexor muscle (Fig. 1D). Its axon enters nerve 2a8, and its dendrites, which differ characteristically from motor terminals, project to the most proximal part of the muscle fibers (Fig. 2D). In the femur and tibia, clusters of sensory neurons are associated with sensory structures resembling chordotonal organs (Fig. 1D). In the femur, groups of sensory neurons are associated with two connective strands (Fig. 1D, 2F). One of the strands (associated with six to nine neurons) is attached to the apodeme of the tibial extensor muscle. The other strand (associated with two to four neurons) spans the femorotibial joint and is attached laterally to the tibia (Fig. 1D). In the tibia, five neurons are associated with a strand that spans the tibiotarsal joint to be attached to the lateral tarsus (Figs. 1D, 2A, see inset).
Remodeling of the larval leg sensory system
The adult leg develops within the larval leg. In M. sexta, like in other lepidoptera, the epidermis of the adult leg develops from cells of the larval leg epidermis that constitute the differentiation center (Kim, 1959; Miles and Booker, 1993). These cells begin to proliferate during the last larval instar and radiate out under the cuticle of the larval legs to form a new, highly folded epidermis. By early stage W2, the imaginal epidermis encloses the larval neuromuscular and tracheal systems and begins to retract from the larval cuticle (Fig. 3A). Biocytin staining of nerve 2a revealed a larval-like sensory pattern of innervation. The cell bodies of the sensory neurons innervating cuticular proprioceptors were detached from the larval cuticle and became associated with the retracted imaginal epidermis (Fig. 3A–D). The dendrites of these neurons elongated considerably to innervate their sensory organs (Fig. 3D, see arrows). Extracellular recordings from the main leg nerve (2a) during this stage of development revealed trains of action potentials in response to mechanical displacement of hair sensilla or spines (Fig. 6A), which suggests that dendritic elongation does not prevent transduction. In addition, tactile stimuli applied to a single hair or to a group of hairs elicit reflex responses similar to those recorded in the intact larval leg (stage L2, not shown; see also Kent and Levine, 1988), suggesting that the sensory neurons maintain proper connections with their targets within the CNS and that the underlying circuits remain functional. During this period, the number and position of the sensory neurons supplying the femoral and tibial chordodonal organs remained unchanged (Fig. 3B,C), but their apodemes were detached from the larval leg cuticle (not shown).
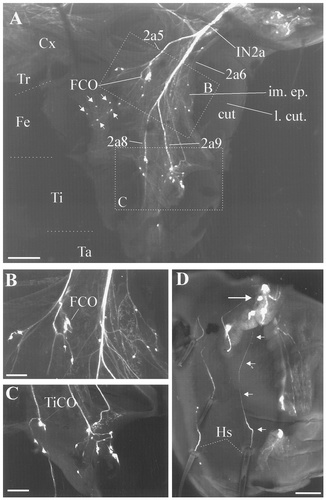
Confocal photomicrographs of biocytin-filled nerve branches of the prothoracic leg in an early stage (wandering day 2; W2) larva. A: The newly formed (imaginal) epidermis (im. ep.) of the adult leg begins to retract from the larval cuticle (l. cut.). The larval cuticle was dissected along the medial part of the leg, as in the L2 stage preparations, but the imaginal epidermis remained intact. Four secondary sensory branches (2a5, 2a6, 2a8, and 2a9) can be seen. Note that the cell bodies of the larval sensory neurons are associated with the imaginal epidermis (arrows). For abbreviations, see Figure 1. B,C: Higher magnification of areas in the femur and tibia marked in A. The FCO and TiCO are present in the imaginal leg, but their tendons have detached from the larval cuticle. The cell bodies of the sensory neurons in the tibia begin to aggregate in the medial aspect of the imaginal leg. D: Confocal photomicrograph of the medial part of the tarsus from a different preparation. Note that the dendrites of the sensory neurons supplying larval exteroceptors have elongated considerably (arrows), remaining attached to their target organs. HS, hair sensilla. Scale bars = 500 μm in A; 100 μm in B–D.
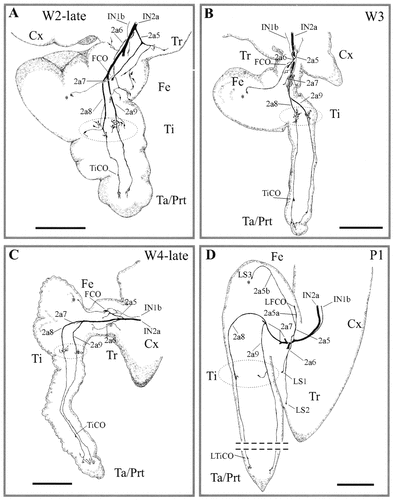
Overview of leg sensory nerve remodeling during the last larval and first pupal stages. All camera lucida drawings are from biocytin-stained preparations. Note the differences in the scale bars. A–C: The imaginal legs have been removed from the larval cuticle. The larval sensory branches and sensory neurons concentrate in the mediocentral regions of the imaginal leg. Encircled areas indicate the tibial sensory neurons. During the 2 last larval days (W3 and W4, late stages), the imaginal leg has grown and elongated considerably. The tarsal larval sensory neurons remain localized at the distalmost part of the imaginal leg. Note that the TiCO neurons have migrated to the imaginal tarsus. Similarly, two larval femoral sensory neurons (asterisk) have migrated to the border of the imaginal tibia. D: Two days after pupation (pupal stage 1; P1), the imaginal leg has reached its final length but is not yet segmented. The basic features of the sensory innervation pattern remain larval-like. Two larval sensory neurons (LS1 and LS2) are localized in the imaginal trochanter. The larval FCO neurons, together with the LS3 neurons (see also asterisk), remain in the femur. Three neurons remain in the tibia, and four neurons, together with the larval tibial chordotonal neurons, are localized in the imaginal tarsal/pretarsal segment. For abbreviations, see Figure 1. Scale bars = 1 mm in A; 2 mm in B–D.
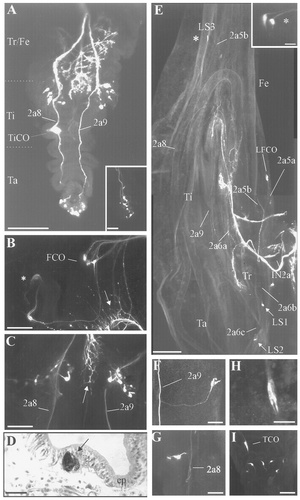
Confocal photomicrographs of biocytin-stained nerve branches of W3, W4, and P1 stage preparations. A: Wholemount, nondissected preparation of a stage W3 imaginal leg. Note that the tibial and tarsal larval sensory neurons have concentrated in the mediocentral region of the developing leg. Inset: A group of six sensory neurons at the tip of the leg (from a different preparation). B,C: Confocal images from late W4 stage preparations of femoral (B) and tibial (C) imaginal segments showing the larval sensory neurons that persist within the developing leg prior to pupation. Asterisk in B indicates two sensory neurons that have migrated close to the femur/tibia border. Arrow shows the axons and terminals of the leg motoneurons. D: Cross section from the tibial imaginal segment of a late W4 preparation stained with hematoxylin and eosin. Note that the cell body of a sensory neuron (arrow) remains within the epidermal cell layer (ep). E: Low-magnification confocal photomicrograph of stage P1 preparation. Few larval sensory neurons (FCO, LS1, LS2, and LS3) are apparent. Inset: The two distalmost LS3 neurons that persist at the femur/tibia border. F–I: Confocal images showing the three larval sensory neurons that persist at the tibia (F,G), the larval TiCO neurons (H), and the group of sensory neurons that persist in the tarsus. Note that the TiCO has migrated close to the group of the six sensory neurons at the tip of the imaginal leg. For abbreviations, see Figure 1. Scale bars = 250 μm in A; 100 μm in B,C; 500 μm in E; 20 μm in D,F–H.
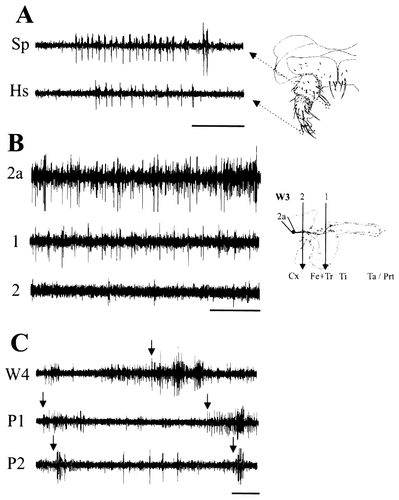
A: Examples of responses to mechanical stimulation of larval sensory neurons innervating hair sensilla (Hs) and spines (Sp) from early stage W2 preparations. During this period of development, the dendrites of these sensory neurons have elongated considerably, but the neurons remain capable of generating spikes. B: Extracellular recordings from nerve IN2a at the level of trochanter/coxa border showing spontaneous activity. The number of spikes is reduced after a lesion between the femur and the tibia (lesion 1), and spikes are almost eliminated after a lesion between the coxa and the femur (lesion 2), suggesting that spikes recorded from nerve 2a are produced by groups of sensory neurons located in each leg segment. By this stage of development (stage W3), the sensory neurons are not associated with their target organs. C: Extracellular recordings from nerve IN2a from last larval and early pupal stage preparations. Spontaneous activity and bursts of activity in response to tactile stimuli (arrows) applied to the epidermis in the vicinity of the cell bodies of the persistent larval sensory neurons are apparent. For abbreviations, see Figure 1. Scale bar = 1 second.
The imaginal leg completes its retraction from the larval cuticle and begins to grow and elongate considerably during the last 2 days of the fifth larval instar. Biocytin staining of the peripheral nerves revealed that the four primary sensory pathways (2a5, 2a6, 2a8, and 2a9) persist within the imaginal leg. However, the number of the sensory neurons decreases substantially (Table 1). For example, of the 21 neurons supplying external sensory organs in the femur of an early fifth instar larval leg (stage L2), only 13 ± 3 sensory neurons remain by stage W2b, and only 10 ± 2 neurons remain by late stage W4 (Table 1; also compare Figs. 1D–4C). During the last 2 larval days, the sensory neurons are not associated with external proprioceptors, and their elongated dendrites degenerate (Figs. 4A–C, 5A– C). The sensory neurons are concentrated in groups in the medial region of the imaginal leg segments (Figs. 4A–C, 5A–C). However, two of the femoral chordotonal organ sensory (FCO) neurons migrate toward the femorotibial border (see Figs. 4A–C, asterisk; Fig. 5B). Similarly, the axons of the tibial chordotonal organ (TiCO) neurons, together with the tarsal/pretarsal group of neurons, elongate considerably to occupy the distal-most region of the developing leg (Fig. 4A–C, Fig. 5A, inset). Cross sections from late larval (Fig. 5D) and early pupal (not shown) preparations revealed that the cell bodies of the sensory neurons that supply exteroceptors in the larva are located at the outer cell layer of the imaginal epidermis. Although, during this time of development, the remaining larval sensory neurons are not associated with sensory organs and do not serve any function, they can be active spontaneously (Fig. 6B). It was not possible to stimulate specific neurons, but spontaneous activity and bursts of activity were apparent in response to tactile stimuli applied to the epidermis in the vicinity of the cell bodies (Fig. 6C).
| Stage | No. of preparations | No. of neurons | ||
|---|---|---|---|---|
| Femur | Tibia | Tarsus/pretarsus | ||
| L2 | 8 | FCO, 21 | TiCO, 21 | 9 |
| W2b | 9 | FCO, 13 ± 3 | TiCO, 15 ± 2 | 6 ± 2 |
| W3 middle | 8 | FCO, 11 ± 3 | 16 ± 2 | TiCO, 6 |
| W4 late | 10 | FCO, 10 ± 2 | 13 ± 2 | TiCO, 6 |
| P2 early | 16 | FCO, 4 ± 2 | 3 | TiCO, 6 |
| P3 | 12 | FCO, 21* | 2 | TiCO, 4 |
- 1 Two larval femoral sensory neurons were localized in the imaginal trochanter. L, larval day; W, wandering day; P, pupal stage; FCO, femoral chordotonal organ; TiCO, tibial chordotonal organ.
After pupation, the imaginal leg begins to unfold, and, by stage P1, it reaches its final length. Although fewer larval sensory cells were present in retrograde biocytin stainings of the peripheral nerves of stage P1 and P2-early preparations (Table 1), all of the major sensory branches were apparent by this stage of development. Nerve branch 2a6 had shifted to the imaginal trochanter. It carried the axons of two larval sensory neurons that are designated here as larval sensory neuron 1 and 2 (LS1 and LS2; Figs. 4D, 5E). Six larval FCO neurons were present in the proximal imaginal femur (Fig. 5E). Their axons, together with the axons of the other two chordotonal organ neurons that migrate close to the femur/tibia border (designated here as larval sensory neurons 3; LS3; Figs. 4D, 5E), run through two collateral branches (2a5a and 2a5b) of nerve 2a5. Three larval sensory neurons survive larval-to-pupal transition in the imaginal tibia (Fig. 4D, circled area; Fig. 5F,G). Two groups of three larval neurons each, together with the larval TiCO neurons, are located at the tip of the developing adult leg (Figs. 4D, 5H,I). Their axons project to the CNS through branches 2a8 and 2a9. Similar to what occurs in late larval stages, several of these neurons are active spontaneously and can respond to tactile stimulation of the nearby epidermis (Fig. 6C).
Birth of adult sensory neurons
To determine the birth dates of new-adult sensory neurons, BrdU was injected into late larval stage (W0–W4) and early pupal stage (P0–P6) animals. The injected animals were left to develop normally to stages P10 or P14, then the legs were dissected, sectioned, and processed for BrdU immunocytochemistry. BrdU incorporated by the nuclei of dividing epidermal cells should be diluted due to many rounds of division. In contrast, BrdU still should be detectable in the nuclei of adult sensory neurons if the latter derive from precursors undergoing relatively few divisions. Although BrdU incorporation by the nuclei of epidermal cells and by myonouclei still was detectable in late pupal stages, the strongly labeled, large nuclei of the adult sensory neurons could be distinguished easily (Fig. 7). Neurogenesis within the legs occurred in a proximal-to-distal fashion. BrdU injections into animals (n = 15) at early pupal stages (from P0 to P2-early) and dissection 8–10 days later (stage P10) revealed labeled nuclei of sensory neurons in the trochanter, femur, and tibia and, occasionally, in the tarsus (Fig. 7A,B). BrdU injections into animals (n = 17) at stages P2–P5 revealed BrdU incorporation by the nuclei of sensory neurons in the tibia and tarsus (Fig. 7C). BrdU injections into late fifth instar larvae or into pupae later than stage P5 did not result in any labeling (data not shown). Because BrdU in the late larva or pupa remains available for approximately 2 days (unpublished data; L.A. Oland, personal communication), the above findings suggest that adult sensory neurons derive from epidermal cells that undergo a single or a few divisions between stages P2 and P5.

Confocal photomicrographs from cross sections (A,B) and longitudinal sections (C) of stage P10 (A,B) and P12 (C) preparations showing 5-bromodeoxyuridine (BrdU) incorporation by the nuclei of adult sensory neurons. A,B: Images from cross sections of trochanter/proximal femur (A) and proximal tibia (B). Arrows indicate nuclei of sensory neurons showing BrdU incorporation. Arrowheads show BrdU incorporation by the nuclei of some epithelial cells and by some myonuclei. C: Image taken from a longitudinal section of the third tarsal segment of a P12 stage preparation showing BrdU incorporation by the nuclei of five sensory neurons. cut, Cuticle; sc, scales. mHP, medial hair plate neurons. Scale bars = 100 μm.
Development of the adult pattern of sensory innervation
The different classes of sensory organs of the adult prothoracic legs and their underlying sensory neurons have been described previously (Kent and Griffin, 1990). The establishment of the innervation pattern is described here. During the first 3 days of the pupal stage, the adult leg epidermis is separated gradually from the pupal cuticle, and the first signs of segmentation appear by stage P3. The imaginal leg becomes fully segmented during the next 3 days. The main sensory pathways are established within 1 day (stages P2-late and P3; Fig. 8A,B), and during the next 3 days (until stage P6), most of the adult sensory neurons grow axons into the CNS (Fig. 8C). During these early pupal stages, the sensory organs have not yet differentiated, making it difficult to identify specific neurons, but hair-plate and chordotonal organ neurons can be distinguished easily due to their unique appearance and arrangement. Another caveat is that the data presented here are based solely on biocytin fills. During the critical time of early pupal development, in which most of the larval neurons degenerate and new-adult neurons differentiate, there is always the possibility that the absence or relocation of neuron(s) may be due to the failure of the dye to incorporate into all of the neurons. This problem could be resolved by using, in addition to diffusable markers, other molecular tools, such as antibodies against neuronal proteins, that are not available currently for Manduca.
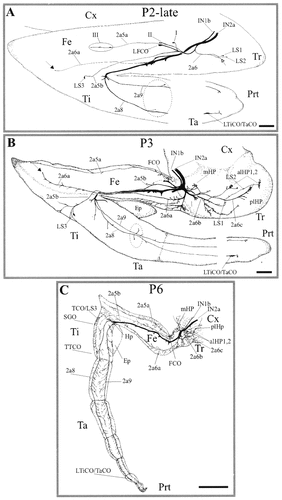
Pattern of sensory innervation in the developing adult leg. Camera lucida drawings of biocytin-stained preparations. A: New sensory neurons in the trochanter (open arrow) and the femur (groups I, II, and III; solid arrow in the distal femur) were stained in the developing leg by late stage P2. LS3 neurons have migrated in the femorotibial border. Two larval sensory neurons with axons in nerves 2a8 and 2a9 persist in the tibia (encircled area), and two groups of neurons, together with the larval tibial chordotonal organ neurons (LTiCO/TaCO, which becomes the tarsal chordotonal organ), are localized at the distalmost part of the leg. B: The pattern of sensory innervation in a stage P3 preparation. At this time of development, a large number of new sensory neurons are stained in the trochanter, femur, and proximal tibia. The axons of trochanteral hair plate sensory neurons, together with other neurons, run in two nerve branches established by LS1 and LS2 neurons. In the femur and tibia, new sensory axons run in the previously established sensory pathways 2a5a, 2a5b, 2a6a and 2a8, and 2a9, respectively. Solid arrow in the distal femur indicates the new neuron, the same as in A, that is the precursor of nerve branch 2a6a. C: Encircled area in the tibia indicates the two larval sensory neurons. The pattern of sensory innervation becomes adult-like 3 days later (stage P6). alHP1 and alHP2, anterolateral hair plates 1 and 2; mHP, medial hair plate; plHP, posterolateral hair plate; SGO, subgenual organ. For other abbreviations, see Figure 1. Scale bars = 2 mm in A,B; 4 mm in C.
Biocytin backfills of nerve IN2a revealed that the axon of the first adult sensory neuron in the developing leg reaches the CNS by middle stage P2. Its cell body is situated at the distal femur (not shown) adjacent to the LS3 neurons, and its axon runs together with the LS3 axons (nerve 2a5b). By late stage P2, a single neuron in trochanter and three groups of neurons appear almost simultaneously along the medial axis of the femur (Figs. 8A, 9A). The most proximal group (group I) consists of five to seven neurons. Together with the persistent larval FCO neurons, group I neurons participate in the formation of the FCO in the adult leg (Consoulas, Rose, and Levine, in preparation). The second group of neurons (group II) is comprised of four cells with axons joining the preexisting nerve branch 2a5 (Figs. 8A, 9A). The most distal group (group III) is comprised of three neurons. Their axons travel toward and fasciculate with the axons of group II neurons (Figs. 8A, 9A). At this stage of development, LS3 neurons migrate distally to the proximal tibia (Figs. 8A, 9A), leaving behind the new-adult sensory neuron at the distal part of the femur (Fig. 8A; Fig. 9A, arrow). This axon then becomes the precursor of the new-adult sensory pathway 2a6a.
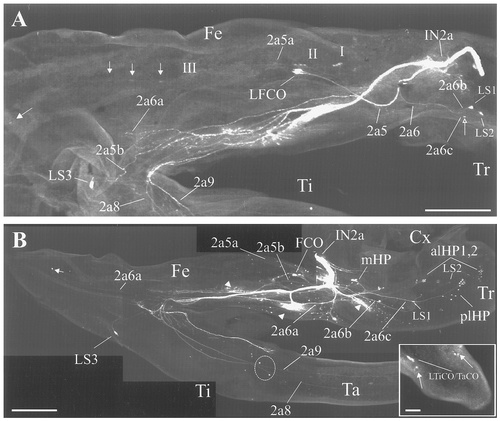
Confocal photomicrographs of biocytin-labeled nerves of stages P2-late (A) and P3 (B). Images were made from intact leg preparations by projecting 120–200 optical sections of 10 μm in depth each. A: By stage P2-late, two new neurons are stained in the trochanter (open arrow) and in the distal femur (solid arrow), and three groups of neurons are stained in the mediolateral femur (Groups I, II, and III). Note that LS3 neurons have migrated to the femorotibial border. B: Pattern of sensory innervation in the developing leg at stage P3. Arrowheads indicate the terminal processes of motor neurons. Two sensory branches (2a6b and 2a6c) in the trochanter, three branches (2a5a, 2a5b, and 2a6a) in the femur, and two branches (2a8 and 2a9) in the tibia, tarsus, and pretarsus carry the axons of all persistent and new-adult sensory neurons. Inset: Larval sensory neurons at the tip of the developing leg. LFCO, larval FCO neurons. For other abbreviations, see Figure 1. Scale bars = 500 μm in A,B; 100 μm in inset.
By stage P3, newly emerged sensory neurons could be stained in the trochanter, femur, and tibia of the imaginal leg with biocytin introduced into the CNS (Fig. 8B; Fig. 9B, inset). In the trochanter, the axons of the posterolateral hair plate and anterolateral 1 hair plate neurons project toward and fasciculate with the axons of the preexisting LS1 and LS2 neurons to form two adult sensory nerve branches (designated here as 2ab and 2ac). The medial and anterolateral 2 hair plate neurons and other sensory neurons appear with a short delay, and their axons join 2ab and 2ac branches (Figs. 8B, 9B). New-adult sensory neurons that are located at the posterolateral, lateral, and anterolateral parts of the femur project axons through nerve 2a6a. Nerve 2a5a carries the axons of the persistent larval and new sensory cells that supply the FCO, together with the axons of the sensory neurons located in the medial and anteromedial regions. The axons of the sensory neurons located in the posteromedial part of the femur join nerve branch 2a5b, which was established previously by the axons of LS3 neurons (Figs. 8B, 9B). In the tibia, two larval sensory neurons persist until this time of development (Figs. 8B, 9B, encircled areas). Two pairs of sensory neurons, together with the larval tibial chordotonal neurons, are apparent at the distalmost part of the leg (Fig. 9B, inset).
During the next 4 days of pupal development, more sensory neurons differentiated within the developing leg (Fig. 10). The full compliment of adult sensory neurons and most of their target-sensory organs became apparent by stage P8. A total of seven sensory nerves carried the axons of all of the sensory neurons (Fig. 10A–C). In the trochanter, hair plate, campaniform, and hair sensilla, neurons projected their axons through nerves 2a6b and 2a6c (Fig. 10B). It is interesting to note that the larval sensory neurons LS1 and LS2 that were apparent in the previous stages were not stained in any biocytin fill of the peripheral nerves of later stages (Fig. 10A,B,H). In the femur, sensory axons projected through three nerves; the preexisting larval nerve (2a5b), which was established by the LS3 axons, and the two new sensory nerves (2a5a and 2a6a), which were established by the axons of early adult sensory neurons (Fig. 10A). In the tibia, tarsus, and pretarsus, adult sensory axons projected through one of the two preexisting larval nerves, 2a8 and 2a9 (Fig. 10E–G). This pattern of sensory innervation remained unchanged throughout the pupal stage and in the adult.
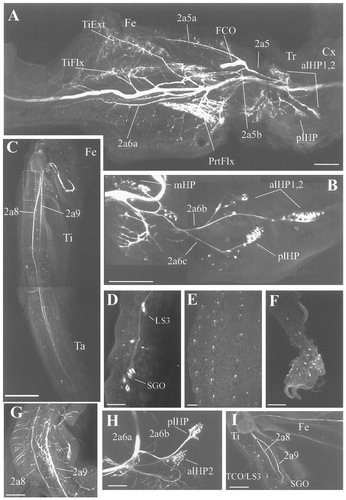
Confocal photomicrographs showing the pattern of biocytin-filled peripheral nerves in wholemount preparations. A,B: Innervation of the trochanter (Tr) and femur (Fe) of stage P4 prothoracic legs. A: The leg was dissected along the lateromedial axis. Major sensory and motor nerve pathways are apparent. TiExt, tibial extensor muscle; TiFlx; tibial flexor muscle. B: Closer view of the innervation in trochanter (from another preparation). Note that LS1 and LS2 larval sensory neurons are not present. C: Confocal image showing the two larval sensory branches (2a8 and 2a9) that carry the axons of sensory cells in the distal part of the leg (tibia and tarsus). A number of sensory neurons, including the subgenual organ (SGO) and LS3 neurons, are missing (area in box) because their axons run through nerve 2a5b, which was not stained. D: Backfill of nerve 2a5b reveals the sensory neurons that supply the subgenual and tibiotarsal (LS3 neurons) chordotonal organs. E,F: New sensory neurons in the last tarsal segment and pretarsus. The two sensory branches, 2a8 and 2a9, are absent in this nondissected preparation due to the poor penetration of indocarbocyanine-streptavidin. G–I: Sensory innervation of tarsus (first segment; G), trochanter (H), and lateral tibia (I) of a stage P8 leg. By this time in development, almost all of the adult sensory neurons are present, and the adult pattern of sensory innervation has been established. Note that LS1 and LS2 neurons are absent in the trochanter (H). In contrast, LS3 neurons are present in the tibia innervating the TiTaCO (H). For other abbreviations, see Figure 1. Scale bars = 1 mm in A; 500 μm in B,C,G; 100 μm in D–F,H.
Biocytin fills in late pupal stages and in the adult revealed that, in contrast to LS1 and LS2 larval neurons, LS3 neurons survive metamorphosis to innervate the tibiotarsal chordotonal organ (Fig. 10D,I). Six larval FCO neurons survived metamorphosis and, together with new neurons, comprised the new organ in the adult leg (Fig. 10A; Consoulas, Rose, and Levine, unpublished observations). The larval TiCO neurons migrate to the last tarsal segment to become the tarsal chordotonal organ. Finally, the six larval sensory neurons (two in the tibia and four in the tarsus) persist into the adult stage to innervate hair sensilla.
Assessment of persistent sensory neurons
Biocytin stainings of the peripheral nerves in late larval preparations revealed that, despite the degeneration of the larval leg, many of the larval sensory neurons become associated with the imaginal leg and survive until the end of the last larval instar. After pupation, most of the larval sensory neurons degenerate but, few of them survive to the adult. Although biocytin fills of these neurons at every stage during the larval-to-pupal transition strongly support the conclusion that these larval neurons persist to the adult, there is always the possibility that these larval neurons are replaced by similarly located adult neurons that were not detected in BrdU preparations. To ensure that these neurons are larval and not newly born adult sensory neurons, the lipophilic vital dye, DiI, was injected into a central area within the prothoracic ganglion in early fifth instar larvae (stage L2). After the injection, the animals were left to develop normally to late larval or early pupal stages. In early stage W3 preparations, DiI labeling was detected in some of the sensory nerve branches and in almost all of the sensory cell bodies (Fig. 11A). In late larval or early pupal preparations (W4 and P3), DiI had almost disappeared from nerve branches but remained in the sensory cell bodies (Fig. 11E,F). For these stages, to ensure that DiI staining was localized in the cell bodies of neurons, animals that were injected with the dye at stage L2 were dissected at late larval (stage W4) and early pupal (stage P3) stages, and the peripheral nerves of the same preparations were then backfilled with biocytin (Fig. 11B,C). Colocalization of DiI and biocytin in most of the sensory cell bodies was apparent (Fig. 11D). Although definite proof that some larval neurons persist to the adult could be gained by detecting DiI staining in the adult leg, unfortunately, DiI disappears completely from preparations older than pupal stage P3. Nevertheless, the above observations, in combination with the biocytin fills and the BrdU labeling experiments, strongly support the notion that these larval sensory neurons persist to the adult stage (see below).

Confocal photomicrographs of sensory neurons stained with 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (DiI; A,C,E,F) and biocytin (B). Crystals of DiI were deposited within a central area of the prothoracic ganglion of stage L2 larvae, and the animals were left to develop and were dissected in late larval and early pupal stages. A: Wholemount preparation showing DiI staining of sensory neurons in a stage W3 preparation. Note that DiI is detectable in sensory nerves 2a8 and 2a9. B–D: Tibial sensory neurons that were stained previously with DiI (C) were counterstained with retrograde biocytin labeling that was revealed with streptavidin-fluorescein (B) in a stage W4 preparation. The image in D is composed from the images in B and C. Note that most of the sensory neurons were stained with DiI. Arrow in B and C indicates a sensory neuron that did not stain with DiI. E,F: Confocal images from stages P0 (E) and P3 (F). Arrows indicate sensory neurons that were stained previously with DiI in the imaginal tibia (E) and tarsus (F). Note that DiI barely is detectable in sensory nerves (arrowhead in E). For abbreviations, see Figure 1. Scale bars = 250 μm in A; 100 μm in B–F.
DISCUSSION
This study sought to investigate the remodeling of the leg sensory system in Manduca sexta and the formation of the adult sensory pathways. Given the differences in form and function between the larval leg and the adult leg (Johnston and Levine, 1996; Consoulas et al., 1997) and in the classes of sensory organs and their innervation (Kent and Griffin, 1990; Kent et al., 1996; this study), many, if not all, larval sensory neurons were expected to die during metamorphosis and be replaced by new sensory neurons in the adult leg. Indeed, most of the larval sensory neurons follow the fate of their target sensory organs and are replaced by new neurons and organs in the adult leg. Some larval sensory neurons, however, survive metamorphosis.
The remodeling of the sensory system occurs simultaneously with several other aspects of adult leg development (Kent et al., 1995; Consoulas et al., 1996, 1997; Consoulas and Levine, 1997, 1998a) and coincides with elevations in the ecdysteroid titer (Bollenbacher et al., 1981), suggesting the possibility of hormonal regulation. In the CNS of Manduca, neurogenesis and cell death are controlled hormonally during metamorphosis (Truman and Schwartz, 1984; Weeks and Truman, 1985; Booker and Truman, 1987). Even more elaborate processes, such as the remodeling of the central arborizations of sensory neurons, are under the direct influence of 20-hydroxyecdysone (Levine et al., 1986; Levine, 1989). Whether larval leg sensory neurons undergo programmed cell death in response to direct hormonal signals or in response to indirect influences, such as hormonal regulation of epidermal cells, peripheral glia, or sensory organ degeneration, remains to be determined. The onset of adult sensory neuron birth coincides with the onset of the divisions of neighboring epidermal and muscle cells (Consoulas et al., 1997) and, similarly, may be under hormonal control.
Previous studies (Kent and Griffin, 1990; Kent et al., 1996) and this study have revealed the type, innervation, and arrangement of the internal and external proprioceptors in the larval and adult prothoracic legs. Hair, spines and campaniform sensilla, chordotonal organs, and multiterminal junctional receptors are common for the legs of both stages, but their number and placement in the leg segments differ. There are also sensory organs that serve adult-specific functions, such as the hair plates in the trochanter and tibia, which detect changes in the relative position of the adjacent segment, or the multiply innervated, scattered, and spine-associated chemosensilla in the distal leg segments (Kent and Griffin, 1990). Larval legs lack an articulated trochanter and do not serve any chemosensory function. Thus, differences in the classes, variety, and positions of sensory organs in the legs likely reflect differences in leg architecture, articulation, function, and, thus, requirements for sensory feedback.
Although the vast majority of sensory organs and neurons in the adult legs are born during metamorphosis, 6 tibial hair sensilla and 13 chordotonal organ neurons persist from the larval to the adult stage to supply similar sensory structures. Although the persistent sensory neurons serve the same function in both sets of legs, it remains unclear why these particular neurons survive to serve in the adult leg. Both persistent neurons and degenerating neurons lose their larval target sensory organs before pupation, suggesting that survival or death of the neurons cannot be related to the fate of their sensory organs. Another, and perhaps the most suitable, explanation for the persistence of these particular larval sensory neurons is that they may play a role in guiding the newly born adult sensory neurons to the CNS (see below).
Degeneration of the larval sensory neurons
Most of the larval sensory neurons degenerate within a period of 5 days (stage from W2b to stage P3; see Table 1) during the larval-to-pupal transition. The neurons do not degenerate immediately after the loss of contact with their associated sensory organs but degenerate a few days later. LS1, LS2, and some tibial and tarsal neurons degenerate 5 days later. These neurons may persist for this additional time to facilitate the establishment of new sensory branches (see below), but most do not appear to serve this role in the developing leg. At present, there is not a suitable explanation for the delayed degeneration of these larval sensory neurons.
Larval sensory neurons that supply exteroceptors (hair and campaniform sensilla and spines) remain functional for an additional day after the formation of the imaginal leg. They retain their mechanotransduction capability by growing elongated dendrites that span the distance between the cell bodies, which are localized in the retracted imaginal epidermis, and their target-sensory organs, which are located at the larval cuticle. Dendritic elongation of sensory neurons supplying mechanosensory hairs of the prolegs during the molt also has been shown (Jacobs and Weeks, 1990). Not only the sensory neurons but also circuits within the CNS remain functional, because the legs can exhibit larval-like reflexes in response to sensory inputs. It remains unclear why limited leg motor function is preserved for 2 additional days after the completion of the puparium cell (Dominick and Truman, 1986).
During the prepupal stages, the axons of the larval sensory neurons retract, which results in the grouping of the cell bodies within central regions of the imaginal segments. Subsequently, the axons of particular groups of neurons (LS3, TiCO, and tarsal/pretarsal neurons) undergo a phase of expansion as the imaginal leg grows and elongates considerably during the last larval and first pupal days. These initial phases of axonal retraction and expansion may represent passive responses to the shrinkage and later expansion of the developing imaginal epidermis. Alternatively, active migration may account for the large-scale relocation of the LS3 neurons. Similarly, axonal processes of leg motoneurons undergo similar phases of retraction and expansion as they lose their larval target muscles and become associated with imaginal tissue during elongation of the imaginal leg (Consoulas et al., 1996).
Persistence of larval sensory neurons and their role in the adult
Small numbers of larval sensory neurons that occupy strategic positions in the imaginal segments survive the larval-to-pupal transition. They participate in the establishment of the adult sensory pathways by providing a route to the CNS for the growing axons of the adult neurons. Evidence that larval sensory neurons persist in the pupa was based on 1) biocytin fills of peripheral nerves at frequent intervals; 2) DiI labeling of the sensory system of intact, early last instar larvae and examination for stained neurons of the same animals at later developmental stages; and 3) electrophysiological recordings of spontaneous sensory activity. These approaches, together with the fact that no adult neurons are born before stage 2 in the pupa, leave little doubt that the neurons identified in this study are persistent larval sensory neurons. Other examples of larval sensory neurons that persist into the pupal stage have been described in Manduca. Specific larval sensory neurons in abdominal segments survive to innervate hairs within the gin traps (Bate, 1973; Levine et al., 1985; Levine, 1989). Similarly, the abdominal stretch receptors (Tamarkin and Levine, 1996) as well as other larval thoracic and abdominal sensory neurons survive metamorphosis (Consoulas, unpublished observations). Larval sensory neurons associated with leg and eye imaginal discs also persist through metamorphosis in diptera (Jan et al., 1985; Tix et al., 1989a,b; Lakes-Harlan et al., 1991b). Also, an array of larval sensory neurons persist in each segment of Drosophila to become functional adult neurons (Shepherd and Smith, 1996). Thus, the survival of sensory neurons, like many motor and interneurons (see above), may be a general feature among holometabolous insects.
Do larval sensory neurons survive metamorphosis because they are required for adult behavior? In Manduca, gin trap sensory neurons become capable of mediating a pupal-specific defense reflex (Levine et al., 1985, Waldrop and Levine, 1989, 1992), and the abdominal stretch receptor organ survives metamorphosis to provide information about the length of the abdomen to persistent motoneurons and interneurons (Tamarkin and Levine, 1996). In the dipteran, Phormia regina, two sensory neurons that are associated with the Keilin's organ (mechanoreceptor) in the larva survive metamorphosis to innervate campaniform sensilla in the adult (Lakes-Harlan et al., 1991a,b). Similarly, in Drosophila, multidendritic and stretch receptor neurons survive metamorphosis to perform the same function in the adult (Shepherd and Smith, 1996; Smith and Shepherd, 1996). Larval FCO, TiCO, LS3 neurons and six tibial and tarsal neurons (that supply campaniform sensilla) survive to adulthood (this study; Consoulas, Rose, Levine in preparation) to serve the same function and participate in the locomotor behavior of the adult (Johnston and Levine, 1996).
Role of persistent larval sensory neurons in the establishment of the adult sensory pathways
A common feature of the developing nervous system in a broad range of organisms is that later developing neurons fasciculate with certain nerve pathways that are established by earlier developing neurons, known as pioneers, to reach their target area within the CNS or in the periphery (LoPresti et al., 1973; Bate, 1976; Raper et al., 1983; Goodman et al., 1984; Eisen et al., 1986; Kuwada, 1986; Dodd et al., 1988; Ghosh et al., 1990; Peinado et al., 1990; Stanier and Gilbert, 1990; McConnell et al., 1994; Gan and Macagno, 1995; Jellies et al., 1995). During metamorphosis, newly emerged sensory neurons arise far from the CNS and, unlike during embryonic development, must span distances that, in the Manduca leg, are in the range of centimeters.
The data presented here suggest that persistent sensory neurons may provide guidance cues for adult sensory neuron growth and, thus, may act as pioneers. Adult sensory axons always fasciculate with preexisting larval sensory nerves. In other holometabolous insects, persistent neurons may guide adult sensory axons to the CNS. In dipterans, for example, larval neurons that are associated with the leg (Tix et al., 1989a; Lakes-Harlan et al., 1991b) and eye (Tix et al., 1989b) imaginal discs, and neurons that innervate internal proprioceptors in thoracic and abdominal segments (Shepherd and Smith, 1996) survive metamorphosis and provide an axonal pathway to the CNS for the newly generated sensory neurons. Persistent neurons may be necessary for guiding the new axons, or new axons simply may prefer to use a preexisting larval nerve for navigation.
Flies homozygous for mutant alleles of the disco gene have defects as the larval Bolwing's nerve, which may explain the failure of the adult retinular neurons to innervate the optic lobe (Steller et al., 1987). Similarly, in Tenebrio, ablation of larval sensory neurons disrupts the growth of adult sensory neurons (Breidbach, 1990). In contrast, the pattern of nerve pathways formed in ectopic leg discs of Antennapedia flies seems to be unaffected by the absence of larval neurons (Tix et al., 1989b), and, in the absence of pioneer neurons, peripheral nerves in the embryonic grasshopper limb can be established successfully by neurons that appear later (Keshishian and Bentley, 1983b).
Newly emerged neurons have several choices and must make specific decisions. New adult axons in the tibia and tarsal segments, for example, grow along larval nerves 2a8 and 2a9 (Figs. 4, 9). Similarly, adult axons in the tibia and femur fasciculate with the axons of LS3-persistent neurons (nerve 2a5b; see Figs. 4, 9). In these cases, new sensory neurons that appear in the vicinity of larval sensory nerve branches make the decision to use these larval pathways as a scaffold for reaching the CNS. In contrast, nerve branches 2a5a and 2a6a (Fig. 9) are newly generated branches. They are established not by larval axons but by the axons of a few adult sensory neurons that appear earlier than the follower sensory neurons. These early adult sensory neurons, therefore, may play the same guiding role as the persistent larval sensory neurons. The formation of branches 2a6b and 2a6c is of particular interest. New sensory axons in the trochanter grow toward and fasciculate with the axons of LS1 and LS2 neurons, which disappear immediately after the formation of the adult pathways. These larval neurons have many of the characteristics of the embryonic pioneer neurons (Bate, 1976; Keshishian and Bentley, 1983a). They are present in the imaginal trochanter earlier than the adult neurons, their axons serve as a substrate for follower adult neurons, and they eventually degenerate. The only feature that differentiates these neurons from pioneer neurons is that, prior to metamorphosis they supply functional mechanoreceptors.
In conclusion, this study describes the establishment of the sensory pathways in the adult leg. It can be used as a tool in designing experimental perturbations that may allow a better understanding of the mechanisms underlying axon outgrowth and neural circuit remodeling during postembryonic development.
Acknowledgements
The author thanks Dr. R.B. Levine for the space, the materials, the continuous motivation to complete this study, and the critical reading of the article. He also thanks Maria Anezaki for her excellent technical assistance.




