Honeycomb-like structure of the intermediate layers of the rat superior colliculus, with additional observations in several other mammals: AChE patterning
Abstract
The aim of the present study was to reinvestigate the stereometric pattern of acetylcholinesterase (AChE) activity staining in the intermediate layers of the superior colliculus in several mammalian species. A pioneering study in the cat and the monkey by Graybiel (1978) stressed the regular arrangement of AChE staining in the deep collicular layers. According to her description, made in the frontal plane, the enzyme was arranged in a mediolateral series of patches, the cores of which tended to line up in the longitudinal axis of the structure, so they formed roughly parallel bands. As exhaustive a description as possible of the AChE distribution was undertaken in the rat by compiling observations in the frontal, sagittal, and tangential planes. It emerged that AChE-positive elements are organized in the form of a conspicuous honeycomb-like network that is divided into about 100 rounded compartments, over virtually the full extent of the intermediate layers. The generality of the rat model was then tested in other rodents such as mouse and hamster and also in cat and monkey. For these species we resorted to a single tangential cutting plane, which proved to be more appropriate for disclosing such a modular arrangement. The data revealed that in all species AChE staining followed the same architectural plan and identified the striking similarity in the number of compartments that compose the various honeycomb-like lattices. In conclusion, the present findings support a unified model of the AChE arrangement within the intermediate layers of the mammalian colliculus; the model comprehensively incorporates the classical description of the patchy and stripy features of the enzyme distribution. We hypothesize here that the modular AChE arrangement might be the anatomical basis for collicular vectorial encoding of orienting movements. J. Comp. Neurol. 419:137–153, 2000. © 2000 Wiley-Liss, Inc.
The concept of modularity in the brain arose from the seminal work of Woolsey and Van der Loos (1970), Asanuma and Rosen (1972), Hubel and Wiesel (1977), and Kaas et al. (1981), based on the existence of cytoarchitectonic and functional subunits in the cerebral cortex. Modules were designated for decomposition of both the proximal and distal sensory world into physical information such as the location, color, orientation, and movement of stimuli on the one hand and on the other, for fine muscular command.
During the last two decades, physiological and anatomical studies have provided convergent evidence for a modular arrangement of the deep superior colliculus (SC) in a variety of mammals (including primates, carnivores, and rodents). The SC or tectum is a laminated structure in the roof of the midbrain containing alternating cellular and fibrous layers (Kanaseki and Sprague, 1974; Tokunaga and Otani, 1976). Based on histological and functional considerations, the structure is classically divided into superficial and deep parts (Casagrande et al., 1972; Edwards, 1980). The superficial SC comprises three main layers: the stratum zonale, the stratum griseum superficiale (SGS), and the stratum opticum (SO). These layers are primarily involved in visual functions (see review by Stein and Meredith, 1991).
Briefly, they received their main inputs from the retina, visual cortex, lateral geniculate, and parabigeminal nuclei (Graybiel, 1972, 1975; Swanson et al., 1974; Hubel and Wiesel, 1977; Chalupa and Rhoades, 1979; Edwards et al., 1979; Graham, 1982; Mize, 1983). Their efferent connections are in turn directed to vision-related stuctures in the diencephalon (the lateral pulvinar/posterior complex, the geniculate nucleus), the pretectal area, and the deep SC (Caldwell and Mize, 1981; Holstedge and Collewijn, 1982; Huerta and Harting, 1983; Sugita et al., 1983; Grantyn et al., 1984; Rhoades et al., 1989). In the superficial SC, neuronal reactivity is predominantly related to onset-offset of stationary visual stimuli and also to moving cues (Schiller and Koerner, 1971; Goldberg and Wurtz, 1972; Tiao and Blakemore, 1976). The deep SC is composed of the stratum griseum intermediale (SGI), the stratum album intermediale (SAI), the stratum griseum profundum (SGP), and the stratum album profundum (SAP). As extensively reviewed elsewhere (Huerta and Harting, 1984; Grantyn, 1988; Sparks and Hartwich-Young, 1989; Stein and Meredith, 1991), these layers are mainly involved in multimodal integration and premotor functions. They process a variety of sensory, motor, and associative information arising from about 40 cortical and subcortical centers.
Concerning the sensory reactivity in the deep SC it is, by contrast with that of the superficial layers, visual, somatic (including tactile, proprioceptive, and nociceptive submodalities), and auditory. These inputs converge to these layers in a topographic fashion, and the various sensory maps they form are shown to be functionally registered (Gordon, 1973; Dräger and Hubel, 1975; Stein et al., 1976b; Tiao and Blakemore, 1976; Chalupa and Rhoades, 1977; Finlay et al., 1978; Donaldson and Long, 1980; Harris et al., 1980; McHaffie et al., 1989; Redgrave et al., 1996). In rodents for instance, the tactile and nociceptive reactivities of the face and the nasal visual field overlap in the rostral SC; the limb and trunk representations coincide with the auditory and temporal visual fields in the caudal SC. Likewise, the upper visual field and the tactile representation of the upper head, neck, and back are superimposed in medial SC, and lower visual and somatosensory fields are mapped together in the lateral portion of the structure (Dräger and Hubel, 1975; Tiao and Blakemore, 1976; Chalupa and Rhoades, 1977; Finlay et al., 1978). Thus, visual and/or somatic and/or auditory information coming from a given location in the environment appears to be processed in the same area of the collicular sensory matrix and even to converge at the level of single neurons (Meredith and Stein, 1986; Stein, 1998). A possible explanation for such a sensory alignment would be to ensure a coherent superposition of the physical descriptors of multimodal objects in the environment.
The deep SC also contains premotor neurons that, through extensive projections to motor-related structures in the brainstem, the spinal cord, and the diencephalon, promote orienting responses (Harting, 1977; Harting et al., 1980; Rhoades and Della-Croce, 1980; Holcombe and Hall, 1981; Grantyn and Grantyn, 1982; Murray and Coulter, 1982; Chevalier and Deniau, 1984; Redgrave et al., 1986; Yamasaki et al., 1986; Cowie et al., 1994; Muto et al., 1996). In monkey, they are predominantly gaze orientation involving eye or combined eye-head movements (Robinson, 1972; Stryker and Schiller, 1975; Cowie and Robinson, 1994), whereas in cat and rodents, they also comprise movements of pinnae, whiskers (Syka and Radil-Weiss, 1971; Harris, 1980; McHaffie and Stein, 1982) and even of the entire body (Northmore et al., 1988; Dean et al., 1989). The direction and amplitude of orienting movements are spatially encoded in the deep SC. Focusing on gaze control, which has been the most studied feature of the collicular physiology, early stimulation studies led to the concept of a motor map in deep SC in cat (Syka and Radil-Weiss, 1971; Stein et al., 1976a; Roucoux et al., 1980; Harris, 1980; Paré et al., 1994), monkey (Robinson, 1972; Stryker and Schiller, 1975), and rodents (McHaffie and Stein, 1982). To summarize, localized electrical stimulation of the intermediate layers induces foveating visual “grasping” by conjugate contraversive eye movements toward a particular region of the contralateral visual space.
The direction and amplitude of saccade basically depends on the location of the stimulated site. For example, stimulation in the anterolateral region of SC, produces forward saccades with a downward component, whereas caudomedial stimulation induces backward saccades with a clear upward component. Concerning more specifically the amplitude of saccades, it has generally been observed that they are small when evoked from anterior sites and large and combined with head movements when induced caudally (Syka and Radil-Weiss, 1971; Roucoux et al., 1980; Northmore et al., 1988; Cowie and Robinson, 1994; Paré et al., 1994).The notion of a motor map in SC has also been substantiated by chronic unit recordings in awake monkeys performing orienting tasks. From these studies emerged the concept of a motor field; briefly, neurons in intermediate layers discharge before saccades, and they do so maximally for saccades of a particular direction and amplitude (Wurtz and Goldberg, 1972; Sparks et al., 1976). Thus, cells are topographically organized in intermediate layers, according to their movement field.
Finally, a major advance in the physiology of the deep SC has been provided by the observation that sensory and motor maps align, even if the way in which they do so is still unknown (Sparks, 1988). As initially shown in monkey by Schiller and Stryker (1972), local stimulation of the deep SC produces a saccade that moves the fovea to the region of the visual field represented in this area. Furthermore, convergent observations were drawn from recordings in behaving monkey, showing that cells with both sensory and motor responses, display overlapping (but not necessarily co-extensive) movement and receptive fields (Wurtz and Goldberg, 1972). In conclusion, the deep SC appears suitably equipped with a modular organization of the sensorimotor linkages to perform grasping movements of the cephalic captors and even body displacements toward attractive cues in the environment.
The concept of modularity in the deep SC, and in particular in the well-developed intermediate layers, has also been substantiated by anatomical data, which have provided decisive evidence for a discontinuous cyto- and chemo-architecture of these layers. Viktorov (1968) was the first to draw attention to the clustering of large multipolar neurons lying in the cat SGI, suggesting that such clusters may constitute functional subunits for orientation. This observation was later confirmed by retrograde labeling of different classes of efferent collicular neurons (Castiglioni et al., 1978; Huerta et al., 1981; Murray and Coulter, 1982; Yamasaki et al., 1986). In parallel, there is a patchy innervation of most, if not all, of the collicular afferents (from cortex, Goldman and Nauta, 1976; Künzle and Akert, 1977; Leichnetz et al., 1981; Segal et al., 1983; Clemo and Stein, 1983; from subcortical sensory relays, Kudo and Niimi, 1980; Tashiro et al., 1980; Huerta et al., 1981; Kudo, 1981; Huerta and Harting, 1982; Harting and Van Lieshout, 1991; from basal ganglia, Jayaraman et al., 1977; Graybiel, 1978a; Beckstead et al., 1979; Harting and Van Lieshout, 1991; Redgrave et al., 1992; from cerebellum, Kawamura et al., 1982; from hypothalamus, Rieck et al., 1986). Patches in the intermediate layers are regularly described in the frontal plane as a series of groups of either terminals or neurons with generally increased size from medial to lateral SC. In cat, for instance, the trigemino-collicular terminals segregate in a series of mediolateral patches with diameters ranging from 50 to 500 μm and spaced about 150 μm apart (Tashiro et al., 1980; Huerta et al,. 1981). In the same species, the nigrocollicular innervation distributes in 300–500-μm-wide clumps (Graybiel, 1978a). The frontal eyefield-related patches in monkey range from 300 to 500 μm, with equivalent space intervals (Künzle and Akert, 1977). Finally, in rat, neurons projecting to the thalamic intralaminar nuclei form clusters ranging from 80 to 220 μm, and the intervening gaps vary from 80 to 100 μm (Yamasaki et al., 1986).
The idea of collicular compartmentalization has been reinforced by the neurochemical finding of a regular distribution of a number of neuroactive substances in the intermediate layers such as somatostatin (Spangler and Morlay, 1987), enkephalin (Graybiel et al., 1984; Miguel-Hidalgo et al., 1989; Mize, 1989; Graybiel and Illing, 1994), cytochrome oxidase (Sandell, 1984; Wiener, 1986), choline acetyltransferase (Ross and Godfrey, 1985; Beninato and Spencer, 1986; Hall et al., 1989; Hashikawa, 1989; Illing, 1990; Henderson and Sheriff, 1991; Jeon and Mize, 1993), parvalbumin (Illing et al., 1990), substance P (Miguel-Hidalgo et al., 1989; Behan et al., 1995), hexokinase (Sandell, 1984), and others (Illing, 1996).
Among the impressive number of markers currently available to delineate patterning in the SC, acetylcholinesterase (AChE) represents the most frequently used. In the deep SC, the presence of AChE was shown to be associated, at least for a part, with the cholinergic projections arising in the pedunculopontine and dorsolateral tegmental nuclei (Woolf and Butcher, 1986; Hallanger and Wainer, 1988; Jones and Webster, 1988; Wallace and Fredens, 1988; Hall et al., 1989). The intracollicular distribution of this enzyme has been well documented in cat (Ramon-Moliner, 1972; Graybiel, 1978b; Illing and Graybiel, 1985), rat (Sandell, 1984; Ross and Godfrey, 1985; Beninato and Spencer, 1986; Toga and Santori, 1995), mouse (Wiener, 1986; Wallace, 1986), monkey (Graybiel, 1978b), and human (Graybiel, 1979). In spite of the powerful three-dimensional (3D) digital imaging techniques that have recently been introduced to visualize the stereometric arrangement of the AChE in rat (Toga and Santori, 1995), the descriptions were promising but remained incomplete. In fact, it is in cat that the most detailed description is available, starting with the pioneering studies by Graybiel (1978b) and Illing and Graybiel (1985). According to these authors, AChE labeling appears, when examined in the frontal plane, as a series of regularly spaced dense patches about 200–600 μm wide, at 300–600-μm intervals, extending from the lateral to medial aspects of the intermediate gray layer. This pattern is most clearly visible in the caudal third of the SC. Further forward it tends to become less regular. Rostral patches are irregular in shape and frequently fuse so that the regular spacing may eventually become fully disrupted. A vertical arrangement of the enzyme has also been identified. The AChE patches are frequently split into dorsal and ventral tiers, sometimes with the two parts linked by a wisp-like AChE extension. When reconstructed in the dorsal view from serial frontal sections, the cores of patches tend to line up so as to form longitudinal stripes that seem to radiate from a major anastomotic node, near the caudal pole of the SC, where AChE forms intermingled latticeworks.
Surprisingly, in the course of routine studies in rat, in which AChE activity was systematically reconstructed from tangential sections, we discovered some unexpected characteristics. AChE staining identified nearly perfect circular distributions in the rostral portion of the intermediate layers. This consistent observation was puzzling because it partially conflicted with the well-accepted description of AChE distribution in cat and monkey (Graybiel, 1978b). Following this observation, we carefully reexamined the stereometric pattern of the AChE activity in rat and then extended our comparison to other mammalian species (hamster, mouse, cat, and monkey). In the rat, we attempted to visualize the 3D architecture of the cholinesterase domains by reconstructing the enzyme labeling from serial sections cut in frontal, sagittal, and tangential planes. For the other species, the study was limited to a reconstruction of labeling from tangential sections.
Taken together, the present findings support a unified model of the AChE arrangement within the intermediate layers of the mammalian SC. Furthermore, this model comprehensively incorporates the classical description of the patchy and stripy features of the enzyme distribution.
MATERIALS AND METHODS
We studied the distribution of AChE activity in the superior colliculus of 30 adult male albino rats (Sprague-Dawley), 3 male adult mice (C57BL), 2 adult male golden hamsters (Syrian hamster), 2 adult male cats (Felis domestica), and 1 adult male monkey (Macaca fasciculata).
After an overdose (160 mg/kg, i.p.) of sodium pentobarbital (Nembutal, Sanofi), animals were perfused transcardially with a 0.9% saline wash followed by a fixative solution of 3% paraformaldehyde and 0.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) and finally cryoprotected with a solution of 10% sucrose in 0.1 M phosphate buffer (pH 7.4) at 4°C. The brains were kept overnight in 30% sucrose in phosphate buffer (pH 7.4) at 4°C.
The rat brains were sectioned at 40–60 μm on a freezing microtome, in either frontal, sagittal, or tangential planes, whereas those of the mice, hamster, cat, and monkey were cut only in the tangential plane, at 40–60 μm. For the cat and monkey, the left and right colliculi were separated on the inter-collicular midline and sectioned separately in a slightly rotated plane.
Floating sections were then processed for AChE histochemistry according to a slightly modified protocol of Geneser-Jensen and Blackstadt (1971) using as the developing medium a 10% potassium ferricyanide solution. The sections were mounted onto chrome-alum-coated slides, dehydrated through alcohols and xylene, coverslipped, and examined using brightfield microscopy.
The resulting pattern of staining was observed at ×100 magnification factor and drawn with a camera lucida. Serial-section reconstructions were assembled in each plane as follows. For the sake of clarity, we remind the reader that the mammalian SC is shaped approximately like a slightly flattened portion of a sphere in which the main vascular processes course in a radial direction toward the SC surface. We chose, after Illing and Graybiel (1985), to use them as fiducial landmarks for the stereometric reconstruction of the enzyme domain in each of the species studied. In the sagittal and frontal planes, we lined up the corresponding segments of the blood vessels, section after section. In the tangential plane this time, the cross sections of the blood vessels were vertically aligned through the depth of the deep SC. Consequently, in the tangential plane the procedure straightened the radial vascular trajectory and resulted in a flattened but very useful view of the SC.
An alternative procedure for the tangential reconstruction was attempted in two rats. It consisted of the use of artificial marks to assemble the serial collicular sections. After being fixed with the aldehyde solution, the rats were repositioned in the stereotaxic frame. Two holes were then stereotaxically drilled by the vertical motion of a metallic electrode through the pretectum and the inferior colliculus (atlas of Paxinos and Watson, 1982). The two holes were kept well visible on each section of tissue and thus could be reliably used as reference marks to align the sections. This procedure was not, however, retained for further reconstructions because owing to the collicular curvature, it resulted in complex AChE pictures difficult to analyze without the help of 3D imaging computer software.
Shrinkage due to dehydration was evaluated only from rat tangential sections, as follows. In the same animal we compared pictures produced by AChE staining in both wet and dry tissue. Immediately after the staining procedure, the wet sections were rapidly photographed (using a standard Leica camera) at ×100 magnification. A photomontage of each individual section was prepared from a set of silver prints that we used for drawings. After dehydration and mounting, the same sections were drawn with a camera lucida and the distribution and measurements of the AChE deposits compared with the first set of wet images.
For processing, the figures were prepared with Adobe PHOTOSHOP from scanned (1200 dpi) drawings and silver-printed photomicrographs. The contrast of certain photomicrographs was sometimes altered to obtain a uniform tone for the photomontages presented.
All experimental protocols were approved by the French Office of Agriculture, Fisheries, and Food (authorization no. 6805) and complied with the veterinary regulations on the use of laboratory animals.
RESULTS
AChE patterning in intermediate layers of the rat SC
Frontal plane.
The enzyme labeling appears in this plane in two different staining densities that we have defined as the “dense” and “diffuse” AChE domains respectively (Fig. 1).
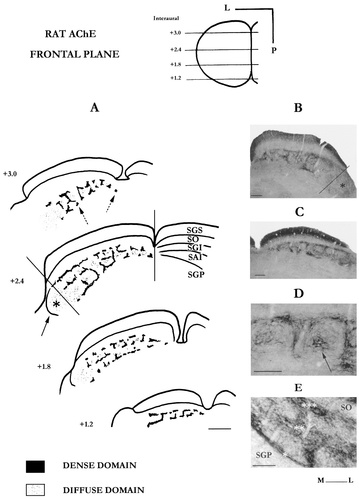
A: Drawings of coronal sections showing the AChE pattern at four selected levels of the left superior colliculus. Dashed arrows (section +3.0) point to some of the cut fascicles in the ventral AChE tier. The oblique and vertical lines (section, +2.4) indicate the orthogonal projection of the superficial layers onto the deep colliculus. Such a projection is meaningful since it conforms to the trajectories of the interlaminar axons running from the superficial to the deep SC, as shown by Rhoades et al. (1989). The asterisk points to the flank area, which is outlined by a thin AChE sheet (solid arrow). Note that the dense AChE geometrical pattern only develops where there are overlying visual layers. B,C: Photomicrographs illustrating the AChE labeling at rostral (+3.0) and caudal (+1.5) levels, respectively, in the right superior colliculus. Asterisk and broken line in B point to the flank. D: Magnification of the AChE staining at a rostral level. The arrow points to the diffuse AChE domain in SAI layer. E: Photomicrograph showing the three AChE tiers of the dense domain at the +2.4 level. Compared with the ventral tier (*), the intermediate (**) and the dorsal (***) ones show a fleecy texture. SGS, stratum griseum superficiale; SO, stratum opticum; SGI, stratum griseum intermediale; SAI, stratum album intermediale; SGP, stratum griseum profundum. Scale bars = 500 μm in A; 200 μm in B and C; 250 μm in D; 100 μm in E.
The dense AChE domain.
This appears predominantly as a heavily stained fibrous system that constitutes a well-defined regular network throughout the intermediate layers. In the caudal pole of the rat SC, where the intermediate layers are limited to the SGI, the AChE labeling forms two semi-parallel AChE tiers of about 40 μm thickness each, which delineate the dorsal and ventral limits of the layer, respectively. They are linked by regular vertical AChE fibrous fascicles or strands (30–50 μm thick), giving the labeling a ladder-like appearance and dividing the SGI into a mediolateral row of three to four square compartments (with 150–250 μm sides). On a given section, their contours are generally seen as a fragmented AChE line continuous with the adjoining sections. In some of the frontal sections, the ventral AChE tier appears broken up into regularly spaced small stained dots, taking on the appearance of cut fiber tracts from which the vertical AChE strands often arise. In the rostral two-thirds of the SC, the AChE staining contrast decreases, but the geometrical arrangement is maintained for the SGI and is even extended into the underlying SAI (Fig. 1). Three AChE tiers are visible here and roughly delineate the SGI and SAI boundaries. The ventral tier spreads at the interface between the SGP and SAI layers whereas the intermediate and the dorsal ones mark the SAI/SGI and the SGI/SO boundaries, respectively.
Compared with the ventral tier, which appears as predominantly fibrous, the dorsal and intermediate tiers show a rather fleecy texture suggestive of the presence of terminal fields (Fig. 1E). As for the vertical strands in which fibers and more moderate terminal fields intermingle, they course through the intermediate layers in a radial direction with respect to the curved SC surface; they follow the trajectory of blood vessels, with which they are often parallel. On some sections, they seem to be at the origin of the intermediate and dorsal tiers through lateral arborizations. Thus, the horizontal and vertical dense AChE strands together form in the rostral two-thirds of the SC a geometrical frame dividing both the intermediate strata into two sets of aligned compartments. However, this double-decker organization clearly blurs in the rostralmost pole, where the intermediate AChE tier becomes difficult to distinguish. The same observation can be made for the AChE organization near the midline, where the SGI and SAI greatly reduce in thickness and fuse. Finally, the enzymatic grid pattern is absent in the anterolateral portion of the intermediate layers, designated as the “flank” by Wiener (1986), i.e., the area that protrudes beyond the overlying superficial layers. This region is only outlined by a thin enzymatic sheet that seems to be a lateral continuation of the dorsal tier (Fig. 1A).
The diffuse AChE domain.
This is well developed only in the rostral SC, where the intermediate strata are in two layers. We systematically distinguish a faint and dispersed AChE labeling filling the spaces delineated by the dense AChE frame, as well as the flank (Fig. 1A). As a rule this labeling shifts from one section to another, making its precise arrangement difficult to identify in the frontal plane. It is sometimes seen right in the middle of the densely stained compartments, clinging near the edges or even absent. Under higher magnification, we noted that the diffuse-AChE domain coincides with rich fibrous areas. The ventral SGI and the SAI are, in rat, regions where richly fibrous areas run along the longitudinal axis of the SC. Thus, the diffuse-AChE domain spreads into the interstices between non-AChE fibers, which reduces its visibility still further in the frontal plane (Fig. 1D). Finally, in three rats, some sparse and weakly AChE-stained medium to large-sized perikarya were encountered in the lateral SAI. For the sake of clarity, we will maintain in the following sections the nomenclature for the AChE domains set out here from observations in the frontal plane. So we will refer to the compartment frame as the dense AChE domain and to its scattered contents as the diffuse-AChE domain.
Sagittal plane.
In this plane, the two distinct AChE domains are still obvious but now the visibility of the diffuse domain has improved (Fig. 2).
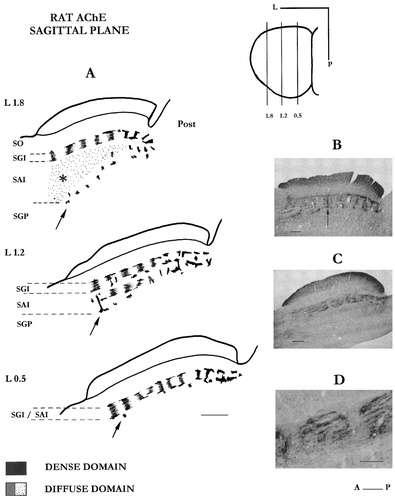
A: Drawings of three selected sagittal sections, in a left superior colliculus. Arrow points to the series of AChE-positive fiber tracts, easily distinguished in the lateral colliculus. Note that the lateral SAI (asterisk) is homogenously stained. B,C: Photomicrographs illustrating the features of the AChE labeling at lateral 1.2 and 0.5, respectively. The arrow in B points to a vertical strand of the dense domain rising from the ventral AChE tier. D: Magnification of the AChE blocks in the anterior SAI, showing their typical streaked appearance due to the presence of non-AChE positive fibers. For abbreviations, see Figure 1. Scale bars = 500 μm in A; 300 μm in B,C; 200 μm in D.
The dense-AChE domain.
A pattern similar to that described for the frontal plane is observed for the caudal third of the SC. AChE labeling is seen in the SGI as a heavily stained fibrous network that composes a one-layered series of four to five square compartments with sides of 150–250 μm. Rostralward, the dense frame, while present, becomes harder to detect, probably due to its out-of-plane orientation and weakening of its staining. In this plane, neither of the dorsal and intermediate AChE tiers are easily distinguishable because they are often confused with the longitudinal strips of the diffuse domain (see below). Only the ventral tier remains discernible as a fairly regular series of AChE-positive fiber tracts. They form a longitudinal line, accurately delineating the boundary between SAI and the underlying SGP from which, in some cases, the vertical strands were seen to arise (Fig. 2A,B). Although a partial reconstruction of the fiber network has been undertaken from sagittal sections, the best view is from the tangential plane. In fact, in the sagittal plane, it is the well-structured diffuse-AChE domain that is the most striking.
Diffuse-AChE domain.
As in the frontal plane, the diffuse domain is seen primarily developed in the rostral two-thirds of the intermediate layers. It displays more often a series of regular blocks that vertically segment both the intermediate strata. Every block is traversed longitudinally by non-AChE fibers, giving it a striated appearance (Fig. 2C,D). In the more medial SC, the AChE staining pattern is a one-layered series of rectangular blocks (100–150 μm thick and 50–300 μm long) separated by free enzyme zones. When followed from section to section, each AChE block regularly splits in two lateral fragments that move away and further fuse to again form a single block. Thus, the interblock interval shifts center to center from 0 to 200–250 μm. At the mid-level of the SC, the AChE blocks organize in two aligned sets lying in the SGI and the SAI layers. In the lateral SC, the upper set of blocks remains visible only in the SGI, and the lower set in the SAI tends to lose its segmental arrangement and becomes an evenly stained area. This even texture will further rule the enzyme staining in the flank area where the SAI layer predominates.
Tangential plane.
This plane was crucial for the integration of the frontal and sagittally derived observations into a logical framework. Indeed, the tangential plane gave the best overall picture of the complementarity of both dense and diffuse AChE domains (Figs. 3, 4A). In rat, taking into account the curvature of the SC and the thickness of the intermediate layers, 10–12 serial sections (50-μm-thick) are generally required from dehydrated tissue, to reconstruct the picture of the horizontal enzymatic arrangement. Both enzymatic domains combine to form a conspicuous honeycomb-like network of about 100 ovoid and rounded compartments.
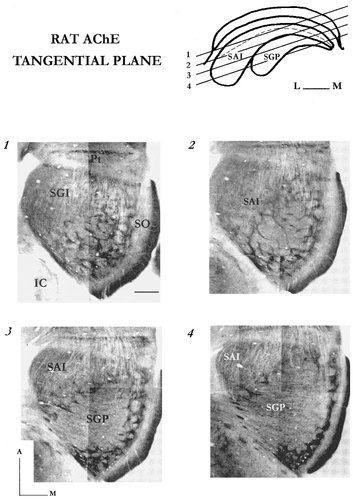
Photomicrographs showing selected tangential views of the AChE patterning (1–4 in the template). Pt, pretectum; IC, inferior colliculus. For other abbreviations, see Figure 1. Scale bars = 500 μm.
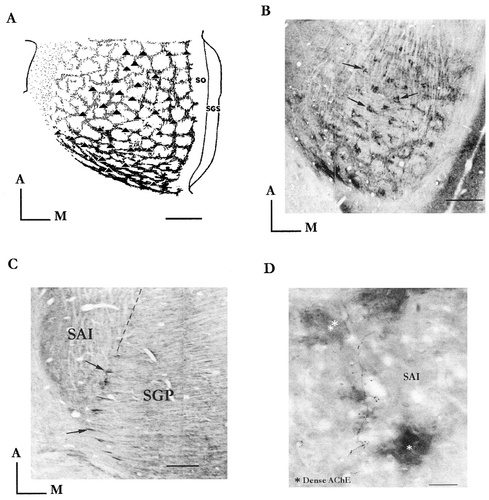
A: Full reconstruction drawing of the AChE lattice in the rat. The black and gray labeled contours stress the decreasing gradient of the AChE staining from caudal to rostral SC. The dotted area in the anterolateral sector marks the flank, where the AChE staining is evenly distributed. We have superimposed on the lattice the array of the dense AChE profiles (solid triangles) corresponding to the vertical strands of the frame as they appear when cut in the tangential plane. Note that their periodic arrangement fits that of the honeycomb-like compartments. B: Illustration in the tangential plane of some of the dense AChE profiles (solid triangles in A) that composed the array of the vertical frame division. The arrows point to some of them. C: Arrows point to some of the 12–13 parallel AChE fascicles entering the intermediate layers at the SGP/SAP interface. D: Biocytin-labeled axon and ending profiles in intermediate layers of a neuron of the pedunculopontine cell-group. Note in this frontal view that all along its course from the ventral (*) to the intermediate dense AChE tier (**), the axon gives off terminals with boutons in the interbundle spaces within the SAI layer. These terminals probably participate in the diffuse AChE domain. White blotches are longitudinally running fiber bundles, as they appear when cut in the frontal plane. For abbreviations, see Figure 1. Scale bars = 500 μm in A–C; 100 μm in D.
It is in the caudal SC that the lattice shows its heaviest staining contrast. Here the enzyme latticework is composed of dense cross-linked bands (40-μm-thick). They delineate 15 or so ovoid compartments (150–200 μm axis) that roughly compose four transverse rows. In the rostral two-thirds of the SC, in agreement with the observations made in the frontal and the sagittal planes, the AChE staining fades, but the compartmentation is maintained. Both the dense and diffuse domains join to form a fairly regular matrix of about 80 rounded compartments with thick contours (50–100 μm) surrounding enzyme-free hollow centers (150–300 μm diameter, the largest being near the medial edge of the SC).The compartments are distributed with a spacing of 200–350 μm from center to center. In this plane, the dense and diffuse domains align perfectly in the depth of the intermediate layers so that the transition from one to the other is not obvious. The vertical strands of the dense domain are nevertheless well identified since they compose an array of heavily stained AChE profiles (40–60 μm in size) regularly spaced by 150–300 μm in any direction (Fig. 4A,B). Their number could not be fully determined, because they often surround blood vessels like a sheath, making their identification sometimes ambiguous. Similar to the other planes, these vertical strands clearly originate from the ventral AChE tier.
In the tangential plane, this tier appears as a fibrous plexus extending from caudal to rostral SC composed of a series of 12–13 regularly spaced parallel fascicles (200–250-μm interval). They enter laterally into the intermediate layers at the SGP/SAI interface and course in a transverse direction toward the collicular midline (Fig. 4C). During its course each fascicle is seen to be periodically (150 to 250–300 μm) at the origin of a lateromedial series of several vertical fibrous strands. We sometimes clearly observed that the 12–13 AChE fascicles also give off a net of divergent secondary branches, spreading this time in a horizontal direction at the SGP surface. They radiate from the bifurcating nodes where the vertical strands arise, and they cross-link the series of nodes of a given fascicle with that of the next (see Fig. 6A). So the ventral AChE tier appears to form latticework whose arrangement reflects the overlying lattice.
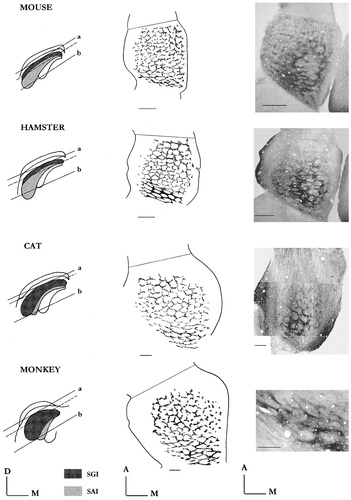
The AChE honeycomb-like pattern in four mammalian species. Reconstruction drawings (intermediate column), from a series of N tangential sections selected from a to b level (left column). For the mouse N = 8, for the hamster N = 12, and for the cat and monkey N = 20. Photomicrographs of sampled sections (right column). Their approximate level is indicated by the broken line in the left column (drawings). Note: the monkey photomicrograph shows the AChE lattice in the caudal SC, due to the plane of section, which revealed through successive sections only partial views of the gridwork. Scale bars = 500 μm.
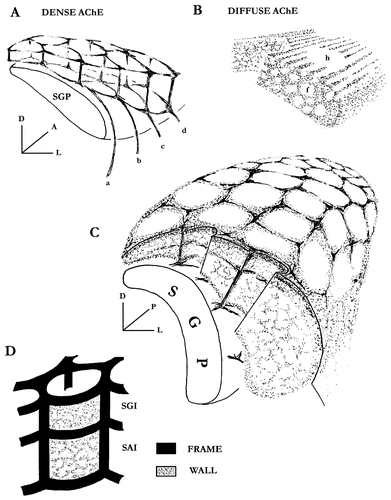
The honeycomb-like model in the rat. A: Architectural principle ruling the dense AChE frame as illustrated in the caudal SGI. (a, b, c, and d, are AChE feeder fascicles). B: In SAI, the diffuse AChE domain spreads between the longitudinally coursing fiber bundles (f), periodically sparing rounded domains to form the hollow-compartment centers (h). C: A three dimensional model for the AChE arrangement in intermediate layers. D: The dense and diffuse AChE domains combine to constitute the frame and the wall components of every compartment. For abbreviations, see Figure 1.
Finally, the honeycomb-like net spreads over the full extent of the intermediate layers with the exception of the flank area, where the AChE staining is evenly distributed.
An estimate of the amount of shrinkage due to dehydration has been carried out from tangential sections. It is approximatively 15% following measurements of the internal and external diameters of the honeycomb compartments in the wet and dry tissue, respectively.
AChE patterning in other mammals
Although frontal, sagittal, and tangential planes are all required to gain a satisfactory picture of the AChE system, the study in rat showed us that the tangential plane was the most appropriate to reveal its basic arrangement. Thus, we characterized the main features of the AChE patterning in a limited sample of mouse, hamster, cat, and monkey using only this plane. Because of the close similarity of the results, data from all species will be reported in the following section (Fig. 5).
The same basic honeycomb-like pattern emerged from each of the species under investigation. As observed in rat, a rostrocaudal gradient of the AChE staining density was always obvious in the three rodent species, but this gradient was more pronounced in cat and monkey. In these latter species, the AChE labeling becomes faint and even disappears in the most anterior sector of the intermediate layers. However, the basic lattice arrangement is the same in all species; a sharp dense labeled latticework is present in the caudal SC, whereas there is a less pronounced labeling of the rostral compartments. On the other hand, from our material in cat and monkey, the anteromedian portion of the lattice, where the large well-rounded compartments predominate in rodents, the AChE labeling gives a less well-defined picture. In monkey, for instance, the contours of the compartments are not as well delineated as in rodents; the AChE features are rather patchy, but nevertheless have a regular arrangement that complies with the general architectural plan of the lattice. Finally, as in rat, the orderly arrangement of AChE-positive tracts entering the SC were observed in mouse, hamster, and cat. They again supply the vertical distribution structures of the gridwork. Unfortunately, we have no direct data on this relationship in monkey.
One further observation of interest arises from the present comparative study. Whereas the longitudinal and transverse dimensions of the SC increase from 1.5 to 6 mm from mouse to monkey, the number of honeycomb compartments in each species remains approximately the same. The number of compartments ranged from 95 to 100 for rat, 85 to 100 for hamster, 80 to 100 for mouse, 80 to 90 for cat, and 90 to 95 in monkey. Thus, the relative size of the individual compartment varies in a manner correlated with the SC dimensions. In cat and monkey, for instance, the maximum size of compartments is 450–500 μm (internal diameter), whereas it never exceeds 200 μm in mouse.
DISCUSSION
The present study was aimed at reinvestigating the stereometric pattern of AChE-related labeling in the intermediate layers of the SC in rat and several other mammals. The findings have led us to conclude that this enzymatic marker demonstrates the same pattern in all species examined. It consists of a regular honeycomb-like lattice of AChE-positive elements throughout virtually the whole extent of the intermediate layers. Such an arrangement was first suspected in cat and monkey by Graybiel (1978b) and then reinvestigated in the cat by Illing and Graybiel (1985); unfortunately, the entire pattern was not systematically revealed. In cat, for instance, these authors stressed as a basic arrangement for AChE a dense anastomotic grid-like node in the caudal SC that rapidly disorganizes rostralward to become a vanishing longitudinal band-like network. We propose that the reason for such a discrepancy between our observations is that these authors lacked pieces of the AChE jigsaw puzzle. The complexity of the 3D enzymatic network is such that it requires a comparison of views in frontal, sagittal, and tangential planes and a complete reconstruction in each of them. Indeed, the three planes are complementary since separately they show only a part of the lattice and cannot reveal the others. Any reconstruction of profile from a single plane can be misleading. In these classic papers, the tangential view of AChE labeling was inferred from frontal sections, and the reconstruction probably failed to take into account what we call in the present report, the diffuse AChE domain. This domain is found predominantly in the anterior two-thirds of the SC, and its visibility is weak in the frontal plane. Although in both studies the same AChE protocol has been followed, it cannot be excluded that minor “tweakings” of protocol that unavoidably take place in different ways, in different labs, might account for somewhat different results.
The honeycomb-like model in rat
In every plane, the AChE displays two different staining densities, a dense and a diffuse one, thus defining two main structural domains in the intermediate layers. Both conjugate to compose a honeycomb-like arrangement divided into about 100 rounded compartments with internal diameters ranging from 150 to 300 μm (Fig. 6). Due to shrinkage, these values on dry tissue underestimate by at least 15% those expected on fresh tissue.
The dense domain originates ventral to the SC probably in the pedunculopontine area and the laterodorsal tegmental nucleus, both known to provide cholinergic input to the intermediate collicular layers (Beninato and Spencer, 1986; Woolf and Butcher, 1986; Jones and Webster, 1988; Wallace and Fredens, 1988; Hall et al., 1989). This innervation enters the SC laterally at the boundary between SAI and SGP in the form of a series of 12–13 regularly spaced fiber fascicles. As they course horizontally toward the collicular midline, each of them extends regularly spaced vertical strands that then arborize once (caudally) or twice (rostrally) within the depth of the intermediate layers to compose a one- and two-layered series of compartments, respectively (Fig. 6A). It is noteworthy that the number of AChE feeder fascicles fits that of the transverse rows of compartments (N =12–13) that can be counted from rostral to caudal SC. This suggests that each fascicle would participate in the delineation of a limited number of compartments, probably organized in a discrete mediolateral row. Based on observation of its textural appearance, the dense frame domain seems to be structurally heterogeneous. Its ventral tier appears to be primarily composed of fibers and would indeed correspond to the feeder system of the AChE lattice. By contrast, both the intermediate and dorsal tiers and also to a lesser degree the vertical strands, where fibers and terminals are mingled, would be part of the dense domain where cholinergic synaptic transmission and related AChE activity might be expected. Finally, from the present observations, this domain would constitute the frame division of the AChE honeycomb-like lattice.
As for the diffuse-AChE domain, it appears that although it is detectable in frontal and sagittal sections as well, this AChE compartment displays its actual arrangement only from the tangential plane. Predominantly developed in the anterior two-thirds of the intermediate layers, it obeys and completes the architecture of the frame by constituting the wall of every compartment. Its textural appearance suggests that it is mainly constituted by AChE-containing terminals dispersed in the spaces between the longitudinally coursing fiber bundles (Fig. 6B). As a consequence, in addition to the frame, it is probably a compartmental subdivision for cholinergic synaptic contacts. Thus, the frame and the wall together constitute, through the intermediate layers, vertical cylinders with clear cut AChE-free hollow centers (Fig. 6C,D).
The origin of the diffuse-AChE domain is not clarified in the present study. If we cannot rule out the possibility of an intrinsic origin, taking into account the presence of some AChE positive-cell bodies scattered in the lateral intermediate layers, we have to accept the fact that only one extrinsic AChE source has so far been clearly identified in the form of 12–13 AChE ventral fiber fascicles. From preliminary studies, we propose that these latter also supply the diffuse-AChE domain. Several well-made points on fiber tracts and frames and terminals support this view. Micro-deposits of biocytin in the cholinergic pedunculopontine area result in an axonal labeling that can easily be followed up to the SC. Some axons were reconstructed and we saw that all of them enter the intermediate layers, taking the route of the ventral fibrous AChE tier before terminating in either the frame and/or the diffuse-AChE compartment, where bouton-like profiles are seen (Fig. 4D). Hence, the diffuse domain as well as the dense frame would have a common origin at least in the cholinergic pedunculopontine area.
Finally, we observed that the rat lattice does not extend into the flank, which, according to Wiener's description (1986), is a region that protrudes beyond the overlying superficial layers where correlatively cells are predominantly driven by orofacial somatic input (Wiener and Hartline, 1987). By contrast, cells distributed anywhere else in the intermediate layers have multimodal reactivities that combine somatic and/or visual and/or auditory inputs (Gordon, 1973; Dräger and Hubel, 1975; Stein et al., 1976b; Tiao and Blakemore, 1976; Chalupa and Rhoades, 1977; Finlay et al., 1978; Donaldson and Long, 1980; Harris et al., 1980; McHaffie et al., 1989; Redgrave et al., 1996). The visual input, in particular, is mapped according to the retinotopy in the superficial layers. Since both direct axonal connections from the superficial to the deep SC (Grantyn et al., 1984; Mooney et al., 1988; Rhoades et al., 1989; Hilbig and Schierwagen, 1994) and dendritic field expansion of deep collicular cells into the the superficial layers (Moschovakis and Karabelas, 1985; Rhoades et al., 1987; Moschovakis et al., 1988) are suspected to account for part of the visual reactivity in the intermediate layers, one may imagine that the latticed area would gain from a topological alignment with the superficial layers.
The honeycomb-like arrangement in other mammals and functional considerations
It emerges from the present comparative investigation that the honeycomb-like arrangement of the AChE system, seen in rat, is shared by other mammalian species (Fig. 5). Although the architectural principle revealed is fundamentally similar throughout species, the vertical staining pattern may be somewhat different in each. This point has not been investigated in the present study. Indeed, there is throughout species a differential development of the SGI and SAI layers that will influence the relative size of the upper and lower compartments. It is well documented that rodents have an enlarged SAI compared with cat and monkey, since it is primarily devoted to the snout, which is their dominant sensory apparatus (Dräger and Hubel, 1975; Tiao and Blakemore, 1976; Chalupa and Rhoades, 1977; Finlay et al., 1978; Chevalier et al., 1984; Wiener and Hartline, 1987; Westby et al., 1990).
Another striking similarity across the species under investigation is the decreasing gradient of AChE staining from caudal to rostral SC. A proposal by Illing (1988, 1996) suggests that the SC mapping of eye position vectors and related retinal topographies are determining factors for the presence and steepness of such a gradient. The existence of a pronounced AChE gradient responsible for the area of ill-defined or even deleted staining in the rostral SC seems to be a characteristic of animals with frontally oriented eyes. This author stressed that, in contrast, animals with laterally oriented eyes such as the pig, cow, or rabbit showed a clear and well-delineated AChE pattern through the whole extent of the intermediate layers. The area centralis or the fovea of animals with frontally positioned eyes is topographically relayed to the rostral SC, which is also a zone with high degree of binocularity (Schiller and Koerner, 1971; Goldberg and Wurtz, 1972; Graybiel, 1975; Tiao and Blakemore, 1976), where electrical stimulation produces the smallest gaze shifts (Robinson, 1972; Roucoux et al., 1980; McHaffie and Stein, 1982; Paré et al., 1994) and where the “fixation cells” are concentrated (Munoz and Guitton, 1991; Munoz and Wurtz, 1993). Considering that the degree of binocularity increases from rodents to cat and monkey, a correlated decrease, or lack, in AChE staining might be expected. This finding is clearly verified in the present study.
Hence the caudal SC, in all the species examined, is a region where the AChE labeling is densest. From physiological investigations we know that it is a zone responsible for large gaze shifts, often requiring the cooperation of eye and head movements (Syka and Radil-Weiss, 1971; Roucoux et al., 1980; Northmore et al., 1988; Cowie and Robinson, 1994; Paré et al., 1994). Thus it appears that there is, in the intermediate layers, a correlation between the AChE density and orienting function according to which the greater the amplitude of gaze deviation elicited from a region, the denser the AChE staining of the honeycomb-like compartments.
Finally, another regional difference in the AChE staining between rodents and higher mammals is that the more medial row of compartments, well delineated in rodents (including rat), is poorly stained in cat and particularly so in monkey (Figs. 3-5). A possible explanation would be that this collicular region, which is concerned with eye/head movements to the upper field (Tiao and Blakemore, 1976), plays a crucial role in rodents in detection of looming predators (Westby et al., 1990). Indeed, this medial zone, which was shown to be critical to perform effective upward visual grasp and organize behavioral escape responses (Dean et al., 1989), would require a highly elaborated neuronal machinery under cholinergic or related modulation.
On the other hand, the most striking finding in the comparative study is that the number of compartmentsin the honeycomb-like network lies within the range of 80–100 for all species studied. Two potential methodological problems, including the variation in the plane of sections and the well-known fickleness of AChE staining, may be suspected in biasing the reconstruction and counting. Even so, the number of compartments observed for each species varied only within a relatively narrow range whereas the linear size of the SC greatly changes. For instance, the linear size of the monkey SC is four times that in mouse. Thus, a mean value of 90 might closely approximate the actual number of compartments in the mammalian SC. This suggests that the collicular AChE mapping might be governed in these species by a common genetic program.
From AChE patches to compartments
The discontinuous distribution of AChE activity in the intermediate layers is traditionally described in the literature by a variety of terms such as patches, clumps, puffs, or clusters. It is clear from the present model that the patches correspond to a series of well-defined AChE compartment modules. Thus, whatever the plane or orientation in which the SC is observed, the AChE labeling always appears as a discontinuous and regularly repeating pattern. For instance, in frontal sections, a row of patches is a series of AChE-rich walls separated by AChE-free zones corresponding to the centers of compartments. Since the AChE arrangement is that of side by side vertical cylinders, one can understand why AChE patches and intercalated free zones vary in size and show spatial phase displacement from one section to the next. This notion is supported by observations, in particular in cat. In this species, the AChE displays in the frontal plane a series of patches about 200–600 μm wide, spaced at 300–600 μm intervals (Graybiel, 1978b; Illing and Graybiel, 1985). Similar data are apparent for the patchy distribution of afferents arising from the cholinergic pedunculopontine area from the pictures by Harting and Van Lieshout (1991). It is obvious that the greater interval observed by these authors in cat between the cholinergic related patches closely fit the internal diameter (450–500 μm) of the AChE compartments that we report here in the tangential plane in the same species. Thus we propose that AChE compartments or even “chambers” should be a more accurate terminology than that of “patchy feature” to designate the AChE arrangement in the intermediate SC of the rodents, cat, and monkey.
What does an AChE compartment mean?
It has long been known that the deep SC is a critical component of the neural circuitry that processes orienting movements of eyes, head, pinnae, vibrissae, and even the whole body toward novel and behaviorally significant cues (Huerta and Harting, 1984; Grantyn, 1988; Sparks and Hartwich-Young, 1989; Stein and Meredith, 1991). Besides orientation, defensive movements such as escape or avoidance have also been described in rodents (Northmore et al., 1988; Dean et al., 1989). The premotor cells involved in the generation of these orienting movements are organized topographically in the intermediate layers and form a map of motor space. Converging onto this neuronal matrix, somatic, visual, and auditory signals are also aligned topographically and thereby form maps of sensory space. Finally, it is generally accepted that the motor and sensory maps are in functional register so that in SC, sensory space would be transformed in motor space and hence in “grasp orienting responses” (Sparks, 1988). Since physiologically the intermediate layers appear to be a high-resolution matrix for the generation of directed orienting movements, there is a possibility that the AChE lattice reflects this organization. In keeping with that hypothesis, one may put forward the proposal that “AChE compartments” function as orienting modules. Each compartment would contain the pool of neurons reactive to various sensory inputs from a location in sensory space and that through efferent connections with brainstem motor circuitry would perform the spatiotemporal transformation required to trigger orienting movements of the appropriate direction and amplitude. With reference to the orienting vector that could be logically expected in each compartment or group of compartments, careful studies combining single unit recordings of sensory and motor fields and AChE staining are clearly required.
However, to guide further experiments, a proposal is presented Figure 7. By drawing one's inspiration from the motor map for eye movement set up by Roucoux and Crommelinck (1976), we drew in the movement vectors we hypothesize to be present throughout the cat AChE lattice. So, compartments in the mid-rostral SC, where the central vision is represented, would be responsible for forward gaze shifts of small amplitude, whereas those in the caudal SC would trigger large eye movements combined with head deviation for the retina's eccentricity beyond 30°. The latter region would correspond to the most heavily stained rows of compartments. Finally, saccades governed by compartments lying parallel to the midline would have a clear upward component, whereas those evoked by the lateral compartments would have a downward component.
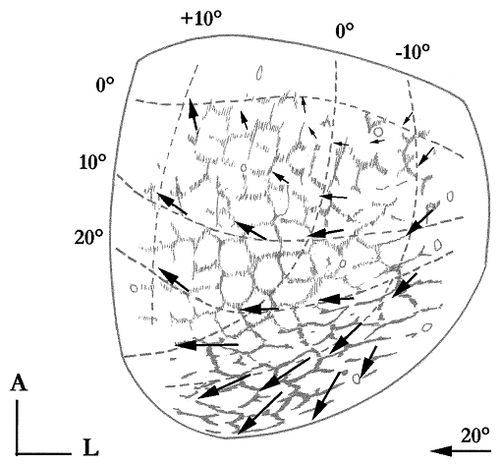
Movement vectors for eye deviation expected in some compartments of the cat AChE lattice. Some pertinent horizontal and vertical meridians of the retinotopic map in superior colliculus (adapted from Feldon et al., 1970) overlie the AChE lattice. The distribution of the movement vectors is inspired by the motor map described by Roucoux and Crommelinck (1976). They represent the eye saccades one would expect, assuming that the activity in a given compartment evokes a saccade bringing the gaze to the point of the visual field, which is retinotopically projected in the same compartment.
All these assertions rely on further evidence that AChE is a relevant marker for disclosing the very structure of the collicular matrix for orientation. In following up this idea, further research should investigate a series of modular markers, such as calretinin, parvalbumin, and cytochrome oxidase, for instance, which constitute with AChE a mosaic of complementary instead of overlapping domains (Wallace, 1986; Wiener, 1986; Illing, 1996). Likewise, some somatic afferents in the intermediate layers such as those from the bulbospinal junction, the somatosensory area SIV, and also visual afferents from the lateral suprasylvian cortex were shown in the cat to form complementary pictures with the AChE-rich domain (Illing and Graybiel, 1986). An important question is how these afferents and chemoarchitectural domains are distributed with respect to the AChE lattice. With regard to this, we report data in a companion paper in favor of a single modular structure that links AChE with the alignment of some of the major input and output pathways of the intermediate SC.
Acknowledgements
The authors are grateful to Dr. G.W.M. Westby for critical comments on the manuscript and to Dr. J.M. Deniau for fruitful discussion.




