Ultrasound screening for fetal chromosome anomalies
Abstract
Ultrasound evidence for aneuploidy may be found in almost every organ of the fetus and can be used to modify the risk of aneuploidy. The diagnosis of these minor anomalies on second-trimester ultrasonography will increase the risk of an abnormal karyotype whereas the absence of these findings may reduce this danger. The most specific and most ominous isolated markers for fetal aneuploidy are nuchal findings (edema or cysts), indicating the need to obtain a fetal karyotype in all cases irrespective of maternal age or results of biochemical serum screening. Hyperechoic fetal bowel is apparently also a strong indicator of fetal aneuploidy. Other isolated sonographic markers may increase the risk of an abnormal karyotype three- to ninefold. Most sonographic markers for aneuploidy specify an increased risk for Down syndrome, but choroid plexus cysts are apparently more specific for trisomy 18. Along with other screening methods, ultrasound screening for fetal aneuploidy should be used routinely to identify additional pregnancies at need for evaluation of fetal karyotype. Am. J. Med. Genet. 90:98–107, 2000. © 2000 Wiley-Liss, Inc.
INTRODUCTION
The frequency of chromosome anomalies at birth is calculated as 1 in 165. Despite the well-established association between advanced maternal age and an increased risk of fetal aneuploidy [Hook, 1981], if maternal age (older than 35 years) is used as the only screening tool for selection of pregnancies that need evaluation of fetal karyotype, as many as 80% of chromosomally abnormal conceptions will be missed. Because most chromosomal aberrations in pregnancy occur in women younger than 35 years, there was an urgent need to develop effective screening means to detect pregnancies at higher risk for aneuploidy than expected by maternal age alone.
Malformations affecting multiple organ systems are a major component of fetal aneuploidy syndromes, with the abnormal karyotype having a significant effect on fetal prognosis. Ultrasound diagnosis of major fetal anomalies [e.g., cystic hygroma (Fig. 1), congenital heart defects, or omphalocele] is associated with a high risk of abnormal fetal karyotype (10–80%) and amniocentesis is clearly indicated in these cases [Platt et al., 1986; Williamson et al., 1987]. In the last decade many publications described minor fetal anomalies, which in themselves are meaningless in terms of fetal well being but which serve as ultrasound markers for fetal aneuploidy. In this review, we will illustrate systematically these ultrasound findings and discuss their clinical implication on the risk of fetal aneuploidy.
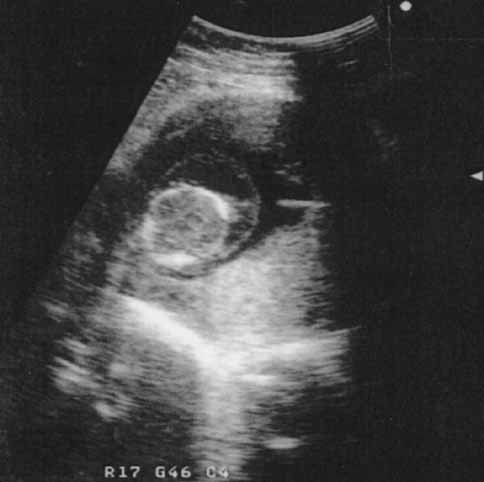
Cervical cystic hygroma in a 13 weeks fetus, associated with trisomy 21.
FETAL HEAD
Choroid Plexus Cysts
The prevalence of choroid plexus cysts, calculated from eight population-based studies [Bromley et al., 1996; Chitty et al., 1998; Denis et al., 1998; Gray et al., 1996; Kupferminc et al., 1994; Morcos et al., 1998; Reinsch, 1997; Walkinshaw et al., 1994] is about 1% (2,188 of 214,545 patients studied). Fetal choroid plexus cysts (Fig. 2 a,b) have been observed on prenatal ultrasonography in 44–50% of pregnancies with trisomy 18 [Denis et al., 1998; Gray et al., 1996] but only in 1.4% of trisomy 21 conceptions [Bromley et al., 1996]. The predictive value of choroid plexus cysts (true fetal aneuploidy among pregnancies identified to be at risk) has been obscured by flaws in population selection and by limitations of ultrasound technology and expertise. Depending on the population studied, a risk of 1–12% for fetal trisomy has been cited [Gray et al., 1996; Gupta et al., 1995; Kupferminc et al., 1994; Maieron et al., 1996; Shields et al., 1996; Walkinshaw et al., 1994]. About three fourths of abnormal fetal karyotypes associated with choroid plexus cysts are trisomy 18 and most of the rest are trisomy 21 [Gupta et al., 1995]. The consensus is that in the low-risk population, isolated choroid plexus cysts carry a risk of 1 in 50–150 cases [Gupta et al., 1995; Shields et al., 1996]. The risk of fetal aneuploidy increases considerably (10.5–12%) by sonographic identification of additional fetal malformations or of other markers for aneuploidy [Maieron et al., 1996; Reinsch et al., 1997]. Advanced maternal age and multiple biochemical markers in maternal serum may also help to identify those patients with isolated CPC that need genetic evaluation and fetal karyotype [Gratton et al., 1997; Maieron et al., 1996]. In a large multi-center study by Chitty et al. [1998], the incidence of aneuploidy in fetuses with isolated CPC was 0.36% when the mother was under 36 years and increased to 2.4% in older women. Gupta et al. [1997] suggested a ninefold increase in risk for trisomy 18 in women with isolated CPC. Based on his data, it appears that a 28-year-old woman with apparently isolated CPC has a 1 in 371 risk for trisomy 18, equivalent to the prior risk of a 40-year-old woman. The size of choroid plexus cysts, bilaterality, or disappearance with progression of gestation does not affect the risk of aneuploidy associated with this ultrasound finding.
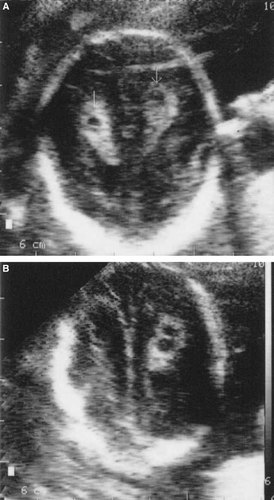
a: Bilateral choroid plexus cysts. b: Double choroid plexus cysts.
Because sonographic markers for fetal aneuploidy may be subtle and difficult to detect, defining CPC as an “isolated” finding depends, in part, on the expertise of the sonographist. Centers with less experience in ultrasound studies should probably resource more often to amniocentesis for this indication. Despite suggestions that performing amniocentesis on second-trimester fetuses with isolated choroid plexus cysts is neither cost effective nor beneficial [Donnenfeld, 1995; Gross et al., 1995], we think that, in most cases, choroid plexus cysts should be considered an indication for evaluation of fetal karyotype. However, as with other ultrasound findings, individual judgment based on clinical data and genetic counseling should be exercised in all cases.
Cisterna Magna
Two separate studies [Rosati and Guariglia, 1996; Watson et al., 1992] attempted to correlate the size of the cisterna magna, measured at the level of the posterior fossa, with results of genetic amniocentesis (indicated for advanced maternal age). A total of 1102 euploid fetuses and 61 fetuses with abnormal karyotype was evaluated. In all the aneuploid fetuses, the size of the cisterna magna was normal. The conclusion from these studies was that transvaginal sonographic measurement of the cisterna magna is easy to perform in early pregnancy but is a poor screening test for the prediction of abnormal fetal karyotype. Pregnancy outcome and postnatal follow-up was also reported on 15 fetuses with isolated enlargement of the cisterna magna (>10 mm) on prenatal ultrasound—all fetuses had normal chromosomes and normal phenotype at birth [Haimovici et al., 1997].
In the third trimester, Chen et al. [1998] found an enlarged cisterna magna in 6 of 19 fetuses with trisomy 18. Despite the known excess of females in fetuses and liveborns with trisomy 18, the sex ratio were reversed in trisomic fetuses with this finding (5 males vs. 1 female). Chen et al. [1998] concluded that prenatal detection of an enlarged cisterna magna associated with intrauterine growth retardation or polyhydramnios should prompt genetic counseling, a careful search for other congenital anomalies, and evaluation of fetal karyotype.
Ear Length
Fetal ear length was studied in 452 pregnancies undergoing genetic amniocentesis [Lettieri et al., 1993]. A linear relationship between ear length and gestational age was found in the second trimester, with 10 of 14 aneuploid fetuses having ear length at or below the 10th centile. The sensitivity and positive predictive value of small ears was 71% and 23%, respectively. The authors concluded that fetal ear length might be useful in the sonographic identification of aneuploid fetuses. These findings need corroboration by additional studies.
Cerebellum
Cerebellum of smaller size has been noted in infants, children, and adults with Down syndrome. The value of hypoplasia of the cerebellum as a screening tool for Down syndrome in the second trimester was evaluated in 42 affected and 1161 euploid fetuses [Rotmensch et al., 1997a]. The diameter of the cerebellum in Down syndrome fetuses was smaller than in normal controls at all gestational ages. A cutoff point of 0.92 for observed/expected differences in transcerebellar diameter yielded a sensitivity of 21%, specificity of 95%, and positive predictive value of 1.66 and 0.56 in high-risk (1 in 250) and low-risk (1 in 750) populations, respectively. Thus, although hypoplasia of the cerebellum is already evident and clinically recognizable in second-trimester fetuses with Down syndrome, size differences between normal and Down syndrome fetuses are apparently too small to be used as a screening tool for aneuploidy [Rotmensch et al., 1997a].
FETAL NECK
Excess soft tissue at the posterior aspect of the neck is a cardinal sign in neonates with Down syndrome. Benacerraf et al. [1985] first showed that the sonographic finding of a thick (>6 mm) nuchal skin fold confers an increased risk for Down syndrome. Since that original report, many studies in the first or second trimester confirmed the positive association of sonographic findings in the posterior or lateral aspects of fetal neck and an increased risk of chromosomal aberrations. Synonyms such as nuchal thickness [Hewitt et al., 1996], nuchal edema [Brun et al., 1994], non-septated nuchal cystic hygroma [Bronshtein et al., 1993a], simple hygroma [Szabo et al., 1995], and nuchal translucency [Brambati et al., 1995; Mahieu-Caputo et al., 1996; Pandya et al., 1995a; Taipale et al., 1997; van Vugt et al., 1996] are used in the literature. In a recent, relatively large study, the sensitivity of nuchal translucency in the first and early second trimester for trisomies 13, 18, and 21 was 62%; the sensitivity for trisomy 21 alone was 54% [Taipale et al., 1997]. However, it is apparent from this study and others that the sensitivity of nuchal findings increases both with size and with maternal age. In the population undergoing chorionic villus sampling, mostly for advanced maternal age, the sensitivity for fetal aneuploidy of nuchal thickness ≥3 mm has been reported as 30% [Brambati et al., 1995] to 57% [Hewitt et al., 1996]. Pandya et al. [1995a] suggested that the risk of trisomy in fetuses with nuchal translucency thickness of 3 mm, 4 mm, 5 mm, and ≥6 mm was 3, 18, 28, and 36 times higher than the respective risk derived from maternal age.
In the first and early second trimester, sonographic abnormalities of the fetal neck remain the most sensitive and specific marker for fetal aneuploidy [Benacerraf, 1996; Vintzileos et al., 1997a]. Even after correction of the risk for trisomy 21 (derived from maternal age combined with biochemical triple serum screening) to 1 in 10,000, the risk conferred by sonographic findings in the nuchal area of the fetus is calculated as 1 in 231, higher than the midtrimester risk for Down syndrome of a 35-year-old [Vintzileos and Egan, 1995]. Thus, it is obvious that in almost all cases, the diagnosis of nuchal translucency, cysts, or edema (Fig. 3 a–c) in the first or early second trimester should be considered an indication to obtain fetal karyotype. Moreover, even if the karyotype is normal, there is still an increased risk for associated fetal structural anomalies, which are found in about 13% of cases [Cha'ban et al., 1996; Montenegro et al., 1997; Sebire et al., 1997]. Fetal loss and other poor pregnancy outcomes are common.
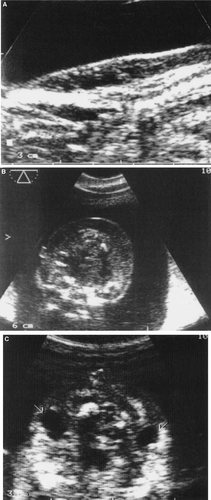
A: Nuchal translucency observed on transvaginal ultrasound at 13 weeks. B: Nuchal edema in a 14-week-old fetus—transabdominal ultrasound. C: Nuchal cysts (“non-septated cystic hygroma”) on transabdominal sonography, 15 weeks of gestation.
FETAL CHEST
Pleural Effusion
The association between pleural effusion and fetal aneuploidy was investigated by Achiron et al. [1995], who added their own experience to cases collected from previously published reports. Eight fetuses with trisomy 21 and 1 with monosomy X were diagnosed among 153 cases of isolated fetal pleural effusion, for a calculated risk of aneuploidy in the cumulative series of 5.8%. The fluid accumulating in the pleural cavity was chylous in most cases, suggesting some abnormality of the lymphatic system, a pathogenic mechanism that has been proposed also for nuchal edema or cysts observed in aneuploid fetuses. Thus, isolated pleural effusion in the fetus should clearly be considered an indication to obtain fetal karyotype.
Echogenic Foci Within the Fetal Heart
Calcification of the papillary muscle is observed on ultrasonography in 16–18% of fetuses with trisomy 21, in 39% of those with trisomy 13 and in 2–4.8% of fetuses with normal karyotype [Benacerraf, 1996]. Trisomy 21 was diagnosed in 13% of high-risk patients with intracardiac echogenic foci and in 2% of the patients without this finding [Manning et al., 1998]. The risk of aneuploidy in fetuses with echogenic foci as an isolated finding was calculated in a prospective study as 1%, indicating that amniocentesis is warranted in these cases [Simpson et al., 1996]. Benacerraf [1996] suggested that the rate of chromosome anomalies in fetuses with isolated echogenic foci in the left ventricle is four times higher than that related to the specific maternal age.
It is apparent from these studies that women carrying fetuses with left ventricular echogenic foci should be informed of the statistically significant association with trisomy 21 [Manning et al., 1998]. The risk is apparently not increased by multiple echogenic foci in fetal heart or involvement of the right ventricle—these findings are most commonly benign [Diddy et al., 1996; Petrikowsky et al., 1996]. However, diffuse echogenicity may be associated with a higher risk of adverse pregnancy outcome [Bronshtein et al., 1996].
FETAL ABDOMEN
Hyperechoic Fetal Bowel
The fetal bowel is considered hyperechoic if its echogenicity is similar to that of the surrounding bone [Bromley et al., 1994]. This finding was observed in association with meconium ileus, peritonitis, intrauterine infection, cystic fibrosis (CF), and fetal aneuploidy. Echogenic bowel has been observed on ultrasonography in 4.8% of fetuses with prenatal diagnosis of Down syndrome [Rotmensch et al., 1997b]. An increased risk of intrauterine growth retardation (14.9%), prematurity (15.3%), and intrauterine death (9%) has also been reported in fetuses with increased bowel echogenicity and normal karyotype [Bahado-Singh et al., 1994; MacGregor et al., 1995]. In a high-risk population, the observed rate of fetal chromosome anomalies (4–12.4%) and of CF (4–25.6%) certainly justifies invasive prenatal testing [Bahado-Singh et al., 1994; MacGregor et al., 1995; Slotnick and Abuhamad, 1996]. In a low-risk population, the risk of fetal aneuploidy associated with hyperechogenic bowel has been calculated theoretically as 1.4% [Bromley et al., 1994]. Thus, it is obvious that evaluation of parental carrier status for CF, exclusion of intrauterine infection, and amniocentesis for fetal karyotype is necessary in all cases with increased echogenicity of fetal bowel. Moreover, even when these tests are normal, this sonographic finding should mandate continuos pregnancy surveillance in a high-risk setup.
Fetal Stomach
Filling of the stomach can be visualized on transvaginal ultrasonography as early as 12 weeks of gestation. After 18 weeks of gestation, a small or absent fetal stomach is associated with a significant risk of chromosome anomalies (4% and 38%, respectively), as well as with increased rate of other structural anomalies and intrauterine or postnatal death [McKenna et al., 1995]. The prognostic significance of this finding earlier in gestation is not clarified yet. However, given that esophageal and duodenal atresia are occasional findings in infants with Down syndrome, a higher rate of aneuploidy should be expected in these cases.
The Gallbladder
The fetal gallbladder can be identified by transvaginal sonography as early as 14 weeks of gestation [Bronshtein et al., 1993b]. Failure to visualize the fetal gallbladder by 15 weeks of gestation should raise the differential diagnosis between gallbladder atresia, which has a good prognosis, and external billiary atresia, for which the prognosis is extremely guarded. Evaluation of bile salts in amniotic fluid should discriminate between these two conditions.
In the second trimester, an enlarged gallbladder has been associated with increased risk of fetal abnormal karyotype, mainly trisomy 18 and trisomy 13 [Bronshtein et al., 1993b; Sepuvelda et al., 1995]. However, additional structural malformations of the fetus were observed in all of these cases. If isolated, an enlarged gallbladder is probably a normal variant.
Fetal Pyelectasis
Pyelectasis has been defined as enlargement of the antero–posterior diameter of the renal pelvis to at least 4 mm before 33 weeks of gestation. After this gestational age, pelvic enlargement of at least 7 mm is considered abnormal [Adra et al., 1995; Benacerraf et al., 1990; Corteville et al., 1992; Wickstrom et al., 1996]. This finding appears in 17–25% of fetuses with Down syndrome [Benacerraf et al., 1990; Corteville et al., 1992] and in 2% of normal pregnancies [Corteville et al., 1992]. Corteville and colleagues calculated the risk of Down syndrome associated with isolated pyelectasis to approximately 1 in 340 affected pregnancies, suggesting that amniocentesis should be performed only in those cases presenting other risk factors (i.e., advanced maternal age or abnormal biochemical serum screening). Others [Wickstrom et al., 1996b] indicated that isolated pyelectasis confers a 3.9-fold increase in the relative risk for Down syndrome and a 3.3-fold increase in relative risk for all chromosome anomalies. Following the identification of mild pyelectasis in chromosomally normal gestations, a significant risk of recurrence in subsequent normal pregnancies (6.1 relative risk) was described by Degani et al. [1997]. This may mean that genetic or environmental factors may be implicated in the predisposition of fetal pyelectasis. The risk of aneuploidy associated with recurrent pyelectasis is probably less ominous than that observed in primary cases.
A significant risk (44%) for development of pathological processes affecting the urinary tract (i.e., uretero–pelvic junction obstruction or vesico–ureteral reflux) was documented in fetuses with isolated pyelectasis and normal chromosomes. Thus, serial ultrasound examinations are necessary to evaluate in utero progression of pyelectasis—the risk of postnatal uropathy was significantly increased by in utero progression, male gender, contralateral pyelectasis, and increased kidney length [Wickstrom et al., 1996a]. Fetuses with an antero–posterior diameter of the renal pelvis larger than 8 mm after 28 weeks of gestation require appropriate urologic evaluation after birth [Adra et al., 1995].
Umbilical Anomalies
The absence of one umbilical artery (Fig. 4) is a relatively common phenomenon—its' incidence is 0.46% in singleton liveborns, 0.8% in multiple gestations, and 6.1–11.3% in infants with chromosome anomalies [Lilja, 1991; Saller et al., 1990]. Fetuses with trisomy 13 and 18 are most commonly affected—single umbilical artery (SUA) is rare among fetuses with trisomy 21 or sex chromosome aneuploidy [Saller et al., 1990]. Thus, in most aneuploid gestations associated with SUA, other structural anomalies will be observed on ultrasonography, pointing out that fetal karyotype should be obtained. Isolated SUA should apparently not be considered an indication for prenatal evaluation of fetal chromosomes [Khong and George, 1992]. However, obstetric evaluation and follow-up in a “high-risk” setup is needed because these fetuses are at increased risk of low birth weight and premature delivery [Lilja, 1991].
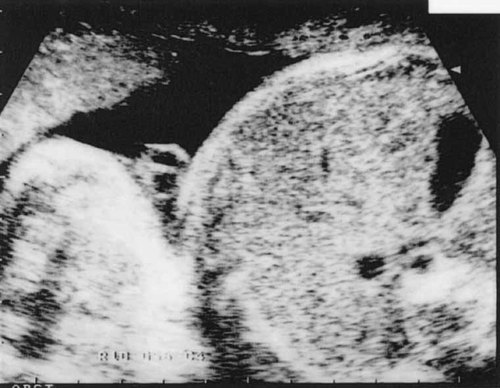
Single umbilical artery (SUA) at the level of the fetal abdomen, at 22 weeks of gestation.
Cystic masses of the umbilical cord have been observed on ultrasonography as early as the first trimester—when persistent into the second and the third trimester, umbilical cord cysts have been associated with congenital anomalies and with lethal aneuploidy, mostly trisomy 18 [Ramirez et al., 1995; Smith et al., 1996]. The diagnosis of umbilical cord cyst in the first trimester should prompt a detailed search for other structural anomalies; amniocentesis should also be considered.
FETAL BIOMETRY
Early intrauterine growth retardation is common in chromosomally abnormal fetuses and is usually observed on ultrasonography early in the second trimester. Previously, we proved in our CVS population that a fetal crown–rump length (CRL) ≥7 days smaller than dates is associated with a significant increase in risk of chromosome anomalies (three times higher than the maternal age-related risk). Moreover, the larger the size–dates discrepancy, the higher the possibility that the aneuploidy affecting that pregnancy is of the severe or lethal type [Drugan et al., 1992; Sorokin et al., 1991]. However, for pregnancies affected by Down syndrome, the difference between measured and expected CRL was not significant.
The short stature and distinct appearance of children with Down syndrome have prompted investigators to evaluate the yield of fetal long bone biometry as a screening tool for antenatal detection of chromosomal abnormalities. Lockwood et al. [1987] first suggested that a shortened femur length, defined as BPD–femur length ratio higher by 1.5 standard deviations than the ratio of unaffected controls, yields a sensitivity of 50% and a false positive rate of 7% in the detection of second trimester fetuses with Down syndrome. Many reports since attempted to substantiate [Dicke et al., 1989] or refute [LaFollette et al., 1989] these findings. Evaluation of fetal humeral length [Rotmensch et al., 1992] or a combination of humerus and femur lengths [Biagiotti et al., 1994] was also used in an endeavor to increase the sensitivity and specificity of fetal biometry as a screening tool for chromosome anomalies. Biometry of four long bones (femur, humerus, tibia, and fibula) apparently yields the best results, with a sensitivity of 63.6% and a specificity of 78.5% [Vintzileos et al., 1996] and may be used to adjust the a priori risk for trisomy 21 in both high-risk and low-risk populations. The authors of the latter study suggest that in the presence of normal biometry of the four long bones, genetic amniocentesis may not be recommended for women younger than 40 years. Johnson et al. [1994] have shown that foot length provides a reliable gestational age control. Determination of femur/foot or femur + humerus/foot ratios appears to confer improved results over long bone biometry, per se. However, additional data are necessary to determine the clinical applicability of these ratios. As of now, long bone biometry does not appear to have an added value to more simple screening tools like the biochemical multiple marker screening test [Owen et al., 1994].
DISCUSSION
In experienced hands, sonography in the early second trimester of pregnancy is a useful screening tool for fetal chromosome anomalies—about 4% of the population screen positive, with a sensitivity of 75% to 80% (Table I). In previously “low-risk” patients with a positive ultrasound screen, 1 in 50 amniocenteses will yield an abnormal karyotype, comparable to the yield of biochemical serum screening [Drugan et al., 1996a]. In a priori “high-risk” patients, the meaning of positive identification of some ultrasound markers for fetal aneuploidy is more ominous [Drugan et al., 1996b], with 1 abnormal fetal karyotype out of every 8 amniocenteses. Moreover, Vintzileos et al. [1997a] suggest that as many as 80% of fetuses with an abnormal karyotype will show on ultrasound some markers of aneuploidy. Thus, it appears that most amniocenteses indicated for advanced maternal age can be safely avoided by lowering the risk of aneuploidy to women with a normal ultrasound study.
| Sonographic Marker | Prevalence | Relative riska |
|---|---|---|
| Choroid plexus cysts | 1.25% | × 9b |
| Nuchal edema or cysts | 4–5% | |
| >4 mm | × 18 | |
| >5 mm | × 28 | |
| >6 mm | × 36 | |
| Lt. ventricular echogenic focus | 5% | × 4 |
| Hyperechoic bowel | 0.6–0.8% | × 14–16 |
| Pyelectasis | 2% | × 3.3–3.9 |
| Fetal biometry | ||
| Short CRLc | 7% | × 3 |
| Short femur length | 4–5% | × 2.7 |
| Short humerus | 4–5% | × 4.1 |
| Short femur and humerus | 2.4% | × 11.5 |
- a Risk for trisomy 21 as calculated in relation to maternal age alone or in combination with biochemical screening.
- b Risk specific for trisomy 18; risk for trisomy 21 is negligible.
- c CRL; crown–rump length.
A question of utmost importance is what gestational age yields the best results for ultrasound screening. Most studies that evaluate ultrasound performance as a screening tool for fetal chromosome anomalies are based on abdominal scans performed late in the second trimester [Benacerraf, 1996; Vintzileos et al., 1997b]. Because many of the sonographic changes defined as potential markers for fetal aneuploidy are subtle, the improved resolution acclaimed by high-frequency transvaginal transducers allows earlier detection of these findings. Moreover, it is apparent that some isolated ultrasound markers for aneuploidy are transient and may disappear later in gestation. Pandya et al. [1995b] reported that in 5 of 6 Down syndrome fetuses with increased nuchal translucency in the first trimester, the nuchal fold thickness was normal on a subsequent scan in the second trimester.
In our own experience (unpublished) with 76 aneuploid gestations, markers for aneuploidy were observed on transvaginal ultrasound early in the second trimester in 68 cases—46 in association with major fetal structural anomalies and 22 isolated findings. Only 2 of the 22 isolated markers for fetal chromosome anomalies were documented on transabdominal sonography performed before second-trimester amniocentesis. Thus, transvaginal ultrasound at 14 to 16 weeks of gestation appears to provide the best time window for detection of sonographic markers for fetal aneuploidy, increasing the sensitivity of ultrasound screening (in our hands) from 63 to 85%. These data are comparable to that published by Nicolaides et al. [1992] and by Snijders et al. [1996] on ultrasound screening with nuchal translucency in the first trimester (10–14 gestational weeks). Sonographic evaluation of the fetus at 14 to 16 gestational weeks is more time consuming and requires more expertise than limited screening for nuchal translucency in the first trimester. However, it pays off by recognition of additional markers for fetal abnormal karyotype (i.e., CPC or pyelectasis) as well as by early diagnosis of other fetal structural anomalies that may affect fetal prognosis.
Ultrasound screening for fetal aneuploidy in the early second trimester allows identification of pregnancies at risk early enough to offer genetic counseling and amniocentesis at the “traditional” gestational age (16–17 weeks). Earlier detection and testing of pregnancies at risk will obviate the need to use more invasive procedures for prenatal diagnosis (i.e., cordocentesis or “late” CVS) with their inherent increased risk of procedure-related pregnancy loss. The latter will be needed when diagnosis of fetal aberrations is made late in the second trimester. Moving prenatal diagnosis toward the first trimester is a trend observed in the last decade, in ultrasound as well as in invasive procedures [Evans et al., 1989].




