Ring chromosome 8 syndrome: Further characterization
Abstract
We describe two de novo cases of extra r(8) confirmed by fluorescent in situ hybridization (FISH). Based on these two and eight additional cases of extra r(8) confirmed by FISH, the phenotype is better documented. One of our patients had minor facial anomalies, near-normal growth, and neurological development. She had a ring in each cell analyzed. The second had minor craniofacial anomalies and growth and mental retardation. He had a small or double-sized ring in each cell. The phenotype of these 10 cases ranges from almost normal in an adult with 10% mosaicism to variable degrees of minor anomalies, growth retardation, and mental retardation overlapping the mosaic +8 syndrome. Am. J. Med. Genet. 90:162–164, 2000. © 2000 Wiley-Liss, Inc.
INTRODUCTION
Extra chromosomes are rare with an incidence of approximately 1 in 2,500 in human population [Warburton, 1991]. Ring chromosomes have been identified for all human chromosomes [Wyandt, 1988]. Those involving chromosomes 13 and 18 are the most commonly reported autosomal rings. Ring chromosome 8 is relatively rare. Traditional cytogenetic methods make it difficult to characterize these marker chromosomes accurately and to correlate genetic and phenotypic data.
Use of fluorescent DNA probes and in situ hybridization (FISH) has been a landmark in diagnosing the origin of these supernumerary markers. An array of marker chromosomes has been characterized by use of this technique [Rauch et al., 1992; Callen et al., 1991]. To our knowledge a total of eight cases confirmed by FISH have been described with ring chromosome 8 [Blennow et al., 1993; Daniel et al., 1994; Melnyk and Dewald, 1994; Butler et al., 1995; Spinner et al., 1995; Rothenmund et al., 1997]. We present two additional cases of ring chromosome 8 diagnosed by conventional cytogenetic techniques and confirmed by FISH in order to better understand the spectrum of phenotypic presentations involving this anomaly.
CLINICAL FINDINGS
Case 1
She was first seen at age 7.5 months. She was the first child born after an uneventful pregnancy to a Caucasian couple. The mother's age was 32 years, and the father was 34 at the time of birth. The marriage was nonconsanguineous. Results of routine ultrasound at 5 months of gestation were normal. She was born by vaginal delivery and was noted to have jaundice in the first week of life, which was thought to be due to breastfeeding.
At the time of examination, she was hypotonic, able to roll over but had poor head control, and could not sit without support. Her length, weight, and head circumference were all at the 25th centile for age. Her head showed mild frontal prominence and ears were low set with overfolding at the superior helical regions. The nose was short and upturned. The baby tended to arch her back during examination. There was a small hemangioma on the root of the nose and another cavernous hemangioma measuring 1cm in the interscapular region. The physical findings were otherwise unremarkable.
At age 2 years her length was at the 5th centile, weight was slightly below the 5th centile, and OFC was at the 10th centile. Developmental progress was satisfactory, and it was thought that her motor skills were at 23 months. She is speaking and putting words together. She has had no health problems.
Cytogenetic analysis from peripheral blood was analyzed by standard GTG banding [Seabright, 1971]. An extra small ring chromosome (approximately ½ the size of G group chromosome) was present in each of the 50 metaphases analyzed. The karyotype was 47,XX,+r (Fig. 1). Centromere specific probes for chromosome 8 and wcp8 (Vysis, Downers Grove, IL) were used to detect the origin of the ring (Fig. 2). The karyotype was amended as 47,XX,+r. ish r(8)(wcp8+, D8Z2+). The FISH procedures were a modification of the manufacturer's protocol [Jalal et al., 1993]. The parental karyotypes were normal.
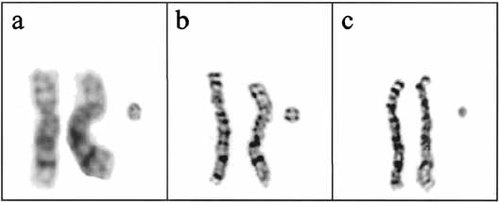
Partial GTG banded karyotype of pair 8 and the ring. a: Pair 8 and ring from patient 1. b: Pair 8 and a large ring from patient 2. c: Pair 8 and a small ring from patient 2.
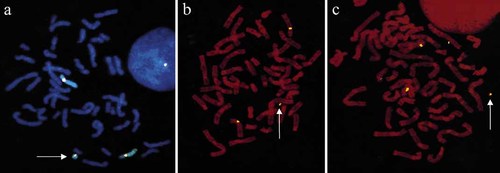
FISH on metaphase spreads for identification of the ring. a: wcp8 and D8Z2 hybridized to pair 8 and the ring 8 from patient 1. b: D8Z2 hybridized to centromeres of pair 8 and ring 8. Two hybridization signals were present in the larger ring from patient 2. c: D8Z2 hybridized to centromeres of pair 8 and the smaller ring of patient 2.
Case 2
Patient 2 was a male born by caesarian section after an uneventful term pregnancy. His parents were of Caucasian decent and nonconsanguineous. There was no known teratogen exposure during gestation.
The baby was referred to the genetics clinic at age 6 months for developmental delay and unusual appearance. Height, weight, and OFC were above the 95th centile for age. There was plagiocephaly with a prominence of left occiput and right forehead, epicanthic folds, highly arched palate, and small nose and lingual frenulum tethering the tip of the tongue. The right ear was cup shaped, and the left helixi was overfolded. There was a hemangioma on the back of the neck. She was developmentally delayed and hypotonic. Computerized tomography (CT) study showed agenesis of the corpus callosum. At age 22 months, the patient's growth was normal for age. Development was at approximately the 12-month level. She started to walk at age 26 months but at age 32 months has only limited speech development.
Cytogenetic analysis showed the presence of one of the two different rings (large, approximately ½ the size of G group and small, approximately 1/4 the size of G group chromosome) in all metaphase cells examined. In 50 cells from phytohemagglutinin (PHA)-stimulated blood, 26 had the large ring and 24 had the small ring (Fig. 1). The karyotype was mos47,XY+r. Three independent cultures from tongue tissue were treated with methotrexate, and 30 cells were analyzed at high-resolution level [Yunis, 1976]. Eighteen cells had the large ring and 14 had the smaller ring.
Parental karyotypes were both normal. FISH was performed on the patient's metaphase cells by centromere-specific probe (Oncor, Gaithersburg, MD) to determine the origin of the rings from chromosome 8 (Fig. 2). The hybridization technique was in close agreement with the manufacturer's recommendation. The karyotype was amended as mos47,XY,+r. ish r(8)(D8Z2+).
DISCUSSION
Trisomy 8 was described by Pfeiffer and later confirmed by chromosome banding technique [Pfeiffer and Leonard, 1973]. The mosaic trisomy 8 syndrome was described by Riccardi [1977] based on a collection of 70 patients. Only one of these patients was nonmosaic trisomy 8. The mean proportion of trisomic cells was 48% from blood cultures compared with 72% in skin fibroblasts in cases where both tissues were analyzed in the same patient. The syndrome comprises mild to severe mental and growth deficiency, prominent forehead, deep-set eyes, hypertelorism with broad nasal root, micrognathia, prominent cupped ears, limb defects such as deep creases in palms and soles, camptodactyly, and abnormal nails. The other abnormalities include long, slender trunk, urethral-renal, and cardiac defects.
Extra marker chromosome including rings of unidentifiable origin occur with a frequency of 1 in 2,500 [Warburton, 1991]. The risk for extra nonsatellited marker chromosome prenatally is 14.7%, 70% of which occur as mosaic [Warburton, 1991]. Ring chromosomes have been identified for all human chromosomes. With the use of FISH to date eight cases, excluding the two cases presented here, are described. The phenotype due to mosaic or nonmosaic extra r(8) range from almost completely normal to several abnormalities shared with trisomy 8 syndrome.
The father of two affected daughters with 10% mosaicism of r(8) was a college graduate with no apparent minor anomalies other than myopia [Rothenmund et al., 1997]. This was our first case where they had minor anomalies and near normal growth and neurological progress at 23 months. The case reported by Melnyk and Dewald [1994] with a nonmosaic extra ring (approximately a third of G group chromosome size) had minor facial anomalies and developmental delay, hypotonia, and mental retardation at age 15 months. In a similar case reported by Butler et al. [1995] the extra ring was present in 41% of cells studied. Our second case had either a small or large ring in every cell. By sister strand crossing-over small rings are expected to occasionally double in size. It appears that the larger ring had a similar origin since it has two α-satellite signals (Fig. 2). Therefore, this case would have four rather than three copies of some genes around the centromere in cells with the large ring. The child had several craniofacial anomalies, growth retardation, and severe mental retardation overlapping with mosaic r(8) syndrome. The remaining five cases have variable degrees of moderate to severe mental and growth retardation, and they share some of the features present in mosaic trisomy r(8) syndrome. Our patients had somewhat variable clinical manifestations.
One of the girls at age 15 years was functioning at a higher level and had fewer autistic-like behaviors [Rothenmund et al., 1997] than the other, but they were both 97% or higher +r(8). This type of observation is not uncommon, especially for mosaic syndromes. Saneto et al. [1998] reported two atypical cases of mosaic +9. These two were mildly retarded, and one patient had strikingly normal facial features with 82% mosaicism of +9, although severe mental retardation is common in such cases. Chang et al. [1990] based on 92 prenatally diagnosed cases of 45,X/46,XY mosaics concluded that “there was no relationship between the percent mosaicism and presence of degree of abnormalities.” However, extra embryonic cells may influence these proportions. The patient described with 10% r(8) mosaicism and essentially normal phenotype [Rothenmund et al., 1997] supports the view that low-level mosaicism is generally associated with a milder clinical picture.




