Promoting resilience to weather-related and seasonal provocations to health in people with multimorbid heart disease: a prospective pragmatic, randomised trial
See Editorial (Hunter)
Abstract
Objective
To investigate whether a multifaceted intervention for building resilience to external provocations to health reduced the number of all-cause hospital re-admissions and deaths of people hospitalised with multimorbid heart disease, compared with standard post-discharge management.
Study design
Single centre, prospective, open, randomised trial with blinded endpoint acquisition and adjudication (REsilience to Seasonal ILlness and Increased Emergency admissioNs CarE, RESILIENCE).
Setting, participants
Adults (aged 18 years or older) admitted as emergency medical patients with multimorbid heart disease to Austin Hospital, a tertiary hospital in Melbourne, 19 November 2020 – 28 July 2022, with planned discharge to home.
Intervention
Standard post-discharge management, as well as the 12-month active management program: home visits by a nurse, specialist clinical review, and tailored recommendations for optimising clinical management and promoting resilience to external provocations; the nurse coordinated the additional care, provided individualised support, and arranged RESILIENCE physician reviews as required. The comparator group received standard post-discharge management only.
Major outcome measure
Proportion of days alive and out of hospital during follow-up (minimum, twelve months) with respect to the maximum number possible.
Results
Of 203 participants (mean age, 75.7 years; standard deviation [SD], 10.2 years; 104 women), 103 were randomly allocated to the intervention group, 100 to the standard management group; median follow-up time was 600 days (interquartile range, 416–681 days). A total of 470 hospital admissions and 3874 days of hospital stay during follow-up were recorded for 138 of the 203 trial participants (68%); 38 people (19%) died during follow-up. The days alive and out of hospital proportion was 86.5% (SD, 25.3 percentage points) for the intervention group and 88.3% (SD, 23.5 percentage points) for the standard management group (adjusted difference, 2.04 percentage points; 95% CI, –4.97 to 8.56 percentage points).
Conclusion
A multifaceted intervention for reducing bio-behavioural vulnerability to external events was ineffective in increasing the proportion of days alive and out of hospital after hospital discharge for people admitted with multimorbid heart disease. However, the program could be modified to improve health outcomes for such people.
Trial registration
ClinicalTrials.gov, NCT04614428 (prospective).
The known: Threats to cardiovascular health posed by climate change are recognised, but few disease management programs aim to modulate exposure to challenging weather conditions.
The new: Our novel intervention aimed to establish greater resilience to external provocations to health for people hospitalised with multimorbid heart disease. It did not increase their time alive and not in hospital after their discharge home.
The implications: Our program was ineffective in achieving its primary aim, but our trial provided proof of concept evidence that a modified program could achieve better health outcomes for people after hospitalisation with multimorbid heart disease.
The global burden of cardiovascular disease is increasing, the estimated attributable mortality rising from 12.9 to 18.6 million deaths per year during 1990–2019.1 This trend reflects the increasing numbers of both men and women with multimorbid heart disease: combinations of cardiovascular disease (coronary artery disease, heart failure, atrial fibrillation) with diabetes, respiratory disease, or renal failure.2-4 Meeting the complex needs of people with multimorbid heart disease, while reducing their need for frequent and expensive hospital admissions, can be challenging even for well resourced health care systems.5 It is therefore crucial that we better understand the factors underlying clinical instability in multimorbid heart disease and develop new strategies for preventing it.
Most reports about cardiovascular events assume that they occur randomly throughout the year, as episodes of clinical instability driven by internal pathophysiological factors. However, numerous studies have identified distinctive peaks and troughs in cardiovascular event numbers, as well as several predictable (eg, the onset of winter6 or Christmas7) and unpredictable (eg, extreme heat waves8) provocations of cardiovascular events. Climate change will generate more weather extremes that will provoke more cardiovascular events.9 People living with multimorbid heart disease are much more likely to experience such events,6 and we have previously reported strong seasonal patterns of repeated hospitalisations and of death among people receiving gold standard multidisciplinary care.10 Responding to single factors (eg, reducing energy poverty11 or providing thermal clothing12) is unlikely to effectively reduce this risk without taking into account the complexity of the bio-behavioural factors that underlie vulnerability to weather events.6
We therefore undertook the REsilience to Seasonal ILlness and Increased Emergency admissioNs CarE (RESILIENCE) trial,13 the first prospective randomised investigation of a multifaceted intervention for building resilience to external provocations to health, to determine whether it reduced the numbers of all-cause hospital re-admissions and deaths among people who had been hospitalised with multimorbid heart disease compared with standard post-discharge management.
Methods
RESILIENCE was a single centre, prospective, open, randomised trial with blinded endpoint acquisition and adjudication.13 The study rationale, design, participant characteristics (including their vulnerability to external provocations to health), and the impact of the coronavirus disease 2019 (COVID-19) pandemic on the study have been reported elsewhere.13 Participant recruitment, delayed by the COVID-19 pandemic, commenced on 19 November 2020 at the Austin Hospital, a 671-bed tertiary referral centre in Melbourne, which has a temperate, oceanic climate.14 The RESILIENCE trial was registered prospectively with ClinicalTrials.gov (NCT04614428; 29 October 2020); clinical management and follow-up of patients was adjusted during the trial when appropriate. We report our study in accordance with the CONSORT guidelines for reporting parallel group randomised trials15 and the template for intervention description and replication (TIDieR) checklist.16
Patients were recruited for the trial during their index admissions by trained research personnel. All people who were admitted to the Austin Hospital as emergency medical cases with diagnoses of multimorbid heart disease, who were aged 18 years or older, living independently in the community within 10 km of the hospital, and provided informed consent were eligible for participation. People who could not provide informed consent, had terminal illnesses, or were to be discharged to long term care facilities were excluded.
Randomisation and masking
An independent data management team implemented a blinded, computer-generated randomised protocol. A pre-determined randomisation sequence with block groups by biological sex randomised participants one-to-one to the intervention or standard management groups.
Procedures
Information about socio-demographic status (based on multifaceted, individual profiling), medical history, hospital treatment, and planned post-discharge management were collected for each participant during their index hospitalisation, as previously described.13 Profiling specifically focused on their vulnerability to external provocations to health (infectious diseases, direct provocations to cardiovascular health related to weather events) from a bio-behavioural perspective,6 including assessment of their behaviour patterns and any history of recurrent hospital admissions during a particular season (defined as more than half of admissions during one season during the preceding twelve months)10 (Supporting Information, part 1; appendix II). After discharge from hospital, all participants had access to high quality specialist and primary care as part of standard management, including standard hospital avoidance programs, outpatient management by specialist physicians, routine follow-up by primary care physicians, subsidised pharmacological treatment, and referral to allied health care services and multidisciplinary chronic disease management programs, as required. Treating physicians received a copy of the hospital discharge summary, as well as information about the trial (including group allocation).
Intervention group participants received standard management as well as the RESILIENCE program, which entailed more frequent clinical surveillance and support during the twelve months after discharge, according to their assessed vulnerability to external provocations to health (Supporting Information, part 1, figure 4).13 Seven to fourteen days after discharge, a qualified nurse with postgraduate training visited the participant at home. A standardised protocol13 was used to assess their home environment, behaviours, and clinical status to identify areas of vulnerability to changes in the weather and other external provocations to health from a multifactorial perspective.6 The RESILIENCE nurse, a qualified physician, and the participant and their family (when appropriate) reviewed the priorities and individual circumstances of the participant at a dedicated clinic (virtual or in-person, as preferred) within 30 days of hospital discharge. Recommendations for optimising clinical management and promoting resilience were enacted immediately or conveyed in a comprehensive report to the participant's health care team. The RESILIENCE nurse coordinated the additional care and provided individualised support during the 12-month active management period, including several home visits and arranging additional RESILIENCE physician reviews if required.
At twelve months, all living study participants were invited to attend a health review, and a summary of the findings and recommendations were sent to the participant's health care team.
Outcomes
Data for outcome measures during study follow-up (until 28 July 2023, the end of the 12-month follow-up period for the most recently recruited participants) were collected from electronic medical records by investigators blinded to group allocation. The primary outcome was the proportion of days alive and out of hospital during complete follow-up with respect to the maximum number possible (29–972 days). Secondary outcomes were event-free survival (ie, did not reach the composite endpoint of any-cause hospital re-admission or death), timing of hospital re-admission and length of hospital stay with respect to weather events and season, and 12-month change from baseline in health-related quality-of-life (EQ-5D17). Further pre-planned secondary outcomes, including more detailed analyses of timing of events relative to weather conditions, specific sub-group comparisons (eg. those with and without chronic respiratory disease), and a health economic analysis (if appropriate), will be reported elsewhere.
Statistical analyses
Based on data from a preliminary study of weather-associated health events in cardiac patients,10 we calculated that 150 participants in each group would provide 85% power (α [two-sided] = 0.05) to detect a 10% change in the primary outcome in the pilot study (0.860 days alive and out of hospital during twelve months’ follow-up; standard deviation [SD], 0.035 days). As a result of the prolonged COVID-19 lockdowns in Melbourne, the recruitment target was revised on 5 May 2022 to 100 participants per group, with extended follow-up to 28 July 2023 (minimum 12-month follow-up).13
Our analyses followed a pre-specified statistical analysis plan (Supporting Information, part 1). Discrete variables are summarised as frequencies and proportions, continuous variables as means with standard deviations (SDs) or medians with interquartile ranges (IQRs).
All efficacy analyses were undertaken on an intention-to-treat basis and blinded to group allocation. The statistical significance of differences between the intervention and standard management groups in the proportion of actual days alive and out of hospital was assessed in negative binomial regression analyses, adjusted for length of follow-up. Survival and event-free survival were depicted in Kaplan–Meier survival curves, and differences assessed in Mantel–Cox log-rank analyses.
The statistical significance of between-group differences in the change in EQ-5D scores from baseline to twelve months was evaluated in an independent samples t test. As the difference was not statistically significant, we report only mean change with 95% confidence intervals (CIs).
The timing of hospital re-admissions and length of hospital stay was assessed in multinomial logistic regression analyses. Model fit was assessed using the likelihood ratio and deviance goodness-of-fit tests; the association between baseline characteristics and hospital re-admissions was assessed using multiple logistic regression (backward stepwise method) using the variables listed in Box 2; 95% CIs were derived from model-based standard errors.
Event-free survival and events during specific seasons were assessed in Cox proportional hazards models (entry model: proportional hazards confirmed by visual inspection) and multiple logistic regression (backward stepwise removal [P > 0.1 in univariate analyses]; age, sex, and group randomisation were included as fixed variables, as were initially all variables in table 1 in the Supporting Information and, for events during specific seasons, time of exposure to the season of interest.
All analyses were performed in IBM SPSS Statistics 28.0.0.0 (190).
Ethics approval
The Austin Health human research ethics committee approved the study (HREC/56509/Austin-2019), and all participants provided written informed consent for participation.
Results
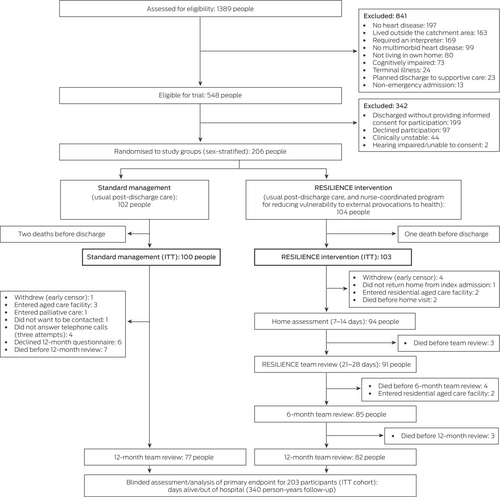
Of 1389 people admitted to the Austin Hospital as emergency medical cases during 19 November 2020 – 28 July 2022 (revised target participant number reached) and assessed for their eligibility to participate in the RESILIENCE trial, 548 were eligible, of whom 206 (38%) consented to participation. As three people died during their index admissions after being randomised to a study group, the intention-to-treat cohort consisted of one hundred people in the standard management group and 103 in the intervention group (Box 1). Median follow-up time after discharge from the index admission was 600 days (IQR, 416–681 days).
The baseline socio-demographic and clinical profiles of the two study groups were similar, but the proportions of participants with histories of heart failure (51% v 43%) or chronic lung disease (40% v 31%) were larger in the intervention group. The mean age of participants in the intervention group was 75.6 years (SD, 10.2 years), in the standard management group 75.9 years (SD, 10.3 years) (Box 2). Iron (60 people, 30%), vitamin D (48 people, 24%), and thyroid function (ten people, 5%) abnormalities identified during the index admissions were treated in both study groups before discharge. Forty participants (20%) had histories of recurrent hospital admissions during a particular season, including ten during winter.
Box 1. Selection and assessment of participants for the RESILIENCE trial at the Austin Hospital, Melbourne, 19 November 2020 – 28 July 2023
ITT = intention to treat.
Box 2. Baseline characteristics of the participants in the RESILIENCE trial at the Austin Hospital, Melbourne, 19 November 2020 – 28 July 2022
| Characteristic | All participants | RESILIENCE intervention | Standard management |
|---|---|---|---|
| Number of people | 203 | 103 | 100 |
| Socio-demographic profile | |||
| Age (years), mean (SD) | 75.7 (10.2) | 75.6 (10.2) | 75.9 (10.3) |
| Sex (female) | 104 (51.2%) | 53 (52%) | 51 (51%) |
| Reliant on public health system | 113 (55.7%) | 59 (57%) | 54 (54%) |
| Living alone | 79 (38.9%) | 43 (42%) | 36 (36%) |
| English not primary language | 41 (20.2%) | 15 (16%) | 26 (26%) |
| Primary school as highest education level | 45 (22.2%) | 22 (21%) | 23 (23%) |
| Risk factor profile/behaviours | |||
| Body mass index (kg/m2), mean (SD) | 29.5 (7.3) | 29.4 (7.2) | 29.6 (7.5) |
| Exercise more than 2.5 hours each week | 89 (43.8%) | 51 (50%) | 38 (38%) |
| Currently or formerly smoked | 111 (54.7%) | 56 (54%) | 55 (55%) |
| Excessive alcohol use* | 26 (12.8%) | 12 (12%) | 14 (14%) |
| Symptoms of depression | 60 (29.6%) | 33 (32%) | 27 (27%) |
| Poor adaptation to weather† | 97 (47.8%) | 49 (48%) | 48 (48%) |
| Previous seasonal hospital admissions | 40 (19.7%) | 23 (22%) | 17 (17%) |
| Behavioural vulnerability | 30 (14.8%) | 18 (18%) | 12 (12%) |
| Physiological vulnerability | 90 (44.3%) | 46 (45%) | 44 (44%) |
| Vaccination history | |||
| Influenza | 155 (76.4%) | 78 (76%) | 77 (77%) |
| Pneumococcal disease | 93 (45.8%) | 46 (45%) | 47 (47%) |
| Varicella | 52 (25.6%) | 31 (30%) | 21 (21%) |
| COVID-19 | 157 (77.3%) | 81 (79%) | 76 (76%) |
| Clinical history (past/index admission diagnoses) | |||
| Hypertension | 146 (71.9%) | 74 (72%) | 72 (72%) |
| Coronary artery disease | 115 (56.7%) | 59 (57%) | 56 (56%) |
| Atrial fibrillation | 101 (49.8%) | 55 (53%) | 46 (46%) |
| Heart failure | 103 (50.3%) | 60 (51%) | 43 (43%) |
| Diabetes | 84 (41.4%) | 40 (39%) | 44 (44%) |
| Chronic lung disease | 72 (35.5%) | 41 (40%) | 31 (31%) |
| Depression/anxiety | 47 (23.2%) | 27 (26%) | 20 (20%) |
| Charlson comorbidity index, mean (SD) | 6.5 (2.7) | 6.7 (3.0) | 6.3 (2.4) |
| Pre-admission profile | |||
| Admitted to hospital during the preceding twelve months | 94 (46.3%) | 49 (48%) | 45 (45%) |
| Temperature (day before admission), °C | |||
| Minimum, mean (SD) | 9.6 (4.2) | 10.4 (4.2) | 8.8 (4.0) |
| Maximum, mean (SD) | 19.8 (5.9) | 20.3 (6.3) | 19.1 (5.5) |
| Dyspnoea | 93 (45.8%) | 47 (46%) | 46 (46%) |
| Chest pain | 68 (33.8%) | 39 (38%) | 29 (29%) |
| Index admission profile | |||
| Season of admission | |||
| Winter (June–August) | 57 (28.1%) | 29 (28%) | 28 (28%) |
| Spring (September–November) | 68 (33.5%) | 30 (29%) | 38 (38%) |
| Summer (December–February) | 37 (18.2%) | 20 (19%) | 17 (17%) |
| Autumn (March–May) | 40 (19.7%) | 23 (22%) | 17 (17%) |
| Acute coronary syndrome | 67 (32.0%) | 36 (35%) | 31 (31%) |
| Acute heart failure | 65 (33.0%) | 30 (29%) | 35 (31%) |
| eGFR (mL/min/1.73m2), mean (SD) | 61.5 (25.7) | 61.8 (26) | 61.2 (25.7) |
| HbA1c (mmol/mol), mean (SD) | 46.3 (13.1) | 45.5 (11.5) | 47.2 (14.6) |
| Anaemia‡ | 78 (38.4%) | 37 (35.9%) | 41 (41%) |
| Vitamin D (nmol/L), median (IQR) | 63.0 (40.5–86.5) | 65.0 (48.0–90.0) | 61.0 (39.0–83.0) |
| Montreal Cognitive Assessment score, mean (SD) | 25 (4) | 25 (3) | 25 (4) |
| HADS anxiety score, median (IQR) | 4.0 (2.0–6.0) | 4.0 (2.0–7.0) | 4.0 (2.0–6.0) |
| HADS depression score, median (IQR) | 5.0 (3.0–7.0) | 5.0 (3.0–7.0) | 4.0 (2.2–6.0) |
| Rockwood Clinical Frailty score, mean (SD) | 3.64 (1.32) | 3.66 (1.28) | 3.61 (1.36) |
| Length of stay (days), mean (SD) | 8.2 (7.2) | 8.1 (6.1) | 8.3 (8.1) |
| Cardiology unit discharge | 110 (54.2%) | 56 (54%) | 54 (54%) |
| General medicine unit discharge | 63 (31.0%) | 36 (35%) | 29 (29%) |
| Discharge pharmacotherapy | |||
| Beta-blocker | 132 (65.0%) | 69 (67%) | 63 (63%) |
| Diuretic | 126 (62.1%) | 65 (63%) | 61 (61%) |
| Renin–angiotensin system inhibitor or blocker | 112 (55.2%) | 55 (53%) | 57 (57%) |
| Clopidogrel and aspirin | 73 (36.0%) | 39 (38%) | 34 (34%) |
| Anticoagulant | 94 (46.3%) | 54 (52%) | 40 (40%) |
| Calcium channel blocker | 35 (17.2%) | 19 (18%) | 16 (16%) |
| Anti-arrhythmic agent | 22 (10.8%) | 14 (14%) | 8 (8%) |
- COVID-19 = coronavirus disease of 2019; eGFR = estimated glomerular filtration rate; HADS = hospital anxiety and depression scale; HbA1c = glycated haemoglobin; IQR = interquartile range; SD = standard deviation.
- * Ten or more standard drinks per week.
- † Based on multifaceted profiling, as described elsewhere.13
- ‡ Based on haemoglobin level by age, and sex: women: < 130 g/L; men aged 60 years or younger: < 130 g/L; men over 60 years of age: < 120 g/L.
RESILIENCE intervention group
During follow-up, 94 of 103 intervention group participants (91%) were visited at home by the RESILIENCE nurse, a median of fourteen days (IQR, 0–23 days) after discharge; 49 people (52%) reported no immediate concerns. Twenty-seven people exhibited poor awareness of weather and climate, seventeen did not have the socio-economic resources to manage areas of concern, and the behaviour of sixteen participants placed them at risk of weather-related cardiovascular events; five people lived in physical conditions that exposed them to weather extremes.
Ninety-one participants attended the RESILIENCE clinic a median of ten days (IQR, 6–15 days) later. All attenders received education and goal-setting advice and agreed to receive weather alerts by text message. More specific measures included adjusting pharmacological therapy (78 participants, 86%), help with applying for home heating or cooling subsidies (42, 46%), organising pertussis (42, 46%), pneumococcal (38, 42%), or varicella (32, 35%) vaccinations, and the commencement of vitamin D supplements (seven, 8%). Vulnerability to external provocations to health was subjectively assessed as being low for 29 participants (32%), moderate for 39 (43%), and high for 23 (25%). Plans regarding areas of concern were communicated to each participant's health care team, and follow-up was tailored to their individual needs and assessed level of risk (sample patient history: Supporting Information, part 2).
Re-admission to hospital and death
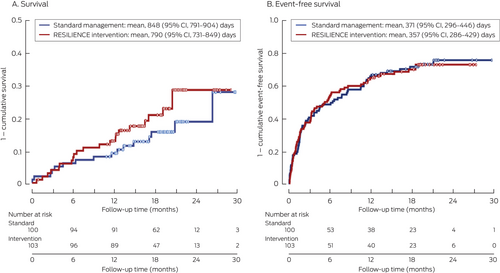
A total of 470 hospital admissions and 3874 days of hospital stay during follow-up were recorded for 138 of the 203 trial participants (68%); 38 people (19%) died during follow-up. The days alive and out of hospital proportion (the primary endpoint) was 89.1% (SD, 22.0 percentage points) for the intervention group and 86.5% (SD, 24.7 percentage points) for the standard management group (adjusted difference, 3.04 percentage points; 95% CI, –0.21 to 6.29 percentage points).
Neither the differences in the overall numbers of deaths in the intervention and standard management groups (22 of 103, 21% v 16 of 100, 16%; P = 0.25) nor the composite endpoint of time to first hospital re-admission or death (72, 70% v 72, 72%; P = 0.97) were statistically significant (Box 3). The number of hospital re-admissions was similar for the intervention and standard management groups (224 v 246; adjusted difference, –2.75%; 95% CI, –0.39% to 5.89%), as were the numbers of days in hospital (1834 v 2040; adjusted difference, 2.63%; 95% CI, –1.13% to 6.39%).
Box 3. Survival and event-free survival* for participants in the RESILIENCE trial: Kaplan–Meier survival analyses
CI = confidence interval.
* No hospital re-admissions or death.
In an analysis adjusted for the variables listed in Supporting Information, table 1, several socio-demographic (living alone) and clinical factors (coronary artery disease, heart failure, diabetes, depressive symptoms, reduced kidney function, low vitamin D level, pneumococcal vaccination), but not group randomisation, were associated with increased risk of hospital re-admission or death during follow-up (Supporting Information, table 1).
Quality of life
The EQ-5D was completed at both baseline and at 12-month reviews by 65 standard management and 81 intervention group participants. For the standard management group, mean self-rated health was 67.2 (SD, 18.9) at baseline and 72.9 (SD, 14.6) at twelve months; for the intervention group, the mean rating was 63.1 (SD, 20.6) at baseline and 69.9 (SD, 17.4) at twelve months. The mean between-group difference in the change between assessments was not statistically significant (standard management v intervention: 1.10; 95% CI, –5.58 to 7.79).
Timing of events
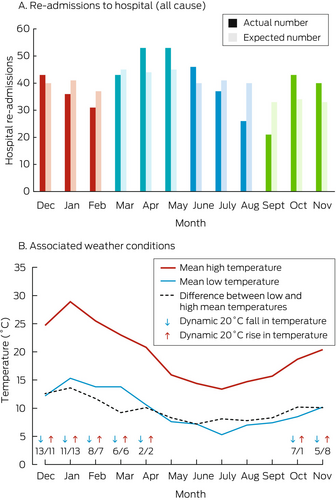
The number of hospital re-admissions peaked during April and May (the transitional months from warmer and dryer autumnal weather to markedly colder and wetter winter conditions in Melbourne), both the raw figures and after adjustment for time at risk based on recruitment date, standardised by applying time-exposure weighting to months of follow-up (Box 4).
Box 4. All-cause hospital re-admissions of participants in the RESILIENCE trial, by month of re-admission (A);* mean high and low temperatures in Melbourne, 19 November 2020 – 28 July 2023, and major weather events during the 72 hours preceding re-admissions (B)
* Red bars: summer; teal: autumn; blue: winter; green: spring.
Of 470 hospital re-admissions, 192 (41%) followed weather events during the preceding 72 hours. One hundred of these events were dynamic temperature changes: 48 warming events (peak temperatures rose by a mean 21.0°C [SD, 3.3°C]) and 52 cooling events (peak temperatures dropped by a mean 20.2°C [SD, 2.9°C]); 63 of these temperature events were during summer. There were also 68 rain periods (64 during autumn/winter) and 24 thunderstorms (21 during summer/autumn).
After adjustment for the time of follow-up, the number of days of hospital stay was lower for the intervention than the standard management group in the three summer months of December (136 fewer days; –3.38 [95% CI, –6.06 to –0.71] days/person), January (138 fewer days; –4.26 [95% CI, –6.84 to –1.68] days/person), and February (130 fewer days; –6.10 [95% CI, –8.91 to –3.29] days/person); the overall difference for the three summer months was –4.10 (95% CI, –6.87 to –1.33) days/person. The numbers of days of hospital stay were higher for the intervention than the standard management group for five of twelve months, but the differences were not statistically significant, nor were the between-group differences significant for any other season (Supporting Information, figure 1 and table 2).
Discussion
We report the first published trial of a nurse-coordinated, multifaceted intervention designed to reduce the bio-behavioural vulnerability of people who have been hospitalised with multimorbid heart disease to weather condition-related ill-health. Our findings challenge the assumption that the management of people with chronic heart disease should be the same all year round. Other studies have reported variations in the incidence of cardiovascular-related events by time of year at the population and clinic levels,7, 18, 19 and our pilot study found that these variations are found even when gold standard multidisciplinary management is provided.10 A new approach to managing people with multimorbid heart disease is therefore warranted.
Based on a bio-behavioural model of vulnerability to climatic conditions and weather events,6 our multifaceted intervention aimed to promote climatic resilience. Many aspects of our intervention (eg, handwashing, vaccinations, social isolation during infection) were adopted as routine practices during the COVID-19 pandemic that disrupted both participant recruitment and the RESILIENCE trial.13 On an intention-to-treat basis, the overall differences between intervention and standard management groups with respect to the primary endpoint (days alive and out-of-hospital) and all-cause hospital re-admission and death were not statistically significant, nor was the difference in change in quality of life among people alive at the 12-month review. The lower than planned study size may not have affected our primary endpoint assessment, given the small differences between groups, but it may have reduced the statistical power of our study to detect differences in the number of re-admissions and recurrent hospital stay. Nevertheless, after adjusting for timing of follow-up, the RESILIENCE intervention was associated with significantly fewer days of hospital stay during the three summer months, during which marked changes in temperature were more frequent.
Specific findings from our study suggest that our novel intervention is worth pursuing further. First, we successfully recruited people with multimorbid heart disease with the bio-behavioural vulnerabilities we expected to find.13 Consistent with other observational studies,20, 21 medical event rates during follow-up varied markedly by month and season. Strikingly, we found that hospital re-admissions were more frequent after dynamic weather events, including storms and acute temperature changes associated with heatwaves and cold fronts. Such dynamic weather events (La Niña reduced weather extremes during the study period) will increase because of climate change,9 including more harmful heatwaves,22 bushfire conditions,23 and thunderstorm asthma,24 adversely affecting an ageing population in which the prevalence of multimorbid heart disease is increasing.25
Our findings reaffirm that dynamic weather conditions with large temperature changes (including cooling events) should not be ignored.6 Multivariate analyses of when events happened could help us better define who is at risk of clinical instability under specific climatic conditions. For example, Australian mortality rates peak in winter, but climate change may alter this pattern.26 We focused on supporting people to cool and heat their homes to maintain homeostasis. Climate change will probably generate the same weather extremes and dynamic changes that provoked higher monthly numbers of hospitalisations in our study.26
If climate change provokes more expensive and potentially fatal events in people with multimorbid heart disease, can we realistically hope to attenuate its harmful effects? Compared with simpler strategies, such as providing weather alerts27 and reducing fuel poverty,28 we applied a multifaceted intervention that, when tailored to each person's profile and circumstances, aimed to increase their number of days alive and out of hospital, but this more holistic approach apparently failed.
However, the rate of hospital re-admissions (one component of the primary endpoint) was significantly lower during the summer months for participants in the RESILIENCE intervention group. Pre-specified analyses of the timing and nature of events will help to place these findings into context and guide future interventions. For example, post hoc analyses found that the RESILIENCE intervention was ineffective for people with chronic respiratory disease (data not reported). We now have sufficient information to better identify who is at greatest risk of weather-related provocations to health and the capacity to refine our intervention to reduce hospital re-admissions (its effect on survival is far less certain). We therefore plan to further assess the proof-of-concept findings reported in this article and undertake a more definitive trial to investigate a phenomenon that contributes to 20–25% excess mortality attributed to cardiovascular disease.6 Climate change means that if our approach is ineffective, another strategy is needed.9
Limitations
Apart from the adverse impact of the COVID-19 pandemic on trial recruitment and delivery of the study intervention, the major limitation of our pragmatic trial was that study group allocation was not masked for participants or health teams. We undertook therapeutic measures when clinical need was identified (eg, iron deficiency) during baseline profiling. Standard management was consequently enhanced for both groups, but we did not monitor levels of routine care or adverse effects of the study intervention. The smaller than planned study size reduced our ability to detect differences in hospital re-admission rates. Causes of death were not known, and we did not perform an interaction analysis to determine whether the effect of the intervention differed by season. As a single centre study conducted in the comprehensive Australian health care system and with the specific weather conditions in Melbourne during the COVID-19 pandemic,29 any interpretation or extrapolation of our findings to other health care systems or locations must be cautious. A detailed and lengthy analysis of who might have benefited from the intervention, together with health economic analyses of the cost of different components of health care and supplementary socio-economic support (eg, subsidised heating or cooling) will be reported elsewhere; this information will inform our plans for testing a revised intervention in a more selected group of patients.
Conclusion
A climate-focused, nurse-coordinated, multifaceted intervention was ineffective in increasing the proportion of days alive and out of hospital for people followed up for at least twelve months after hospital admissions with multimorbid heart disease, compared with standard, post-discharge management. However, given the growing number of people with multimorbid heart disease and bio-behavioural vulnerability to climatic challenges to health, strategies that cost-effectively improve health outcomes should be pursued, especially in the face of climate change.30 Careful analyses of the timing of significant weather events indicated that the efficacy of our intervention could be improved by modifying its components and applying it in a more targeted manner to reduce weather-triggered events.
Acknowledgements
The study was funded by the Medical Research Future Fund (MRF1175865). Simon Stewart is supported by a National Health and Medical Research Council fellowship (GNT1135894). The funders no role in study design, data collection, outcome analyses, interpretation of results, or writing of this report.
We thank Joshua Byrnes, a member of the RESILIENCE Investigator Group, the patients who participated, and the medical and allied health staff at Austin Health for their support of this study.
Open access
Open access publishing facilitated by the University of Melbourne, as part of the Wiley – the University of Melbourne agreement via the Council of Australian University Librarians.
Competing interests
No relevant disclosures.
Data sharing
Trial data (disaggregated) will be available from the corresponding author upon reasonable request.
Received 15 April 2024, accepted 12 December 2024