Circulating Endothelial Cells as Marker of Endothelial Damage in Male Hypogonadism
Abstract
Abstract: Testosterone deficiency has become a frequently diagnosed condition in today's society affected by epidemic obesity, and is associated with cardiovascular risk. Recent studies have established the importance of altered vascular endothelium function in cardiovascular disease. The damage to the endothelium might also cause endothelial cell detachment, resulting in increased numbers of circulating endothelial cells (CEC) within the bloodstream. To evaluate whether hypogonadism could modify CEC count in peripheral bloodstream, we investigated peripheral blood CEC count using the CellSearch System, a semiautomatic method to accurately and reliably enumerate CECs, which are sorted based on a CD146+, CD105+, DAPI+, CD45- phenotype, in a population of 20 patients with hypogonadism. The control group comprised 10 age- and sex-matched healthy participants. CEC count per milliliter was significantly increased in patients with hypogonadism vs the control group. In the group with hypogonadism, an inverse exponential correlation was present between testosterone levels and CEC count per milliliter. A direct linear correlation was present between waist circumference and CECs and between body mass index and CECs. The regression analysis showed that testosterone was the significant independent determinant of CECs. Our results underline that male hypogonadism is associated with endothelial dysfunction. The correlation between CEC and waist circumference underlines that visceral obesity may be synergically implicated in this regulation. Future studies are required to unveil the mechanisms involved in the pathogenesis of testosterone-induced endothelial disfunction, which may provide novel therapeutic targets to be incorporated in the management of hypogonadism.
Testosterone deficiency (TD) has become a frequently diagnosed condition in our current society close to experiencing an obesity epidemic (Ullah et al, 2011). Men affected by TD frequently present associated comorbidities, particularly those involving metabolic syndrome (MetS). Replacement therapy with testosterone may in fact have effects on sexual characteristics, including libido and erectile function, and on metabolism, whereas untreated TD might increase cardiometabolic risk and disease. The association between coronary artery disease/myocardial infarction and male hypogonadism (Khaw et al, 2007) supports the hypothesis that TD negatively affects the cardiovascular system. Receptors for testosterone are variably expressed in the endothelium and vascular smooth cells of different vessels (Hanke et al, 2001). Previous studies in animals showed that testosterone inhibits atherosclerosis plaque development and has a vasodilator effect on coronary arteries in the cholesterol-fed rabbit model (Alexandersen et al, 1999). The molecular mechanisms by which testosterone exerts its effects on the vessel wall are not completely known and remain controversial.
Recent studies had established the importance of altered vascular endothelium function to cardiovascular disease (CVD). Endothelial cells (EC) line the vascular tree and adhere to a basement membrane, with a very low level of cell loss in the peripheral blood in healthy individuals. Circulating ECs (CEC) were first described in the 1970s using methods such as light microscopy, cell morphology, and density centrifugation. CECs can be separated by identification of their surface markers, CD146 adhesion molecule, which is involved in the endothelial junction, where it plays a key role in the control of cell-cell cohesion, permeability, and signalization (Bardin et al, 2001). Vascular ECs respond to numerous pathophysiologic stimuli, such as growth factors, cytokines, lipoproteins, and oxidative stress. The damage to the endothelium might also cause EC detachment, resulting in increased numbers of CECs within the bloodstream (Goon et al, 2006). CECs are considered as cells driven from the intima after vascular damage and are thought to have “sloughed off” vessel walls as a consequence of endothelial damage. Usually absent in the blood of healthy individuals, CECs are elevated in diseases hallmarked by the presence of vascular damage (Blann et al, 2005). Recently, the immunologic measurement of CECs defined in the peripheral blood has been recognized as a useful marker of profound vascular damage and a novel technique for assessment of endothelial injury. Therefore, CECs have been shown to correlate with various endothelial dysfunction and inflammatory markers (Mutin et al, 1999).
CECs are currently determined by several different assay systems, including the flow cytometry and immunomagnetic detection systems. Flow cytometry analysis has some limitations, including standardization between different laboratories and difficulties in fresh blood shipping (Ali et al, 2011). The CellSearch System (Veridex, Raritan, New Jersey) was recently developed to accurately and reliably enumerate CECs, sorted based on a CD146+, CD105+, DAPI+, CD45- phenotype. It is a variant of manual immunomagnetic bead enrichment techniques, and it provides a fully automated collection procedure, which is followed by semiautomated image cytometry. The final enumeration is provided by fully trained laboratory professionals who are able to distinguish the true CECs from the other non-CEC elements. For this reason, this assay shows high recovery and good reproducibility characteristics. As far as CEC is concerned, 2 previous papers demonstrated that CellSearch results are completely identical to those obtained by whole-gene expression analysis (Smirnov et al, 2006; Rowand et al, 2007), confirming the robustness of the CellSearch assay. In fact, CEC assay through the CellSearch system was proposed as a useful tool in monitoring endothelial injury associated with neoplastic and nonneoplastic conditions (Strijbos et al, 2009a,b; Ali et al, 2011).
The aim of the study was to investigate whether hypogonadism could modify the CEC count in peripheral bloodstream.
Materials and Methods
Twenty 25- to 55-year-old male patients with hypogonadism were enrolled consecutively for this study after giving written informed consent. The study was conducted in accordance with the guidelines in the Declaration of Helsinki. The patients were evaluated at least 6 months after neurosurgical operation to remove a nonsecreting pituitary adenoma or craniopharyngioma. Replacement therapies for thyroidal, adrenal, and somatotropic axes were performed when single or multiple deficits were documented. The rationale for studying only men with gonadotropin deficiency was to select a condition of severe hypotestosteronemia without other confounding risk factors. Hypergonadotropic hypogonadal patients were excluded because their testosteronemia is usually not as low as that in individuals with hypogonadotropic hypogonadism, and because hypergonadotropic hypogonadal patients often show normal or increased serum estrogen levels. Hypergonadotropic hypogonadism was then considered a confounding condition. Inclusion criteria were: total testosterone <8.68 nmol/L (<2.5 ng/mL), calculated free testosterone <1.5%, and clinical symptoms of hypogonadism. Exclusion criteria for our study included age <20 years or >55 years, smoking, chronic hypertension, diabetes mellitus, known coronary artery disease, arterial hypertension, autoimmune inflammatory diseases, and previous androgen replacement therapy. The control group comprised 10 age- and sex-matched healthy participants.
Anthropometric parameters, body mass index (BMI), and waist circumference (WC) were evaluated. Serum total cholesterol (TC) and triglyceride levels were determined using an enzymatic method, and high-density lipoprotein cholesterol (HDL-C) level was determined using a direct method. Low-density lipoprotein cholesterol level (LDL-C) was calculated using the Friedewald formula. Glycosylated hemoglobin was determined by ion-exchange chromatography on an ADAMS A1c HA 8160 analyzer (Menarini Diagnostics SpA, Florence, Italy).
A blood sample was collected at 8 am for the determination of testosterone, estradiol (E2), dihydrotestosterone, sex hormone-binding globulin (SHBG), luteinizing hormone (LH), and follicle-stimulating hormone (FSH). Testosterone and E2 were assayed twice by radioimmunoassay with the use of commercial kits by Radim (Pomezia, Italy). LH, FSH, and SHBG were assayed by immunoradiometric methods on a solid-phase coated tube, which is based on a monoclonal double-antibody technique. Dihydrotestosterone was assayed by radioimmunoassay with the use of commercial kits by Chematil (Angri, Italy). Free testosterone was calculated according to the formula proposed by Vermeulen et al (1999).
Sample preparation for isolation of CECs from blood (10 mL) was drawn from patients into evacuated Cell-Save Preservative Tubes (Veridex). The CellSearch system (Veridex) consists of the Celltracks AutoPrep system, which uses the CellSearch Endothelial Cell Kit and the Celltracks AnalyzerII. CellPrep is a semiautomated sample preparation system. The CellSearch Endothelial Cell Kit contains reagents and consumables for the immunomagnetic collection and subsequent staining of CECs and in detail: 3.0 mL of anti-CD146ferrofluid, a suspension of 0.012% magnetic nanoparticles conjugated to a mouse monoclonal antibody that is specific for a cell surface marker present on ECs in a buffer containing 0.3% bovine serum albumin (BSA) and 0.05% ProClin 300 preservative; a 3.0-mL vial of anti–CD105-phycoerythrin/anti–CD45-allophycocyanin (staining reagent) containing <0.0006% mouse monoclonal antibodies specific to CD105 conjugated to phycoerythrin, <0.0013% mouse anti-CD45 monoclonal antibody conjugated to allophycocyanin in phosphate-buffered saline (PBS) containing 0.5% BSA, and 0.1% sodium azide; 3.0 mL of Nucleic Acid Dye containing 0.005% 4',6-diamidino-2-phenylindole, dihydrochloride (DAPI) and 0.05% ProClin 300; 3.0 mL of Capture Enhancement Reagent containing PBS, 0.5% BSA, 0.02% proprietary reagent for controlled ferrofluid aggregation, and 0.1% sodium azide; 3.0 mL of Permeabilization Reagent containing 0.011% proprietary permeabilization reagent and 0.1% sodium azide; 3.0 mL of Cell Fixative containing PBS, 25% proprietary ingredients, 0.1% BSA, and 0.1% sodium azide in buffer; a 3 × 110 mL bottle of Dilution Buffer, containing buffer with 0.1% sodium azide; 16 Conical Tubes (15 mL Conical Centrifuge) and Conical Tube Caps; and 16 Cartridges and Cartridge Plugs.
The 4 mL of blood for CECs was mixed with 6 mL of buffer, centrifuged at 8000 ×g for 10 minutes, and then placed on the CellPrep system. After aspiration of the plasma and buffer layer by the instrument, ferrofluid was added. Ferrofluid consists of a magnetic core surrounded by a polymeric layer coated with CD146 antibody to capture cells. After incubation and subsequent magnetic separation, unbound cells and remaining plasma were aspirated. The staining reagents were then added in conjunction with a permeabilization buffer to fluorescently label the immunomagnetically labeled cells. The fluorescent reagents were added: phycoerythrin-conjugated antibodies that bind to CD105 antibody; an antibody to CD45 conjugated to allophycocyanin; nuclear dye DAPI to fluorescently label the cell; and buffers to wash, permeabilize, and resuspend the cells. After incubation on the system, the magnetic separation was repeated, and excess staining reagents were aspirated. In the final step, the cells were suspended again in the MagNest Cell Presentation Device (Veridex). This process consists of a chamber and 2 magnets that orient the immunomagnetically labeled cells for analysis by using the Celltracks analyzerII. The MagNest is placed on the Celltracks analyzerII, a 4-color semiautomated fluorescence microscope. Image frames covering the entire surface of the cartridge for each of the 4 fluorescent filter cubes were captured. The captured images containing objects that met predetermined criteria were automatically presented in a Web-enabled browser from which a final selection of cells was made by the operator. An event was classified as a CEC when its morphologic characteristics were consistent with those of a cell that exhibits the phenotype correctly: CD146+, CD150+, DAPI+, and CD45.
All descriptive results were expressed as the mean ± SEM. Statistical analysis was performed with SPSS 13.0 software (Chicago, Illinois). Data were not normally distributed and were compared by nonparametric test. Comparison between groups was performed by Mann-Whitney U test. Correlation analysis was performed by Spearman's test. Multiple regression analysis was used to identify independent factors associated with CEC level.
Results
Clinical and laboratory data of patients and controls are reported in Table 1.
| Patients, Mean ± SEM | Controls, Mean ± SEM | Reference Values | |
|---|---|---|---|
| Age, y | 35.30 ± 3.01 | 41.90 ± 2.94 | … |
| BMI, kg/m2 | 30.28 ± 2.10 | 24.02 ± 2.50a | … |
| WC, cm | 106.25 ± 3.67 | 90.12 ± 1.70a | … |
| Glycemia, mmol/L (mg/dL) | 5.12 ± 0.16 (92.20 ± 3.74) | 5.03 ± 0.14 (90.60 ± 2.54) | 3.61-6.11 (65-110) |
| Glycosylated hemoglobin, % | 6.20 ± 1.00 | 5.23 ± 0.80 | 4.3-5.9 |
| TC, mmol/L (mg/dL) | 5.17 ± 0.23 (200.00 ± 8.77) | 4.83 ± 0.33 (187.00 ± 12.77) | 3.36-5.17 (130-200) |
| HDL-C, mmol/L (mg/dL) | 1.37 ± 0.08 (52.89 ± 3.25) | 1.43 ± 0.12 (55.21 ± 4.58) | >1.03 (>40) |
| LDL-C, mmol/L (mg/dL) | 3.36 ± 0.09 (129.85 ± 3.37) | 2.46 ± 0.08 (95.17 ± 3.15)a | <3.36 (<130) |
| TG, mmol/L (mg/dL) | 1.73 ± 0.18 (153.00 ± 16.00) | 1.57 ± 0.22 (139 ± 19.30) | 0.23-1.92 (20-170) |
| FSH, IU/L | 1.90 ± 0.40 | 3.45 ± 2.18a | 2.5-11.0 |
| LH, IU/L | 1.30 ± 0.40 | 4.13 ± 0.81a | 2.5-10.0 |
| Testosterone, nmol/L (ng/mL) | 4.65 ± 0.65 (1.35 ± 0.19) | 16.55 ± 6.55 (4.80 ± 1.90)a | 8.62-28.96 (2.5-8.4) |
| DHT, ng/mL | 0.12 ± 0.09 | 0.37 ± 0.10a | 0.30-0.85 |
| Free testosterone, % | 1.10 ± 0.4 | 2.4 ± 0.20a | 2%-3% |
| E2, pmol/L (pg/mL) | 33.44 ± 5.24 (9.11 ± 1.43) | 106.64 ± 30.10 (29.05 ± 8.20)a | 36.71-128.48 (10-35) |
- Abbreviations: BMI, body mass index; DHT, dihydrotestosterone; E2, estradiol; FSH, follicle-stimulating hormone; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LH, luteinizing hormone; TC, total cholesterol; TG, triglycerides; WC, waist circumference.
- aP < .05
The mean age of patients in the two groups did not differ significantly. BMI, WC, and LDL-C were significantly increased in hypogonadism group. Total testosterone, free testosterone, FSH, LH, and E2 were decreased significantly in patients with hypogonadism when compared with the control group.
We found increased CEC count per milliliter (mean ± SEM) in patients with hypogonadism (17.65 ± 4.76) when compared with the control group (1.03 ± 0.14; P < .05), as reported in Figure 1.
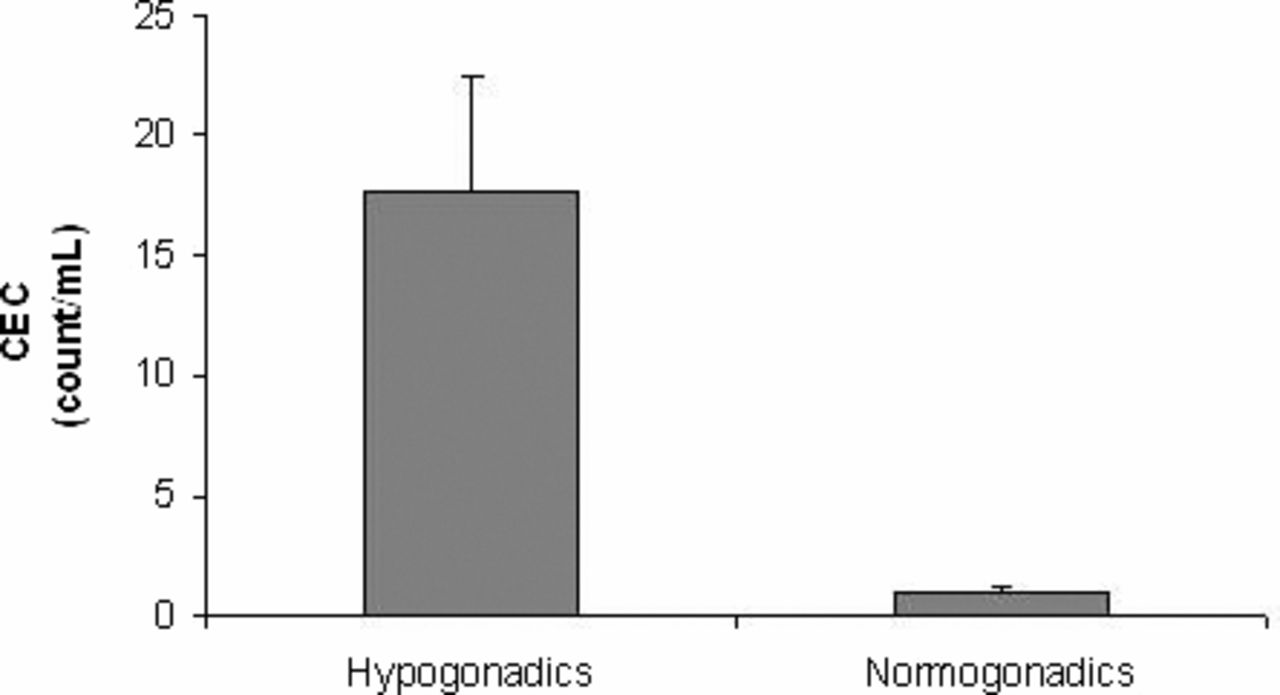
Circulating endothelial cell (CEC) count (mean ± SEM) in patients with hypogonadism (n = 20) and controls (n / 10).
In the hypogonadism group an inverse exponential correlation was present between testosterone levels and CEC count per milliliter (Figure 2). A direct linear correlation was present between WC and CECs and between BMI and CECs (Table 2); an inverse linear correlation was present between testosterone levels and WC (Figure 3). No correlation was present between CECs and the other independent variables in controls.
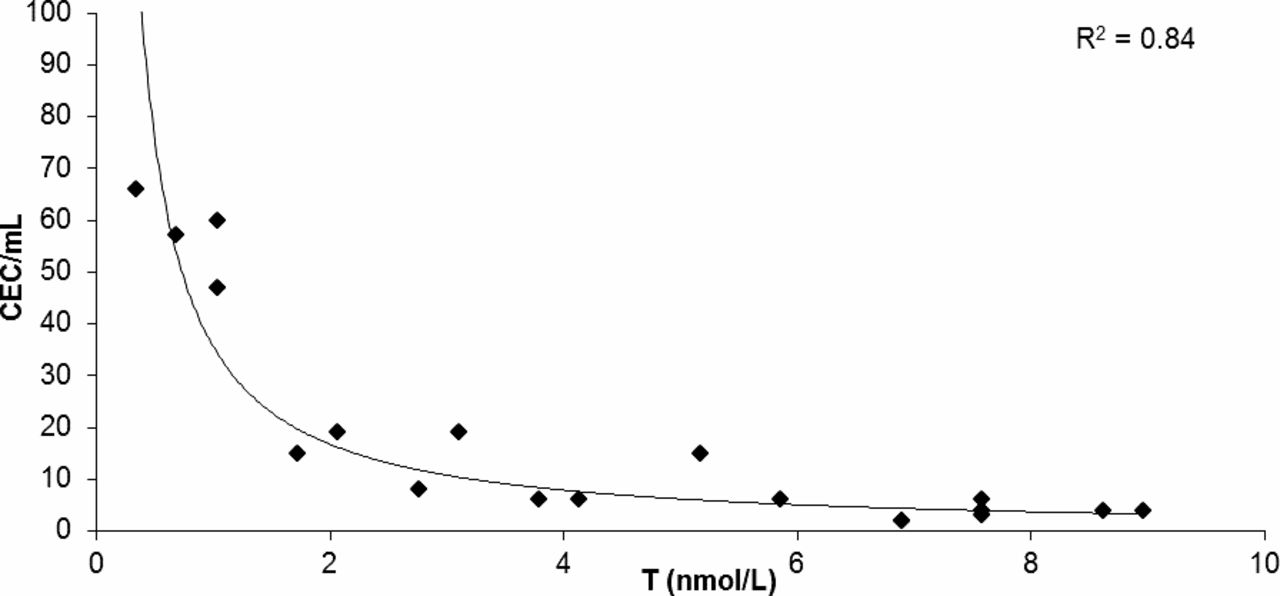
Correlation analysis between testosterone (T) levels and circulating endothelial cell (CEC) count per milliliter in 20 samples by patients with hypogonadism (R2 / 0.84; P < .05).
| CECs | ||
|---|---|---|
| Correlation Coefficient r | Two-Tailed P | |
| Testosterone | –0.867 | .001a |
| WC | 0.779 | .001a |
| LDL-C | 0.307 | .189 |
| BMI | 0.632 | .003a |
| TC | 0.045 | .850 |
| TG | 0.009 | .970 |
- Abbreviations: BMI, body mass index; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; WC, waist circumference.
- aP < .05
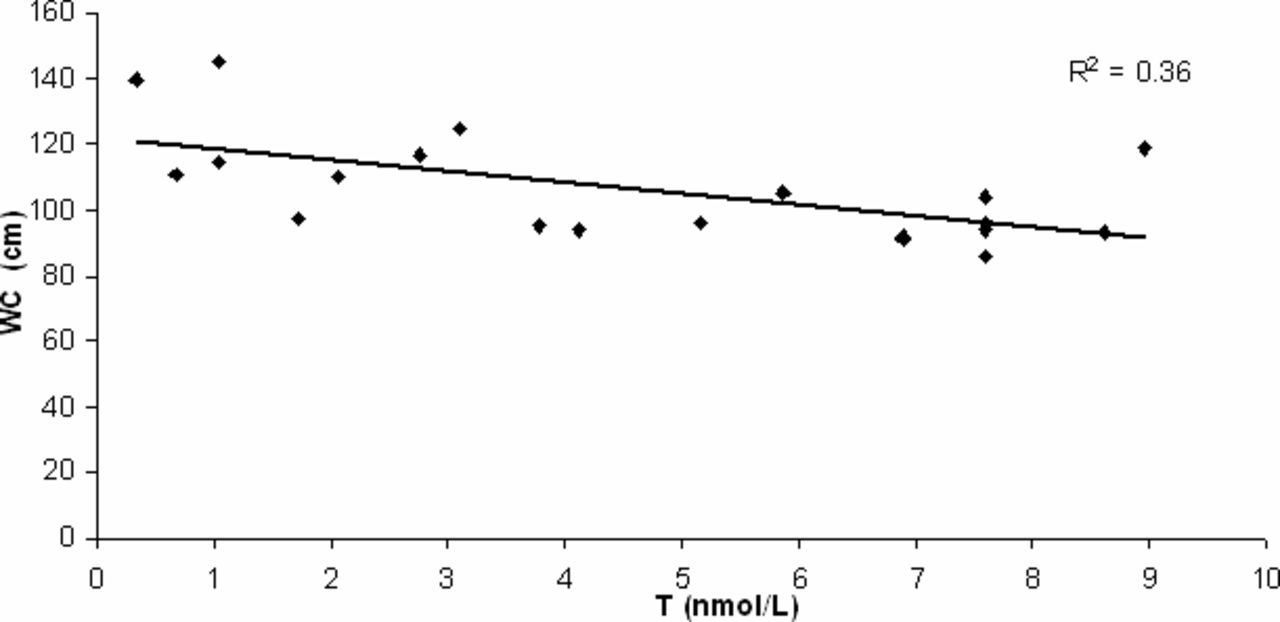
Correlation analysis between waist circumference (WC) and testosterone (T) in 20 samples by patients with hypogonadism (R2 / 0.36; P < .05).
The multiple regression analysis showed the significance of our model (R2 / 0.77; adjusted R2 = 0.71; P < .001). The coefficient values of the independent variables (BMI, testosterone, LDL-C, and WC) showed that testosterone was the significant independent determinant of CECs (Table 3).
| Unstandardized | Standardized | |||
|---|---|---|---|---|
| Coefficients | B | Standard Error | Beta | P |
| (Constant) | 2.014 | 37.595 | … | .958 |
| Testosterone | –14.379 | 4.365 | –0.573 | .005a |
| LDL-C | –0.216 | 0.196 | –0.153 | .286 |
| BMI | 0.041 | 0.808 | 0.011 | .961 |
| WC | 0.584 | 0.343 | 0.450 | .109 |
- Abbreviations: BMI, body mass index; LDL-C, low-density lipoprotein cholesterol; WC, waist circumference.
- aF / 12.867; P < .05
Discussion
Testosterone deficiency affects approximately 30% of men between 40 and 79 years of age. A considerable body of evidence exists that suggests androgen deficiency contributes to the onset and/or progression of CVD.
Clinical and preclinical evidence exists linking endothelial dysfunction to androgen deficiency (Akishita et al, 2007; Miller and Mulvagh, 2007; Foresta et al, 2008). English et al (2000) reported lower concentrations of bioavailable testosterone in men with coronary artery disease compared with men with normal angiograms. Low total and bioavailable testosterone levels were associated with increased risk of aortic atherosclerosis in elderly men (Hak et al, 2002). This association was independent of age, BMI, SHBG, TC, HDL-C, diabetes mellitus, smoking, and alcohol intake. A recent meta-analytic study by Corona et al (2011) among 70 cross-sectional studies reported that lower testosterone and higher E2 levels correlate with an increased risk of CVD and cardiovascular mortality. These data suggest that testosterone could have a “direct” effect on cardiovascular health in the absence of other common risk factors. Although these data showed that hypogonadism is associated with CVD, it is not yet clear whether the reduction in testosterone levels or the resulting increase in hyperglycemia, insulin resistance, and other effects of TD could be main contributors to the pathophysiology of CVD in hypogonadal patients.
Dyslipidemia, obesity, and diabetes are in fact associated with endothelial dysfunction (McVeigh and Cohn, 2003). Mechanisms underlying lipotoxicity include oxidative stress and proinflammatory signaling, whereas the mechanisms underlying glucotoxicity include oxidative stress, advanced glycation end-product formation, the hexosamine pathway, and proinflammatory signaling (Kim et al, 2006).
The close association between hypogonadism and MetS has received more attention. Therefore, the prevalence of hypogonadism in patients with MetS has been shown to be higher both in epidemiologic studies (Araujo et al, 2007) and in a survey of clinical practice (Mulligan et al, 2006). The specific pathogenetic mechanisms linking hypogonadism with MetS appear to be complex and often multidirectional. Visceral obesity can probably be considered a relevant cause of hypogonadism (Corona et al, 2008), but at the same time hypogonadism could be a cause of visceral obesity and insulin resistance, establishing a vicious cycle (Traish et al, 2009). Recently, Filippi et al (2009) demonstrated in an animal model that experimental hypogonadotropic hypogonadism induces an increase in visceral adiposity.
Therefore, a reduction in testosterone levels is associated with serum lipid alterations and obesity both in animals and human men. Androgen deficiency is in fact associated with increased triglycerides, TC, and LDL-C (English et al, 1997). Barud et al (2002) showed an inverse relationship between low testosterone and elevated LDL antibody levels, and after multiple regression analysis they revealed that only testosterone was independently associated. Malkin et al (2004a) reported that proinflammatory factors are associated with the development of atheromatous plaque, and the testosterone-induced reduction of these factors is certainly beneficial to those at risk for CVD. In a subsequent study, the authors reported that testosterone therapy in hypogonadal men with angina significantly reduced TC and tumor necrosis factor α (Malkin et al, 2004b).
In our study we showed a significant increase in CEC count per milliliter in men with hypogonadism vs healthy controls.
The cells designated by the CellSearch Assay as CECs are thought to derive directly from damaged vasculature and can be considered a marker of endothelial damage. Levels of CECs in the bloodstream are indeed predictive of major adverse cardiovascular end points and death in patients with acute coronary syndrome. CECs represent a different population of cells than endothelial progenitor cells (EPC), which seem to be a heterologous population of largely bone marrow-derived large nonleukocyte cells with properties similar to those of embryonal angioblasts, are present at varying stages of development in the peripheral blood (Blann et al, 2005). As such, they represent one of the first specific cellular markers to provide a direct link with the pathophysiology of CVD. In the present study, the significant increase in CEC count in male hypogonadism could represent a clinical predictive marker for cardiovascular risk and confirms the presence of endothelial damage in patients with hypogonadism.
The inverse exponential correlation between testosterone and CECs in the group with hypogonadism confirms the link between hypogonadism and endothelial damage and suggests that testosterone is involved in hormonal regulation of endothelial function. Therefore, the exponential correlation showed that in patients with lower testosterone levels, endothelial damage increases in exponential mode.
Regression analysis showed that testosterone was the strongest predictor of CECs. These data suggest that testosterone has the greatest impact on CEC count. This concept is consistent with findings by Foresta et al (2006), which observed that testosterone modulates the number of EPCs in hypogonadal men. They reported a reduced number of EPCs in hypogonadal men compared with the same number in healthy controls. Differently from EPCs, which decrease in androgen deficiency, the increase in CECs represents a direct index of vascular damage. Testosterone deficiency may in fact induce, as suggested by our data, endothelial damage, as indicated by the increase in CECs, and may simultaneously reduce endothelial repair via reduction in EPCs, as previously reported by Foresta et al (2006). In this way both an increase in endothelial damage and a reduction in endothelial repair could be involved in the pathophysiology of CVD in hypogonadal patients.
The action of androgens could be explained by an altered balance between reactive oxygen species and an antioxidant system. Patients with hypogonadism showed a reduction in total antioxidant capacity and coenzyme Q10, whereas testosterone replacement therapy induces an increase in both antioxidant parameters, as previously reported (Mancini et al, 2008). Furthermore, testosterone on its own stimulates rapid production of nitric oxide through androgen receptor-dependent activation of endothelial nitric oxide synthase in human aortic ECs (Yu et al, 2010). Endothelium-derived nitric oxide has been shown to modulate a variety of vascular functions, including vasodilation, inhibition of EC death, inhibition of platelet aggregation, and attenuation of leukocyte infiltration (Jin and Loscalzo, 2010).
Taken together, our results underline that male hypogonadism is associated with endothelial dysfunction, and the correlation between CEC and WC underlines that visceral obesity may be synergically implicated in this regulation.
In conclusion, CECs represent a direct marker of endothelial damage in patients with hypogonadism. The present study supports previous data about the role of testosterone deficiency, in association with obesity, in the pathogenesis of endothelial dysfunction.
Future studies are required to unveil the mechanisms involved in the pathogenesis of testosterone-induced endothelial disfunction, which may provide novel targets to be incorporated into the clinical management of hypogonadism.




