Activity of the Na,K-ATPase α4 Isoform Is Regulated During Sperm Capacitation to Support Sperm Motility
Supported by NIH grants HD043044 and HD055763.
Abstract
Abstract: The α4 polypeptide is a testis-specific isoform of the catalytic subunit of the Na,K-ATPase, which is essential for sperm motility and fertility. In the present study, we have investigated the regulation of activity of the α4 isoform and the relevance of this event for sperm capacitation. We have performed this by taking advantage of the selective high affinity of α4 for the inhibitor ouabain. Our results show that ouabain-sensitive hydrolysis of ATP and uptake of 86Rb, corresponding to the enzymatic and ion transport activities of α4, respectively, increased during sperm capacitation in a time-dependent manner. Specific labeling of α4 with the fluorescent indicator bodipy-ouabain and immunoblot analysis of biotinylated and streptavidin-precipitated sperm plasma membrane proteins indicated a capacitation- and time-dependent rise in levels of active α4 isoform at the sperm surface. Ouabain inhibition of α4 blocked the increase in total sperm motility and the hyperactive motility pattern characteristic of sperm capacitation. Moreover, interference of α4 activity with ouabain partially prevented the intracellular decrease in Na+ and the plasma membrane hyperpolarization that typically accompany sperm capacitation. In contrast, ouabain inhibition of α4 did not affect the spontaneous sperm acrosomal reaction following capacitation. Together, these results demonstrate that Na,K-ATPase α4 activity is up-regulated during sperm capacitation through mechanisms that involve both increases in molecular activity and levels of α4 at the sperm plasma membrane. This increase in α4 activity helps maintain the changes in motility that are associated with sperm capacitation, emphasizing the biologic relevance of the Na,K-ATPase α4 isoform in sustaining sperm function.
Spermatozoa must tightly regulate their ion composition to function and perform the specific task of fertilizing the egg. To achieve this, the male gamete expresses a series of ion transport systems that are differentially distributed along the plasma membrane (Darszon et al, 1999, 2006). Particularly important is the active ion transporter Na,K-ATPase, which uses the free energy from the hydrolysis of ATP to move 3 Na+ from the intracellular space in exchange for 2 K+ that are taken from the surrounding environment (Kaplan, 2002; Blanco, 2005). The electrochemical gradients for Na+ and K+ that the Na,K-ATPase generates are essential to the physiology of cells, contributing to maintain the resting membrane potential, osmotic stability, and Na+-coupled movement of a variety of solutes and water across the cell plasma membrane (Hoffmann and Simonsen, 1989; Feraille and Doucet, 2001).
The Na,K-ATPase is an oligomer composed of 2 main polypeptides, a catalytic α and a glycosylated β subunit (Morth et al, 2007). The α polypeptide is the subunit primarily involved in ATP hydrolysis, translocation of Na+ and K+, and binding of the steroid ouabain (Jorgensen et al, 2003). Multiple isoforms of the α subunit (α1, α2, α3, and α4) have been identified in mammals, each of which is expressed and regulated in a cell- and tissue-specific manner (Blanco, 2005). The α4 isoform has the most restricted pattern of expression, being specifically expressed in the testis, where it is found in male germ cells (Shamraj and Lingrel, 1994; Blanco et al, 2000). Spermatozoa are abundant in α4, and depending on the species, activity of this isoform constitutes between 50% and 75% of the total Na,K-ATPase of the male gamete (Blanco et al, 2000; Sanchez et al, 2006). Besides α4, spermatozoa also express the α1 polypeptide, which is the catalytic subunit of the Na,K-ATPase ubiquitously present in most cells (Wagoner et al, 2005). The α4 and α1 isoforms have distinct enzymatic properties, as reflected by their different responses to various ligands (Blanco and Mercer, 1998; Blanco et al, 1999; Woo et al, 1999). One of the most divergent functional characteristics between α1 and α4 is the specific sensitivity to ouabain inhibition. Whereas the half maximal inhibitory concentration (IC50) of the α4 isoform for ouabain inhibition is in the nanomolar range, that of α1 is in the micromolar range (Blanco and Mercer, 1998).
Selective blockage of α4 activity with ouabain has been used as a practical tool to distinguish the function of this isoform from that of α1. This pharmacologic approach has allowed determining that α4 and α1 isoforms are not redundant, but instead perform different roles in sperm. Thus, relatively low concentrations of ouabain that only inhibit α4 reduce sperm motility, whereas higher ouabain amounts, which also inhibit α1, do not further affect sperm movement (Woo et al, 2000, 2002; Sanchez et al, 2006; Jimenez et al, 2010). This specific role of α4 in sperm motility appears to depend on the ability of α4 to maintain low intracellular sodium ([Na+]i) levels and a negative membrane potential (Jimenez et al, 2010). The use of transgenic mice, in which the α4 isoform has been either overexpressed or knocked out, has confirmed the role of α4 in sperm motility and showed that this isoform is essential for male fertility (Jimenez et al, 2011a,b).
Although it is clear that adequate function of the Na,K-ATPase α4 isoform is important for sperm fertility, the regulation of α4 activity and the relevance of this event in the functional changes that accompany sperm capacitation are unknown. Sperm capacitation is characterized by an augmentation in motility and the appearance of hyperactivated motility, a particular pattern of sperm movement that is essential for fertilization (Suarez, 2008a). A series of ion transport systems have been shown to play an important role in generating the intracellular ion changes necessary to support sperm capacitation (Visconti et al, 2011). In this work, we show that α4 activity is up-regulated during sperm capacitation through stimulation of the molecular activity and a rise in levels of the α4 isoform at the sperm plasma membrane. In addition, we demonstrate that up-regulation of α4activity is important for the increase in sperm total and hyperactivated motility, helping to maintain the low intracellular Na+ levels and plasma membrane hyperpolarization of capacitated sperm. These results are the first demonstration for regulation of the α4isoform andits relevance for sperm capacitation.
Materials and Methods
Sperm Preparation
All experimental protocols involving animals used in this work were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee. Sprague Dawley rats were purchased from Harlan (Indianapolis, Indiana). Spermatozoa were obtained from the cauda of adult rat epididymides as previously described (Jimenez et al, 2010), in modified Tyrode medium containing: 95 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5.5 mM glucose, 0.27 mM pyruvic acid, 0.25 mM lactic acid, 40 mM Hepes, and 20 mM Tris (noncapacitating medium). Cells were counted with the help of a hemocytometer and used directly, or after processing, for the different assays. Sperm capacitation was obtained after incubation in the above medium, with the addition of 25 mM bicarbonate, 1.7 mM CaCl2, and 0.5% albumin (capacitating medium).
86Rb Uptake Assays
Transport activity of the Na,K-ATPase was determined in whole spermatozoa by measuring the ouabain-sensitive uptake of 86Rb, used as a tracer for K+ as previously described (Wagoner et al, 2005). Cells were incubated at a concentration of 47 × 107 cells per milliliter in noncapacitating or capacitating medium. At different incubation times, cells were transferred to the flux medium, where they became diluted to a concentration of 10 × 106 cells per milliliter. Flux medium contained 50 mM NaCl, 7 mM KCl, 25 mM Hepes (pH 7.4), 2.5 mM MgCl2, 1% bovine serum albumin, and 100 μM bumetanide, in the absence or presence of 1026 M ouabain. Bumetanide was added to inhibit the Na/K/2Cl cotransporter (NKCC1), which moves K+ into the sperm cytoplasm (Wertheimer et al, 2008). This allows the reduction of 86Rb uptake background, facilitating the assessment of Na,K-ATPase—mediated 86Rb transport (Borchgrevink and Ryan, 1988; Wagoner et al, 2005). After adding the cells to the flux medium, the uptake reaction was started by adding 1 μCi/mL 86Rb. After 30 minutes of incubation at 37°C, 100-μL aliquots containing 1 × 106 cells were removed and placed in Spin-X centrifuge tubes (Corning Inc, Corning, New York). Cells were washed with 0.5 mL of ice-cold 116 mM MgCl2 and a 30-second centrifugation at 10 000 ×g. Filters were excised from the tubes and placed in vials with scintillation fluid, and radioactivity was measured. Ion transport specific to the α4 isoform was determined as the difference in 86Rb uptake in the absence and presence of 10−6 M ouabain.
Na,K-ATPase Assays
Na,K-ATPase activity was assayed on sperm homogenates. For this, after incubation in noncapacitating or capacitating medium, sperm samples were sonicated using a Model 500 Sonic Demembranator (Fisher Scientific, Pittsburgh, Pennsylvania). Three cycles of sonication of 5 seconds each, separated by 30-second intervals, were used, maintaining the samples on ice at all times. Na,K-ATPase activity was determined by the initial rate of release of 32Pi from γ[32P]-ATP as described previously (Sanchez et al, 2006). The incubation medium (0.25 mL) contained 120 mM NaCl, 30 mM KCl, 3 mM MgCl2, 0.2 mM ethylene glycol tetraacetic acid, and 30 mM Tris-HCl (pH 7.4) ± 10−6 M ouabain. The assay was started by the addition of ATP with 0.2 μCi of γ[32P]-ATP (2 mM final concentration). Following 30 minutes of incubation at 37°C, the 32Pi-Pi released by the Na,K-ATPase reaction was complexed to molybdate in acidic medium by adding 5% ammonium molybdate in 4N SO4H2. The resulting phosphomolybdate, which is directly proportional to the phosphate present in the sample, formed, and it was extracted with isobutanol as described previously (Beauge and Campos, 1983). Radioactivity in 170 μL of the organic phase was measured by liquid scintillation counting. Enzymatic activity specific to the α4 isoform was determined as the hydrolysis of ATP sensitive to 10−6 M ouabain.
Ouabain-Binding Assay
The ouabain-binding capacity of spermatozoa was measured using the fluorescently labeled form of ouabain, bodipy-ouabain (Invitrogen, Carlsbad, California; Jimenez et al, 2011a). Sperm at a concentration of 2 × 106 cells per milliliter were placed in capacitating medium, and cells were incubated for various lengths of time at 37°C. Bodipy-ouabain (10−8M) was added to the cells during the last 20 minutes of incubation. Then, fluorescence was measured at an excitation/emission of 488/530 nm using an LSRII flow cytometer (BD Biosciences, San Jose, California). Gates were set for the side and forward light scatter parameters to exclude debris and cell clumps. A total of 30 000 spermatozoa were analyzed per sample. The generated data were then analyzed using the BD FacsDiva software (BD Biosciences).
Intracellular Na+ Measurements
Sperm [Na+]i was determined using the cell-permeant nonfluorescent precursor of Sodium Green Tetraacetate (Invitrogen) as described previously (Jimenez et al, 2010). Briefly, spermatozoa (7 × 106 cells per milliliter) were incubated at 37°C for different times in capacitating medium, in the absence or presence of 10−6 M ouabain. Then, 2.5 μM Sodium Green Tetraacetate was added and samples were further incubated for another 30 minutes at room temperature. Cells were washed twice by centrifugation at 300 × g for 5 minutes and suspended in 0.4 mL of fresh capacitating medium. Fluorescence of the samples was measured at an excitation/emission wavelength of 507/532 nm.
Sperm Viability Analysis
To monitor whether cells remained alive under the experimental conditions and fluorescent dyes used, we determined sperm viability. For this, cells were incubated in capacitating medium, and during the last 5 minutes of incubation with bodipy-ouabain or Sodium Green Tetraacetate, propidium iodide was added at a final concentration of 1.2 μM. Then, fluorescence of propidium iodide was determined at an excitation/emission of 488/575 nm by flow cytometry. These experiments showed that sperm remained viable under the different incubation times used, with an average relative viability of 99.7 ± 0.1 for bodipy-ouabain and 99.7 ± 0.07 for Sodium Green Tetraacetate.
Biotinylation Assays
To measure changes in the abundance of the α4 isoform at the sperm plasma membrane, sperm surface proteins were biotinylated and precipitated with streptavidin using the EZ-link Sulfo-NHS-Biotin kit from Thermo Fisher Scientific (Rockford, Illinois). A total of 2 ×106 cells per milliliter were incubated for various lengths of time at 37°C in noncapacitating or capacitating medium. Samples were then incubated with 1 mg/mL biotin for 1 hour at 4°C. Biotin was quenched with 100 mM glycine. Cells were lysed with buffer containing 1% NP-40, 0.25% sodium deoxycholate, 1 mM EDTA, 150 mM NaCl, 50 mM Tris, 1% Triton, and 0.1% sodium dodecyl sulfate (SDS). Cleared lysates were incubated overnight with streptavidin magnetic beads at 4°C. Lysates were then subjected to 8% SDS—polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. The α4 isoform was detected using a specific antiserum (Wagoner et al, 2005). Horseradish peroxidase—conjugated secondary antibodies and chemiluminescence were used for detection. Amounts of α4 were obtained after scanning and quantification of the autoradiographs for signal intensity using the Gel-Pro software (Media Cybernetics Inc, Silver Spring, Maryland).
Sperm Motility Assays
Approximately 3 ×106 cells in 300 μL of capacitation medium were incubated at 37°C for different lengths of time. Samples were treated without and with 10−6 M ouabain to selectively inhibit α4. Then, cells were labeled with 2 μLofa75 μM stock of SITO 21, a green fluorescent nucleic acid stain, which helps track cell movement. After 2 minutes of incubation with the dye, 7-μL aliquots from each sample were taken and placed into a glass cell chamber 20 μm in depth (Leja Products BV, Nieuw-Vennep, The Netherlands). Sperm total and hyperactive motility were determined as described previously (Jimenez et al, 2010) using computer-assisted sperm analysis (CASA). An average of 200 cells per field were captured at a rate of 30 frames per field, and a total of 10 fields in each sample were analyzed. The setup parameters included a cell identification area between 15 and 900 μm2, minimum motility speed corresponding to a straight line velocity of 5 μm/s and progressive motility at a straight line velocity of more than 20 μm/s. Total motility was measured as the average of the entire sperm population in the absence and presence of ouabain. Sperm hypermotility was calculated as previously described, by applying a bivariate analysis using curvilinear velocity (VCL) and linearity (LIN) as the parameters for vigor and progression, respectively (Cancel et al, 2000). Cutoff gates for these parameters were selected based on the distribution of CASA data in control samples at time zero. The cutoff levels for VCL and LIN were set at the 90th (179 μm/s) and 10th (35%) percentiles, respectively. The percent of hyperactivated spermatozoa was defined as the number of cells that exceeded the 90th percentile for VCL and fell below the 10th percentile for LIN, divided by the number of tracked cells, multiplied by 100.
Membrane Potential Assays
Membrane potential of the cells was determined using the fluorescent indicator [DiSC3(5)] (Invitrogen) as previously described (Jimenez et al, 2010). First, sperm samples containing 2 × 106 cells per milliliter were treated for various lengths of time at 37°C, with and without 10−6 M ouabain. Samples were incubated in capacitating media, and then DiSC3(5)atafinalconcentration of 1 μM was added and the samples were incubated at 37°Cforan additional 8 minutes. Sperm were further incubated for another 2 minutes with m-chlorophenyldrazone at a final concentration of 1 μM to collapse mitochondrial potential. After this time period, 2.5 mL of the suspension was transferred into a cuvette with continuous stirring and constant 37°C temperature, and fluorescence at an excitation/emission wavelength of 620/670 nm was recorded. Calibration and membrane potential calculations were performed as described previously (Jimenez et al, 2010).
Sperm Acrosomal Reaction Measurements
Sperm spontaneous acrosomal reaction was determined by following the decrease in acrosin levels in the cells by immunoblotting. A total of 15 × 106 spermatozoa were kept at 37°C for various times in either noncapacitated or capacitated medium. Samples were treated without or with 10−6 M ouabain. At the end of the incubation period, samples were washed twice in phosphate-buffered saline, and then cells were resuspended and lysed with buffer containing 1% NP-40, 0.25% sodium deoxycholate, 1 mM EDTA, 150 mM NaCl, and 50 mM Tris. Cell lysates were then subjected to 10% SDS-PAGE and immunoblotting. An anti-acrosin antibody from Santa Cruz Biotechnology (Santa Cruz, California) was used to identify acrosin in the samples. Horseradish peroxidase—conjugated secondary antibodies and chemiluminescence were used for detection. Acrosin levels were corrected for loading using the housekeeping protein γ-tubulin and were expressed in relative density units and as a ratio of the acrosin amounts of the cells incubated under noncapacitating conditions.
Sperm Protein Phosphorylation Determinations
Tyrosine phosphorylation of sperm proteins was used as an indicator of sperm capacitation. Sperm was capacitated for different lengths of time, collected by centrifugation, and lysed in phosphate-buffered saline buffer containing 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 1 mM orthovanadate, and 1 tablet per 10 mL of the phosphatase inhibitor mixture PhosSTOP (Roche, Indianapolis, Indiana). Then, samples were subjected to SDS-PAGE (10% gel) and immunoblotting. Blots were analyzed for levels of tyrosine-phosphorylated proteins using a specific anti-phosphotyrosine monoclonal antibody, 4G10 (Millipore, Billerica, Massachusetts) and horseradish peroxidase—conjugated secondary antibodies. The same immunoblots were stripped and reprobed with antibodies against α-tubulin. Autoradiographs were scanned and their intensity quantified. The levels of phosphorylated proteins in each sample were expressed as density units relative to the untreated controls and presented as phosphotyrosine-to-tubulin ratios.
Statistical Analysis
Experiments were repeated at least 3 times using a minimum of triplicate determinations. Statistical significance of the differences between samples was determined by Student's t test using Sigma Plot software (Jandel Scientific, San Rafael, California). Statistical significance was defined as P < .05.
Results
Ion Transport and Enzyme Activity of the α4 Isoform Increase During Sperm Capacitation
In a previous report, we determined the activity of the Na, K-ATPase α4 isoform in spermatozoa from several species (Blanco et al, 2000; Wagoner et al, 2005; Sanchez et al, 2006). However, this was performed under noncapacitating conditions and it is unknown whether activity of α4 is regulated to cope with the changes in motility that take place during sperm capacitation. To investigate this, we incubated sperm for different lengths of time in noncapacitating medium or in medium with the addition of bicarbonate, bovine serum albumin, and calcium, which are known to support sperm capacitation (Hernandez-Gonzalez et al, 2006), and we determined the ion transport activity of α4. We measured the uptake of 86Rb by the cells, which is a commonly used indicator of K+ transport through the Na,K-ATPase (Kaplan, 2002). To distinguish the 86Rb uptake of α4 from that of the α1 isoform, we selectively inhibited α4 activity with 10−6 M ouabain, an amount of ouabain that completely blocks the function of α4 but does not affect α1 activity. As shown in Figure 1A, 86Rb uptake dependent on the α4 isoform increased with sperm capacitation in a time-dependent manner (Figure 1A). The 86Rb uptake of cells incubated in capacitating medium at time zero was similar to that of cells incubated under noncapacitating conditions, and no significant changes in 86Rb uptake were observed during incubation of the cells in noncapacitating medium (Figure 1A).
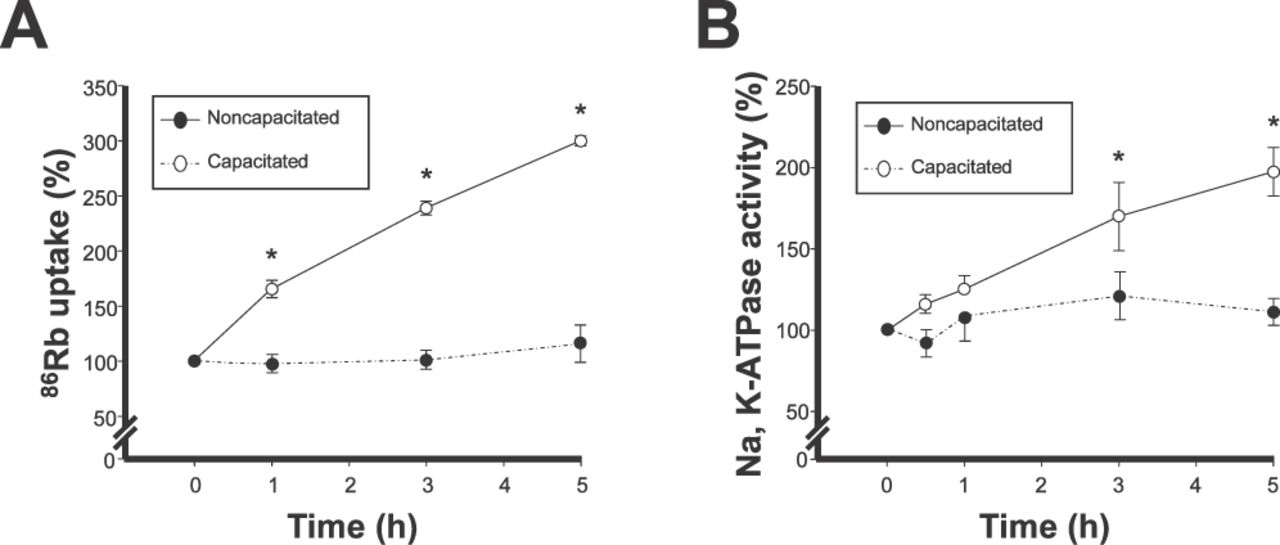
Ion transport and enzymatic activity of the α4 isoform increase during sperm capacitation. (A) 86Rb uptake (used as a tracer for K+) and (B) Na,K-ATPase activity were measured after incubation of caudal epididymal rat sperm in noncapacitating or capacitating medium for the indicated times, on intact sperm, or in homogenates of the cells, respectively. Specific ion transport and hydrolysis of ATP by α4 was measured as that sensitive to 10−6 M ouabain. Values are expressed as a percentage of values obtained at the corresponding zero capacitation time. Symbols are the mean ± SEM of 3 determinations performed in sextuplicate. Asterisks indicate statistical significant differences compared to zero time, with P < .05.
Another characteristic of the Na,K-ATPase is to function as an enzyme, which catalyzes the Na+ and K+ dependent hydrolysis of ATP. We explored whether the function of α4 changes during capacitation by measuring its enzymatic activity. For this, spermatozoa were capacitated for various lengths of time and homogenized, and Na,K-ATPase activity was measured. Specific activity of α4 was determined as the hydrolysis of ATP sensitive to 10−6 M ouabain. As shown in Figure 1B, activity of α4 increased with incubation of the cells in capacitating medium in a time-dependent manner, paralleling the changes observed in ion transport. In contrast, the Na,K-ATPase activity of α4 in sperm incubated in noncapacitating medium remained relatively constant and was not significantly different from that obtained at time zero with capacitating medium (Figure 1B). Together, these results indicate that the α4 isoform is subjected to regulation and that its activity, reflected by its ion transport and enzymatic capabilities, is stimulated as sperm become capacitated.
Amounts of Active α4 Increase at the Sperm Plasma Membrane With Capacitation
Previous studies in somatic cells showed that regulation of the Na,K-ATPase ion transport depends on modification in activity of the transporters already present at the plasma membrane, or on increases in transport molecules at the cell surface, due to their translocation from intracellular stores (Ewart and Klip, 1995; Therien and Blostein, 2000). Our results show that α4 ion transport and enzymatic activity are stimulated during sperm capacitation (Figure 1), suggesting that up-regulation of α4 activity at the plasma membrane of the cells is the mechanism responsible for the effect. However, it is unknown whether mobilization of α4 from intracellular compartments to the plasma membrane represents another way to up-regulate α4 activity in sperm. We explored this by measuring the ability of sperm to bind bodipy-ouabain. Because this fluorescent derivative of ouabain is unable to cross the cell plasma membrane and binds to extracellular sites on the Na, K-ATPase α subunit in a 1:1 stoichiometry, it represents an adequate tool to measure the amounts of α4 isoform at the cell surface. To ensure specific identification of the α4 isoform, bodipy-ouabain was used at a relatively low concentration (10−8 M), which only allows binding to α4 and not to the low—ouabain-affinity α1 isoform. As shown in Figure 2A, binding of the fluorescent ouabain derivative increases in sperm with capacitation time. Figure 2B shows the side and forward scatter dot plot, andFigure 2C depicts the histogram for bodipyouabain fluorescence obtained after 30 minutes and 6 hours of incubation in capacitating medium. These data suggest that sperm capacitation is accompanied by an increase in active Na,K-ATPase sites that can bind ouabain at the cell plasma membrane.
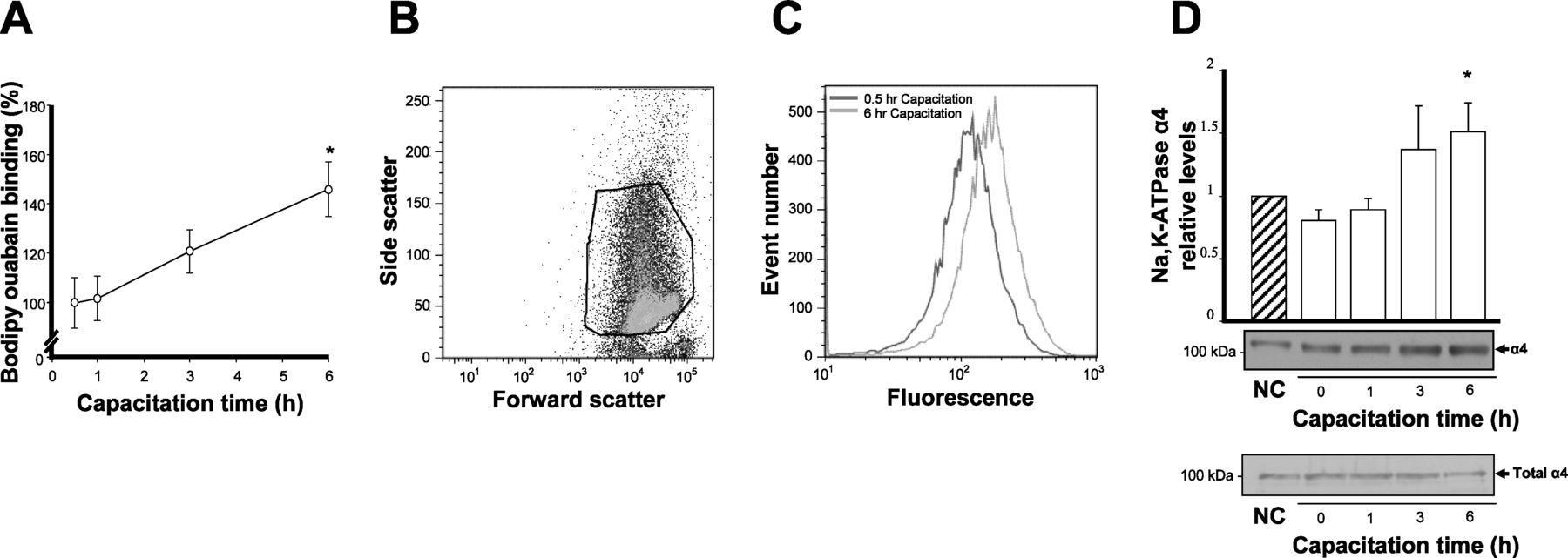
Levels of active Na,K-ATPase α4 molecules increase at the plasma membrane with sperm capacitation. (A, B, C) Ouabain-binding assays. Rat caudal epididymal sperm was capacitated for the indicated times and labeled with bodipy-ouabain. Cells were then subjected to flow cytometry and levels of fluorescence were determined (A). Symbols represent the mean ± SEM of 5 experiments, expressed as percent of the earliest capacitation time point, 30 minutes, which corresponded to the time needed for bodipy-ouabain to bind. A representative dot plot for the forward and side light scatter, and the fluorescence intensity histogram for bodipy-ouabain—labeled events for sperm incubated in capacitating medium for 30 minutes and 6 hours are shown (B, C). (D) Biotinylation/streptavidin assays. Rat caudal epididymal sperm were biotinylated and lysed, and cell membrane proteins were precipitated with streptavidin. Levels of α4 in the samples were quantified by densitometry of the immunoblots. Bars correspond to cells incubated in noncapacitating (NC; striped bars) or capacitating (open bars) media. Values are expressed as percent of the noncapacitated controls and represent the mean ± SEM of 6 experiments. Values significantly different from control are indicated with an asterisk (P < .05). Representative immunoblots for the biotin and streptavidin—precipitated α4 and the total α4 present in sperm before precipitation for the corresponding noncapacitated and capacitated samples are shown at the bottom of the graph.
As another approach to estimate whether changes in α4 amounts are occurring, we determined α4 levels at the sperm surface at different capacitation times using immunoblotting of sperm plasma membrane proteins isolated by biotinylation and streptavidin precipitation. As expected, α4was found in the plasma membrane of both noncapacitated and capacitated sperm. The levels of α4 in noncapacitated sperm were similar to those found at early time points during capacitation; however, as capacitation time progressed, an augmentation in α4 amounts was found (Figure 2D). Although α4 increased at the plasma membrane, total α4 protein content in the whole-sperm homogenates before biotin and streptavidin precipitation remained unchanged (Figure 2D). Taken together, bodipy-ouabain and biotin/streptavidin precipitation experiments suggest that sperm capacitation results in an increase in Na,K-ATPase α4 molecules at the surface of the male gamete.
Activity of the α4 Isoform Is Important for the Changes in Sperm Motility That Accompany Capacitation
The α4 isoform has been shown to be important for the motility of spermatozoa in noncapacitated medium (Woo et al, 2000, 2002; Sanchez et al, 2006). To determine whether the α4 isoform also plays a role in the motility changes that sperm undergo during capacitation, we incubated sperm in capacitating medium for different lengths of time and up to 5 hours, and we measured sperm motility using CASA. To distinguish the function of the α4 from the α1 isoform, we selectively inhibited activity of α4 with 10−6 M ouabain. As shown in Figure 3A, sperm that had not been treated with ouabain showed a time-dependent increase in total motility with capacitation. In contrast, in sperm in which activity of the α4 isoform was specifically inhibited, the capacitationdependent augmentation in sperm total motility was prevented, and even decreased. In addition, incubation in capacitating medium in the absence of ouabain resulted in an increase in sperm hyperactivated motility, as determined by the bivariate analysis of VCL and LIN (Cancel et al, 2000). This is presented in Figure 3B, which shows an increase in the number of cells with higher VCL and lower LIN during capacitation time (notice the shift of the data at time 0 [black dots] and after 4 hours [red dots] of incubation in capacitation medium). These changes in motility pattern indicated that in the absence of ouabain, approximately 25% of the total population of sperm achieved the hyperactivated pattern of motility at 4 hours (Figure 3C, solid line, open circles), and that ouabain inhibition of the α4 isoform prevented sperm hyperactivation (Figure 3C, dotted line, filled squares). These results suggest a central role for the α4 isoform in the motility changes that take place during sperm capacitation.
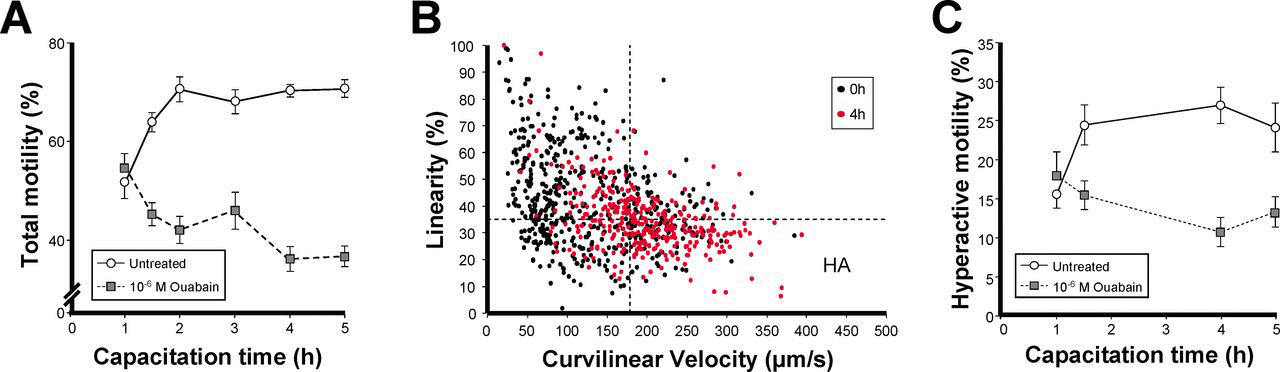
Activity of the α4 isoform is necessary for the motility changes associated with sperm capacitation. Rat caudal epididymal sperm was capacitated for the indicated times and up to 5 hours, and was treated without or with 10−6 M ouabain. Sperm motility was measured using computer-assisted sperm analysis (CASA). (A) Effect of ouabain inhibition of the α4 isoform on sperm total motility. (B)Bivariate analysis of curvilinear velocity (VCL) vs linearity (LIN) as an indicator of hyperactivated sperm. Black circles represent CASA sperm values at the beginning of the experiment (0 hours) and red circles are CASA sperm values after 4 hours of incubation, both in the absence of ouabain. Lines represent the 90th or the 10th percentile cutoff points for VCL and LIN, respectively. Hyperactivated sperm (HA), which exceeded the cutoff of 179 μm/s for VCL and fell below the cutoff of 35% for LIN, is shown in the lower right quadrant. (C) Effect of ouabain inhibition of the α4 isoform on sperm hyperactivated motility over capacitation time. Percent of hyperactivated sperm was calculated from the bivariate analysis for each sample in the absence and presence of ouabain and expressed over time. (A, C) Open circles represent untreated cells and filled squares indicate cells treated with 10−6 M ouabain. Values are the mean ± SEM of 3 experiments.
Activity of α4 Isoform Is Necessary for the Changes in Sperm Intracellular Sodium and Membrane Hyperpolarization During Capacitation
Sperm motility requires the cells to have an appropriate transmembrane Na+ gradient and negative membrane potential. We previously showed that ion transport catalyzed by the Na,K-ATPase α4 isoform maintains those parameters under noncapacitated conditions (Jimenez et al, 2010). During capacitation, spermatozoa undergo a decrease in [Na+]iand become hyperpolarized (Hernandez-Gonzalez et al, 2006; Salicioni et al, 2007). To investigate whether the α4 isoform is a mechanism responsible for these effects, sperm were capacitated for different lengths of time, and [Na+]i and membrane potential were measured in the absence or presence of 10−6 M ouabain. Sodium Green Tetraacetate was used to determine [Na+]i. Sperm viability and motility assays showed that the cells were not affected by the sodium fluorescent dye or the experimental protocols employed (data not shown). This confirmed that the different treatments used did not significantly damage the cells. Figure 4A shows that [Na+]i decreased during capacitation in sperm that were incubated without ouabain, in a time-dependent manner (Figure 4A, white bars). In sperm treated with ouabain, [Na+]i also decreased with capacitation; however, inhibition of α4 partially prevented the progressive decline in [Na+]i in the cells (Figure 4A, gray bars). When membrane potential was measured, the typical hyperpolarization that accompanies sperm capacitation was observed in the cells that were not exposed to ouabain (Figure 4B). In contrast, inhibition of α4 with ouabain hindered the development of sperm plasma membrane hyperpolarization during capacitation (Figure 4B). These results show that activity of the α4 isoform plays an important role in the changes in [Na+]i and membrane potential that are linked to sperm capacitation.
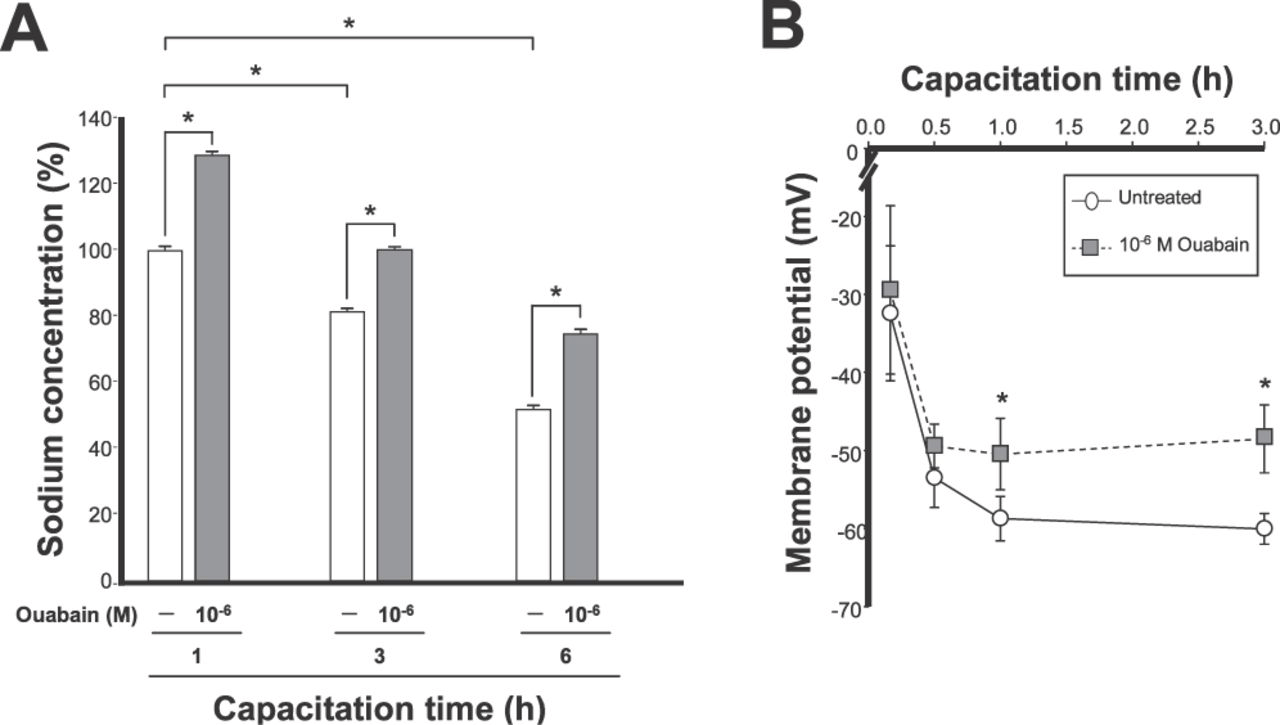
Activity of the α4 isoform is important for maintenance of sperm [Na+]i and membrane hyperpolarization during capacitation. Rat caudal epididymal spermatozoa were capacitated for the indicated times. Cells were treated in the absence and presence of ouabain for the last 30 minutes of incubation. (A) [Na+]i was determined using Sodium Green Tetraacetate. Values are expressed relative to the untreated control at 1 hour of capacitation. Bars correspond to untreated cells (open bars) or cells treated with 10−6 M ouabain (gray bars) and are the mean ± SEM of 3 experiments. Asterisks indicate values with statistically significant differences (P < .001). (B) Sperm membrane potential was determined using the fluorescent indicator [DiSC3(5)]. Symbols represent untreated cells (open circles) or cells treated with 10−6 M ouabain (gray squares) and are the mean ± SEM of 6 experiments. Values significantly different from cells incubated in the absence of ouabain at the corresponding time point are indicated with an asterisk (P < .05).
Inhibition of Na,K-ATPase α4 Activity Does Not Affect Sperm Acrosomal Reaction
Because the α4 isoform has a role in the specific motility patterns that are associated with sperm capacitation, it was of interest to test whether α4 activity is involved in another event of capacitation, such as the acrosome reaction. During this process the contents of the acrosome are released to allow sperm penetration trough the egg zona (Abou-haila and Tulsiani, 2009). We determined the consequences of inhibition of the α4 isoform on the ability of sperm to spontaneously undergo acrosomal reaction upon capacitation in vitro. For this, we incubated sperm for different lengths of time in capacitating medium, treated them in the absence or presence of 10−6 M ouabain, and measured sperm acrosomal status by immunoblotting of cell lysates, following the presence of the acrosomal protein, acrosin. As shown in Figure 5A, the relative levels of acrosin in the samples not exposed to ouabain decreased over time, indicating that acrosomal reaction had proceeded and acrosin was lost from the cells over capacitation time. Acrosin levels also decreased during capacitation in the samples that were incubated with ouabain (Figure 5A). This lack of significant effect of ouabain on acrosin levels suggests that activation of the α4 isoform is not required for sperm to undergo acrosomal exocytosis. The development of the acrosomal reaction indicates that spermatozoa have achieved the capacitated state under our experimental conditions. To confirm this, we used another approach, determining the progression of protein tyrosine phosphorylation in the cells, which is an event closely associated with sperm capacitation (Visconti et al, 2011). As shown in Figure 5B, incubation in capacitating medium showed a time-dependent increase in sperm total protein phosphorylation. These results support the notion that capacitation was advancing in the cells under the conditions used in our assays.
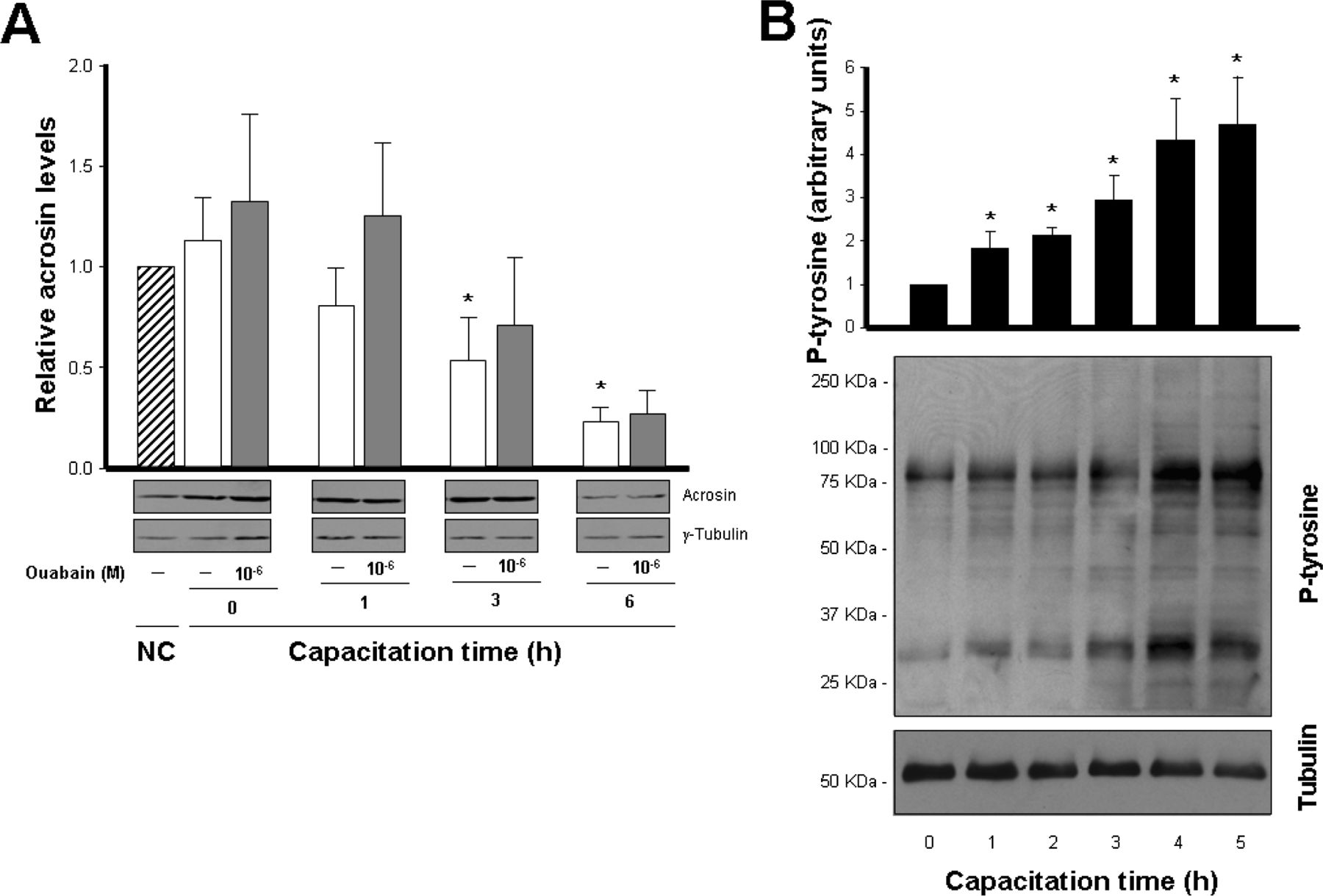
(A) Inhibition of α4 isoform activity does not affect spontaneous, capacitation-induced sperm acrosomal reaction. Caudal epididymal rat sperm were incubated in noncapacitating (NC; striped bars) or capacitating media for the indicated times, in the absence (open bars) or presence (gray bars) of 10−6 M ouabain. Cells were then lysed and acrosin levels were measured by immunoblotting. Data were corrected for loading by comparison with γ-tubulin and were expressed relative to the noncapacitated controls. Bars represent the mean ± SEM of 5 experiments. Values significantly different from the noncapacitated control are indicated with an asterisk (P < .05). Representative immunoblots for acrosin and γ-tubulin are shown. (B)Capacitation-dependent protein tyrosine phosphorylation in sperm. Cells were capacitated for the indicated times, collected, and lysed, and tyrosine protein phosphorylation was followed by immunoblotting. Data were expressed relative to the 0 time point and corrected for loading with α-tubulin. Bars represent the mean ± SEM of 3 experiments, and values significantly different from the 0 hour are indicated with an asterisk (P < .05). Representative immunoblots for phosphotyrosine proteins and tubulin are shown.
Discussion
In the present study, we show that ion transport and catalytic activity of the Na,K-ATPase α4 isoform is up-regulated during capacitation of rat sperm. These results represent the first evidence for the regulation of the Na,K-ATPase α4 isoform. The correlation of stimulation of α4 activity with the acquisition of a spermcapacitated state suggests the relevance of this Na, K-ATPase modulatory mechanism in sperm physiology. Both ATP hydrolysis and 86Rb uptake catalyzed by α4 increase during sperm capacitation, and whereas ATP hydrolysis rises approximately 2-fold, 86Rb uptake increases 3-fold. This quantitative difference in activation between enzymatic and ion transport may reflect dissimilarities in the way each parameter is measured, or in the nature of the samples being tested (ie, whole cells vs cell homogenates). In any case, the level of α4 activation represents a significant stimulatory effect compared with the Na,K-ATPase of other cell types (Blanco, 2005).
Our results also provide insight into the mechanisms by which the α4 isoform is regulated. The increase in catalytic activity of the Na,K-ATPase is known to depend on a rise in the total amount of Na,K-ATPase molecules, or in a stimulation of the molecular activity of preexisting enzyme units (Ewart and Klip, 1995; Therien and Blostein, 2000). Spermatozoa are terminally differentiated cells, which are translationally quiescent and do not synthesize new protein. In this manner, the enhancement of α4 activity in sperm cannot be due to the addition of newly synthesized Na,K-ATPase, but rather must depend on up-regulation of the molecular activity of α4 polypeptides already present. Our data support this possibility, because the increase in α4 activity is accompanied by constant expression levels of α4 in whole-cell homogenates from sperm incubated at different capacitation times (Figure 2D). However, although α4 levels remain unchanged at the cellular level, our results also show that sperm capacitation causes an increase in the amounts of α4 at the sperm plasma membrane. The higher level of α4 at the surface of the cells was confirmed by two independent methods—binding of bodipyouabain and immunoblotting of biotinylated and streptavidin-precipitated membrane proteins, both of which specifically allow quantification of α4 at the sperm surface. Moreover, ouabain binding to the Na,K-ATPase only takes place on catalytically active Na,K-ATPase (Kaplan, 2002), which demonstrates that the recruitment of α4 molecules at the plasma membrane corresponds to functionally competent Na,K-ATPase units. Agreeing with an activation of α4 at the sperm plasma membrane is the increase in 86Rb uptake we observed in the cells during capacitation, which only measures activity of the α4 isoform located at the plasma membrane. This stimulation of α4 activity at the plasma membrane is important, because it is the Na,K-ATPase at the cell surface that is physiologically relevant for the function of the cells.
Because expression of α4 protein does not change during sperm capacitation, the augmentation in α4at the plasma membrane must be the result of incorporation of α4 molecules that were previously present in other sperm cytoplasmic compartments into the plasma membrane. This translocation of Na,K-ATPase molecules to the cell surface is not unique to sperm and has been previously shown for the Na,K-ATPase α isoforms of somatic cells. Thus, in skeletal muscle cells, the quantity of α2 isoform at the plasma membrane is dynamically controlled through hormonal modulation via translocation mechanisms that target and retrieve α2 to or from the plasma membrane from cytoplasmic pools (Ewart and Klip, 1995; Therien and Blostein, 2000).
In muscle cells, the intracellular pool of Na,K-ATPase has been identified as the sarcoplasmic reticulum subjacent to the plasma membrane. At present, an ultrastructural analysis of the distribution of the α4 isoform inside the sperm has not yet been performed. Undoubtedly, additional experiments are required to ascertain the location of the putative reservoirs of the α4 isoform in the sperm cytoplasm, as well as the molecular mechanisms involved in the translocation of α4 from intracellular cell compartments. The capacitation-dependent changes in α4 we observe at the sperm surface agree with experimental evidence from other investigators who have shown that capacitation is characterized by rearrangement of various proteins at the sperm surface (Myles et al, 1987; Jones et al, 1990; Sleight et al, 2005; Boerke et al, 2008). Interestingly, among the proteins found to be redistributed during sperm capacitation is another membrane transporter, the facilitated glucose transporter (Bucci et al, 2010). Besides protein translocation, an alternative mechanism to explain the increase in α4 at the plasma membrane after sperm capacitation could be the unmasking of α4 molecules that are already present at the sperm surface. In this manner, silent α4 isoforms might become active, or available to bodipy-ouabain or the anti-α4 antibody used for detection after changes in association with other proteins, or modification in plasma membrane lipid fluidity, which have been reported to occur during capacitation (Fawcett, 1975). Although further experiments will clarify these possibilities, our data show that activity of the α4 isoform is modulated during sperm capacitation through a combination of both an increase in the number of active α4 molecules available at the sperm surface and a rise in α4 molecular activity.
Previous results have shown that α4 plays an essential role in sperm fertility (Jimenez et al, 2010, 2011a). The present data further indicate that α4 is important for sperm capacitation. Thus, ouabain inhibition of α4 blocked the increase in total motility and the typical high-amplitude and asymmetrical flagellar beat that characterize sperm hyperactivation. Hyperactive motility is essential for the release of sperm from its transient reservoir in the uterine tube before reaching the oocyte, and for the ability of sperm to penetrate the egg zona (Suarez, 2008a,b). Therefore, by helping support hyperactivated motility, α4 may be involved in those events that are essential for sperm fertility. It is important to note that although inhibition of α4 activity coincides with reduction of sperm hyperactivated motility, activation of the α4 isoform may not necessarily cause an increase in hyperactivated motility. Although the present experiments cannot establish a causal relationship between these two events, it appears that sperm hyperactivated motility cannot be maintained without an increase in Na,K-ATPase α4 isoform activity. Our data also show that inhibition of activity of the α4 isoform partially prevents the changes in membrane potential that occur during sperm capacitation. Previous results from our laboratory indicated that the plasma membrane potential of sperm from mice null in the α4 isoform is completely depolarized (Jimenez et al, 2011a). The lower effect on depolarization obtained by ouabain treatment in the current study is unclear, but it may reflect the more drastic effect that the genetic lack of α4 has on membrane potential, compared with the acute inhibition that is achieved with ouabain. Although the pharmacologic and genetic approaches differ in magnitude, they both show that the α4 isoform plays a role in maintaining the membrane potential of spermatozoa. Sperm resting membrane potential and the capacitation-dependent hyperpolarization of sperm are primarily due to an outwardly rectifying K+ current (IKsper), which is mediated by the sperm-specific K+ channel, Slo3 or KSper (Schreiber et al, 1998; Navarro et al, 2007; Santi et al, 2010; Zeng et al, 2011; Lishko et al, 2012). Although the major player responsible for sperm membrane potential is the sperm K+ conductance, our results show that the increase in Na,K-ATPase α4 isoform activity is partially responsible for the changes in membrane potential that occur during capacitation and contributes to the capacitation-induced sperm membrane hyperpolarization. Hyperpolarization of the sperm plasma membrane has been shown to be directly associated with sperm hyperactivation (Darszon et al, 2007; Bailey, 2010). Therefore, up-regulation of α4 activity is a mechanism that sperm use to aid in the capacitation-dependent changes in membrane excitability that are necessary to support hyperactivation.
Experimental evidence from this work and our previous results (Jimenez et al, 2010) demonstrate that α4activity also helps in maintaining the low [Na+]i in the cells and that up-regulation of α4 function further decreases [Na+]i during capacitation. A capacitation-dependent decrease in [Na+]i has also been reported in mouse sperm. This effect has been shown to be secondary to inhibition of the epithelial sodium channel, ENaC (Hernandez-Gonzalez et al, 2006). Our data indicate that besides ENaC inhibition, sperm capacitation also requires the function of the Na,K-ATPase α4 isoform to lower [Na+]i. The role of ENaC and its inhibition may also provide an explanation for the incomplete recovery in [Na+]i,back to noncapacitated basal levels, which we observe after inhibition of α4 with ouabain (Figure 4A). In this manner, it appears that the actions of ENaC reducing Na+ entrance into sperm, together with α4facilitatingNa+ movement from the cells, both collaborate in maintaining the high transmembrane Na+ gradient that is required for sperm capacitation. We have previously shown that maintenance of the Na+ gradient and membrane hyperpolarization are mechanisms through which α4 influences sperm motility (Jimenez et al, 2010). Our current data further indicate the importance of α4in sustainingthe motility changes of capacitated sperm, which contributes at least in part to the infertility of male mice null in α4 (Jimenez et al, 2011a).
In contrast with its effects on sperm motility, we find that activity of α4 is not directly involved in sperm spontaneous acrosomal reaction. Thus, ouabain inhibition of α4 does not significantly affect progression of the acrosomal exocytosis that takes place under our capacitation conditions. This agrees with our previous findings, which showed that overexpression of the α4 isoform in transgenic mice does not significantly influence acrosomal reaction (Jimenez et al, 2011b). Therefore, it appears that the α4 isoform is involved in some aspects of sperm capacitation, such as the regulation of sperm motility, but not in others. This is in accordance with the main localization of this isoform in the mid piece of the sperm flagellum (Blanco et al, 2000; Wagoner et al, 2005; Sanchez et al, 2006).
In conclusion, our results show that spermatozoa rely on regulation of activity of the Na,K-ATPase α4 isoform to help support the [Na+]i, membrane potential, motility changes, and hyperactivation that take place during sperm capacitation, all of which are essential for the fertility of the male gamete.




