Artificial Insemination With Seminal Plasma Improves the Reproductive Performance of Frozen-Thawed Boar Epididymal Spermatozoa
Supported by the Programme for Promotion of Basic and Applied Researches for Innovations in Bio-Oriented Industry and JST grant 12–068 and 12–104.
Abstract
Abstract: Frozen-thawed epididymal spermatozoa have good fertilization capability in vitro; however, their artificial insemination conception rate is less than half of that of frozen-thawed ejaculated spermatozoa. Because the addition of seminal plasma to the thawing solution enhances the in vivo fertilizing ability of frozen-thawed ejaculated spermatozoa, we hypothesized that the reproductive performance of frozen-thawed epididymal spermatozoa could also be improved by the inclusion of seminal plasma. When frozenthawed epididymal spermatozoa were incubated for up to 6 hours, the motility of the sperm significantly decreased in a time-dependent manner. The acrosomal membrane was damaged in the majority of frozen-thawed epididymal spermatozoa. The addition of seminal plasma to the thawing solution significantly decreased the percentage of sperm with abnormal acrosomes and increased their total motility in a dose-dependent manner. Furthermore, the addition of seminal plasma reduced the abundance of a 15-kDa tyrosinephosphorylated protein in frozen-thawed sperm, and the maximum effect was observed at 15% (vol/vol) seminal plasma. When cryopreserved epididymal spermatozoa from 3 different boars were thawed with a 15% (vol/vol) seminal plasma-containing solution, the conception rate and mean litter size obtained by artificial insemination were significantly increased as compared with those in the control without seminal plasma. From these results, we concluded that the addition of seminal plasma to the thawing solution is a key step in obtaining an optimal number of piglets by artificial insemination using frozen-thawed boar epididymal spermatozoa.
Cryopreservation of boar spermatozoa offers an effective means of long-term storage of important genetic material. Many researchers have focused on increasing reproductive performance using artificial insemination (AI) with cryopreserved boar spermatozoa (Roca et al, 2003; Sancho et al, 2007; Flores et al, 2008; Casas et al, 2009; Rath et al, 2009). Recently, we and other groups reported a high conception rate (70%–80%) by AI with frozen-thawed spermatozoa using a modification of the cryopreservation temperature program (Fiser and Fairfull, 1990; Medrano et al, 2002; Kumar et al, 2003), novel cryopreservation extender (Gutiérrez-Pérez et al, 2009; Okazaki et al, 2009a, 2010), or the development of a sperm infusion method (Roca et al, 2003, 2006). Although the best success rates with AI have been recorded in research centers, data from field studies that apply these techniques in commercial settings have also been accumulating (Eriksson et al, 2002).
Serious incidents involving boars with excellent genotypes have occurred; for example, boars have suddenly died or have been injured, making it difficult for semen to be collected. In these cases, collecting the epididymides and then cryopreserving the epididymal spermatozoa has proven to be an effective means to avoid losing the genetic material. It is known that epididymal spermatozoa have a high freezability compared with that of ejaculated spermatozoa (Rath and Niemann, 1997), if motility is used as the marker of sperm quality. Additionally, frozen-thawed sperm show a good response to capacitation inducers such as caffeine or bovine serum albumin (Funahashi et al, 2000; Matás et al, 2010), and a high in vitro fertilization rate has been obtained (Nagai et al, 1994; Kikuchi et al, 1998). However, fertilization rate, conception rate, and litter size after AI using frozen-thawed epididymal spermatozoa are lower than those of frozen-thawed ejaculated spermatozoa (Holtz and Smidt, 1976; Kikuchi et al, 1999; Heise et al, 2010). The reason for the low reproductive performance of frozen-thawed epididymal spermatozoa remains unknown.
In our previous study, the addition of seminal plasma to the thawing solution improved membrane and acrosomal integrity and enhanced the in vivo fertilization capability of ejaculated spermatozoa (Okazaki et al, 2009b). One of the beneficial effects of seminal plasma is that it suppresses phosphorylation of protein tyrosine residues because of decreased calcium uptake during the thawing process (Okazaki et al, 2011). It is known that the phosphorylation of protein tyrosine residues is induced by freezing and/or thawing stress and results in the injury of plasma or acrosomal membranes and reduces the life span of spermatozoa (Watson, 2000; Barbas and Mascarenhas, 2009). This protein phosphorylation—induced sperm damage has been referred to as a spontaneous capacitation-like reaction and causes poor reproductive performance after AI (Ashworth et al, 1994; Watson, 1995, 2000). Therefore, because epididymal spermatozoa showed high motility just after thawing but were susceptible to having increased phosphorylation of protein tyrosine residues, we hypothesized that the addition of seminal plasma to the thawing solution would suppress the phosphorylation of tyrosine residues and preserve long-term sperm quality. We also predicted that the addition of seminal plasma to the thawing extender would improve the reproductive performance of frozen-thawed epididymal spermatozoa after AI, as it did that of frozen-thawed ejaculated spermatozoa as shown in our previous study (Okazaki et al, 2009b). Finally, we postulated that a much higher dose of seminal plasma would be required to maintain sperm function than the dose used with ejaculated sperm in our previous study (10% seminal plasma in thawing solution) (Okazaki et al, 2009b) because epididymal spermatozoa have not been exposed to seminal plasma before freezing.
In this study, we first examined the freezability of epididymal spermatozoa by comparing them with ejaculated spermatozoa from the same boars. The characteristics of epididymal spermatozoa such as phosphorylation of tyrosine residues and acrosomal status after thawing were also examined. In the second experiment, to determine the potential positive effects of seminal plasma during the thawing process of frozen epididymal spermatozoa, we added various concentrations (0, 10%, 15%, and 20% [vol/vol]) of seminal plasma to the thawing solution and analyzed sperm functions, including performance in AI. Based on the data, we proposed an optimal concentration of seminal plasma to be added to the thawing solution when frozen epididymal spermatozoa are to be used for AI.
Materials and Methods
Media
Routine chemicals and reagents were obtained from Wako Pure Chemical Industries Ltd (Osaka, Japan) and Sigma Chemical Co (St Louis, Missouri).
The Modena solution (Funahashi and Sano, 2005), containing 0.15 M d-glucose, 26.7 mM trisodium citrate, 11.9 mM sodium hydrogen carbonate, 15.1 mM citric acid, 6.3 mM EDTA-2Na, 46.6 mM Tris, 1000 IU/mL penicillin G potassium (Meiji Seika, Tokyo, Japan), and 1 μg/mL amikamycin (Meiji Seika), was used as the pretreatment solution (before sperm cooling). The thawing solution was also based on the Modena solution, supplemented with seminal plasma. Niwa and Sasaki freezing extender 1 (NSF1) that consisted of 80% (vol/vol) 0.31 mol/L lactose monohydrate, 20% (vol/vol) egg yolk, 1000 U/mL penicillin G potassium, and 1 mg/mL streptomycin sulfate was used as a freezing extender in this study (Kikuchi et al, 1999). The osmolality of NSF1 was modified to 400 mOsm/kg (the osmolality of the original NSF1 was 300 mOsm/kg), as in our previous study (Okazaki et al, 2009a), and the modified NSF1 was termed as mNSF1. The second dilution (mNSF2) consisted of mNSF1 supplemented with Orvus ES paste (Miyazaki Chemical Sales Ltd, Tokyo, Japan) and glycerol (final concentrations, 0.75% and 2% [vol/vol], respectively), which were added at 5°C before freezing.
To obtain seminal plasma, semen from highly fertile boars (5 boars; more than 80% conception rate with fresh semen) was collected and centrifuged at 700 × g for 10 minutes to remove spermatozoa. The supernatant was further centrifuged at 1500 × g for 30 minutes to completely remove sperm. The seminal plasma was pooled, mixed, and then stored at 280°C. The thawed seminal plasma was added to the thawing solution just before use.
Collection of Ejaculated and Epididymal Sperm
Twelve mature Landrace, Large White, and Duroc boars, bred at Oita Prefectural Agriculture, Forestry and Fisheries Research Center, and aged 15 to 36 months, were used in this study. The boars were housed in individual pens and were fed a commercial diet according to the guidelines for the nutritional requirements for adult boars and received water ad libitum. The Committee of Animal Experiments of Oita Prefectural Agriculture, Forestry and Fisheries Research Center reviewed our research plan. In ejaculated spermatozoa, the sperm-rich fraction was collected weekly from each boar using the gloved-hand technique and was filtered through double gauze. The boars were then slaughtered at a local slaughterhouse 7 to 14 days after having ejaculated, and the testes were collected. The cauda epididymis was flushed using Modena solution, and the collected spermatozoa were frozen as described below.
Sperm Freezing and Thawing Procedure
Collected spermatozoa were frozen as described in our previous study (Okazaki et al, 2009a). The ejaculated semen was directly diluted in the pretreatment solution (1:1, pretreatment solution: semen) and then cooled to 15°C. The harvested epididymal spermatozoa were diluted in pretreatment solution (1 × 109 sperm/mL) and then cooled to 15°C over 2 hours. The diluted sperm were centrifuged for 5 minutes at 800 × g to remove the pretreatment solution. The sperm were gently resuspended in mNSF1 (2 × 109 sperm/mL) and cooled slowly from 15°C to 5°C over 1.5 hours. The sperm suspension was diluted in an equal volume of mNSF2 (final concentration before freezing, 1 × 109 sperm/mL) and then transferred into a 0.5-mL plastic straw. The straws were placed in liquid nitrogen vapor for 10 minutes and finally stored in liquid nitrogen.
To thaw the frozen sperm, the straw was placed in a 60°C water bath for 8 seconds. The frozen-thawed spermatozoa were diluted with 4.5 mL of thawing solution to a final concentration of 1 × 108 sperm/mL.
Evaluation of Sperm Motility and Acrosomal Integrity
Percentages of total sperm motility were measured using a computer-assisted sperm motility analysis system (version 8.1; Hamilton Thorne Biosciences, Beverly, Massachusetts) (Okazaki et al, 2009a).
Sperm acrosomal status was analyzed using fluorescein isothiocyanate—peanut agglutinin as described in our previous study (Zeng et al, 2001). The fluorescent staining of sperm was monitored and photographed using an epifluorescence microscope (ECLIPSE 50i, D-FL; Nikon, Tokyo, Japan). More than 200 cells per slide were counted.
Analysis of Sperm Protein Tyrosine Phosphorylation by Western Blot
Frozen-thawed ejaculated or epididymal spermatozoa were lysed by homogenization in Laemmli sample buffer. The samples were heated to 100°C for 5 minutes, and 20 μL of each sperm extract was loaded in each lane (1 × 106 sperm/lane) of a 10% sodium dodecyl sulfate—polyacrylamide gel. The membranes were blocked in Tris-buffered saline with Tween-20 (TBST; 10 mmol/L Tris [pH 7.5], 150 mmol/L NaCl, and 0.05% Tween-20) containing 5% (wt/vol) bovine serum albumin (Sigma). Blots were incubated overnight at 4°C with an anti-phosphotyrosine antibody (P-Tyr-100, 1:5000; Cell Signaling Technology Inc, Danvers, Massachusetts) or anti–β-actin antibody (1:10 000; Sigma). After the blots were washed in TBST, detection was performed with enhanced chemiluminescence Western blot detection reagents (GE Healthcare, Buckinghamshire, United Kingdom), and the blots were exposed to x-ray film (FUJI FILM, Tokyo, Japan). Specific bands were quantified by densitometric analyses using a Gel-Pro analyzer (Media Cybernetics, Bethesda, Maryland). The experiments were repeated 3 times. A 15-kDa band was detected using the P-Tyr-100 antibody in boar frozen-thawed sperm, the presence of which has been correlated with poor sperm quality (Okazaki et al, 2009b).
AI Test
For the AI study, 32 weaned Landrace sows (aged 12–36 months; approximately 150–250 kg) were used. Estrus detection was performed twice per day (0900 and 1600 hours) by allowing the females nose-to-nose contact with a mature boar and by applying back pressure. Sows showing natural estrus were artificially inseminated (cervix insemination; conventional method) twice per estrus cycle using 50 mL of frozen-thawed epididymal spermatozoa (5 ×109 sperm/50 mL per insemination).
Conception was confirmed by ultrasound diagnosis (SUPER EYE, SSD-500; Aloka, Tokyo, Japan) on day 25 (day 0, day of AI). The conception rate was defined as the number of pregnancies divided by the total number of inseminated sows.
Experimental Design
Experiment 1—
To assess the freezability of epididymal spermatozoa, the total motility of frozen-thawed epididymal spermatozoa was compared with that of frozen-thawed ejaculated spermatozoa from the same boar. The percentage of total motility of frozen-thawed ejaculated and epididymal spermatozoa were measured in the same individual boars.
Experiment 2—
To determine the reason for low reproductive performance after AI using epididymal spermatozoa, the phosphorylation of protein tyrosine residues and acrosome damage in postthawed epididymal spermatozoa were studied. Tyrosine phosphorylation and acrosomal damage were detected by Western blotting or immunofluorescent staining as described earlier. Frozen-thawed ejaculated or epididymal sperm samples were collected following 1- or 3-hour incubation periods after thawing, and changes in protein phosphorylation were examined. For acrosomal damage study, both sperm samples (frozen-thawed ejaculated and epididymal sperm) were collected following a 3-hour incubation period after thawing.
Experiment 3.1—
The effects of adding seminal plasma (10%, 15%, or 20% [vol/vol]) to the thawing solution on the phosphorylation of tyrosine residues (1 and 3 hours), acrosomal damage (3 hours), and total motility of postthawed epididymal spermatozoa (1, 3, and 6 hours) incubated for up to 6 hours after thawing were investigated. After thawing, the spermatozoa were diluted in thawing solution with 10%, 15%, or 20% (vol/vol) seminal plasma and then incubated.
Experiment 3.2—
We examined whether the addition of seminal plasma during the cooling process could also have a beneficial effect on postthaw sperm motility. In the cryopreservation of ejaculated semen, an equal volume of diluent was added, resulting in a final seminal plasma concentration of 50% (vol/vol) (Okazaki et al, 2009a). We therefore tested the effect of the addition of 50% (vol/vol) seminal plasma during cooling on postthaw epididymal sperm total motility. Moreover, a concentration of 15% (vol/vol) seminal plasma, which showed positive effects on sperm functions during thawing in experiment 3.1, was also tested. The collected epididymal spermatozoa were incubated in pretreatment solution with (15% or 50% [vol/vol]) or without seminal plasma during cooling time (2 hours), and then the spermatozoa were frozen as described earlier. Frozen spermatozoa were thawed without addition of seminal plasma.
Experiment 4—
Finally, we performed AI using epididymal spermatozoa diluted with or without seminal plasma to determine whether the positive effects of adding seminal plasma to the thawing solution observed in vitro resulted in increased fertility in vivo.
Statistical Analysis
Data are presented as means ± SEM and were analyzed using 1-way or 2-way analysis of variance (ANOVA) (SAS Institute Inc, Cary, North Carolina). All percentage data were subjected to arcsine transformation before ANOVA. When ANOVA revealed a significant effect, the means were compared using the Fisher protected least significant difference post hoc test and were considered significant when P < .05. The conception rate was analyzed using the χ2 test.
Results
Comparative Analysis of Postthawed Ejaculated and Epididymal Spermatozoa
When the postthaw motility of sperm was examined in experiment 1, the total motility of the epididymis group was approximately 80% after a 1-hour incubation at 37°C. The motility was significantly higher in frozenthawed epididymal sperm than in ejaculated sperm after every incubation period (P < .05; Figure 1). There were no significant differences in total sperm motility among the 12 boars that were studied.
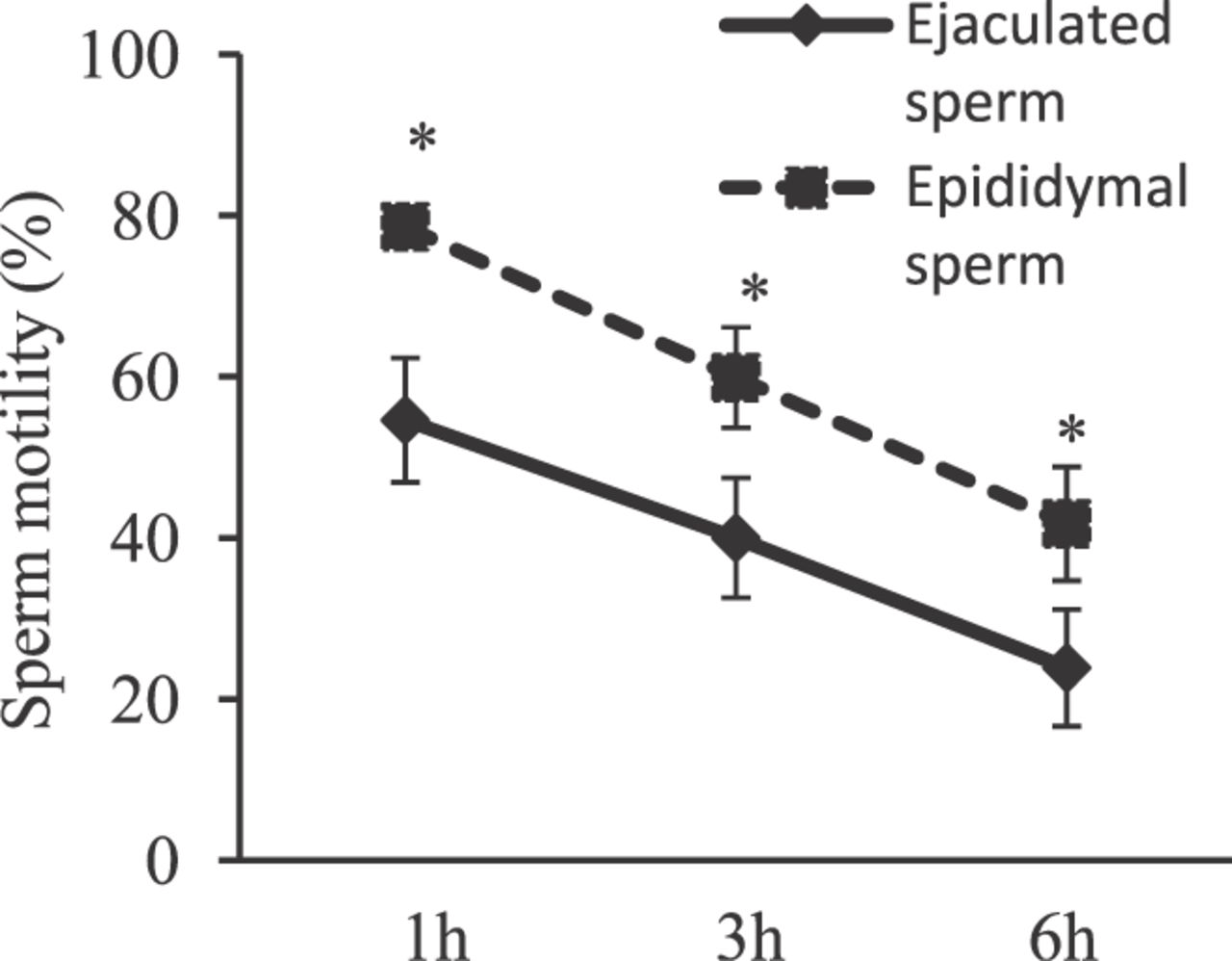
Comparative analysis of postthaw motility in ejaculated and epididymal spermatozoa. Ejaculated and epididymal spermatozoa were both collected from individual boars and then frozen as described in “Materials and Methods.” Postthaw sperm motility was analyzed by computer-assisted sperm motility analysis for up to 6 hours. Values are means ± SEM. Two-way analysis of variance showed that there was no significant difference in the total sperm motility among the 12 boars that were evaluated. However, a significant difference was detected between incubation times (* P< .05).
In the next experiment (experiment 2), to compare the properties of postthawed sperm, we analyzed the tyrosine phosphorylation status of sperm protein and acrosome damage in frozen-thawed sperm. A tyrosine phosphoprotein of approximately 15 kDa was strongly detected in epididymal spermatozoa at 1 hour after thawing and was maintained at 3 hours (Figure 2a). On the other hand, in ejaculated sperm, the same band was only weakly detected, even after 3 hours of incubation. Furthermore, the proportion of spermatozoa with acrosome damage in sperm incubated for 3 hours was significantly higher in epididymal spermatozoa (36.0% vs 15.2% in ejaculated sperm; P < .05; Figure 2b).
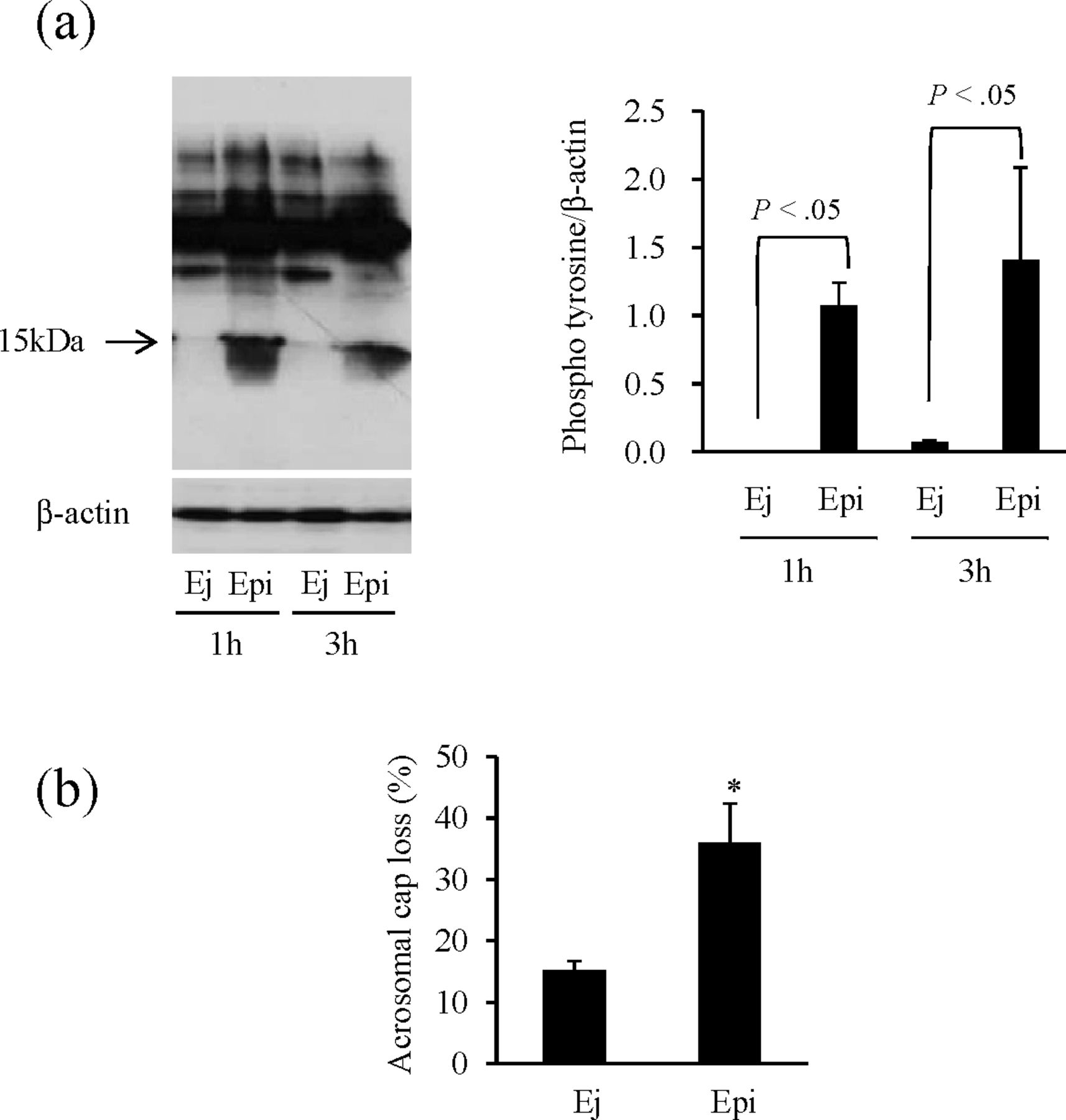
Comparative analysis of postthaw capacitation and acrosomal integrity in ejaculated and epididymal spermatozoa. Frozen-thawed ejaculated and epididymal spermatozoa collected from individual boars was incubated with Modena solution, and each sample was analyzed following a 1- or 3-hour incubation. Protein tyrosine phosphorylation and acrosomal integrity were analyzed by (a) Western blotting (1 and 3 hours) using an anti-phosphotyrosine antibody (P-Tyr-100) and (b) immunofluorescence (fluorescein isothiocyanate—peanut agglutinin; 3 hours), respectively. Values are means ± SEM calculated on the basis of 3 experiments. (a) A significant difference (P < .05) was detected among incubation times. (b) * shows a significant difference (P < .05). E indicates ejaculated spermatozoa; Epi, epididymal spermatozoa.
Effects of Adding Seminal Plasma During Thawing or Cooling Processes on Tyrosine Phosphorylation, Acrosomal Integrity, and Motility of Postthawed Epididymal Sperm
To investigate the effect of seminal plasma on postthaw phosphorylation of sperm protein tyrosine residues, various concentrations of seminal plasma (0, 10%, 15%, or 20% [vol/vol]) were added to the thawing solution (experiment 3.1). Whereas the 15-kDa tyrosine phosphoprotein was readily detected in epididymal sperm at 1 hour in the control (seminal plasma free; Figure 3a), its expression decreased dramatically with the addition of seminal plasma. In addition, a positive effect of seminal plasma on acrosome integrity was observed in a dose-dependent manner (Figure 3b). Although no effect of seminal plasma on total motility of postthawed epididymal spermatozoa was observed after a 1-hour incubation, the total motility of sperm treated with 15% (vol/vol) seminal plasma was significantly higher than that of sperm without seminal plasma by 3 hours after thawing (Figure 3c).
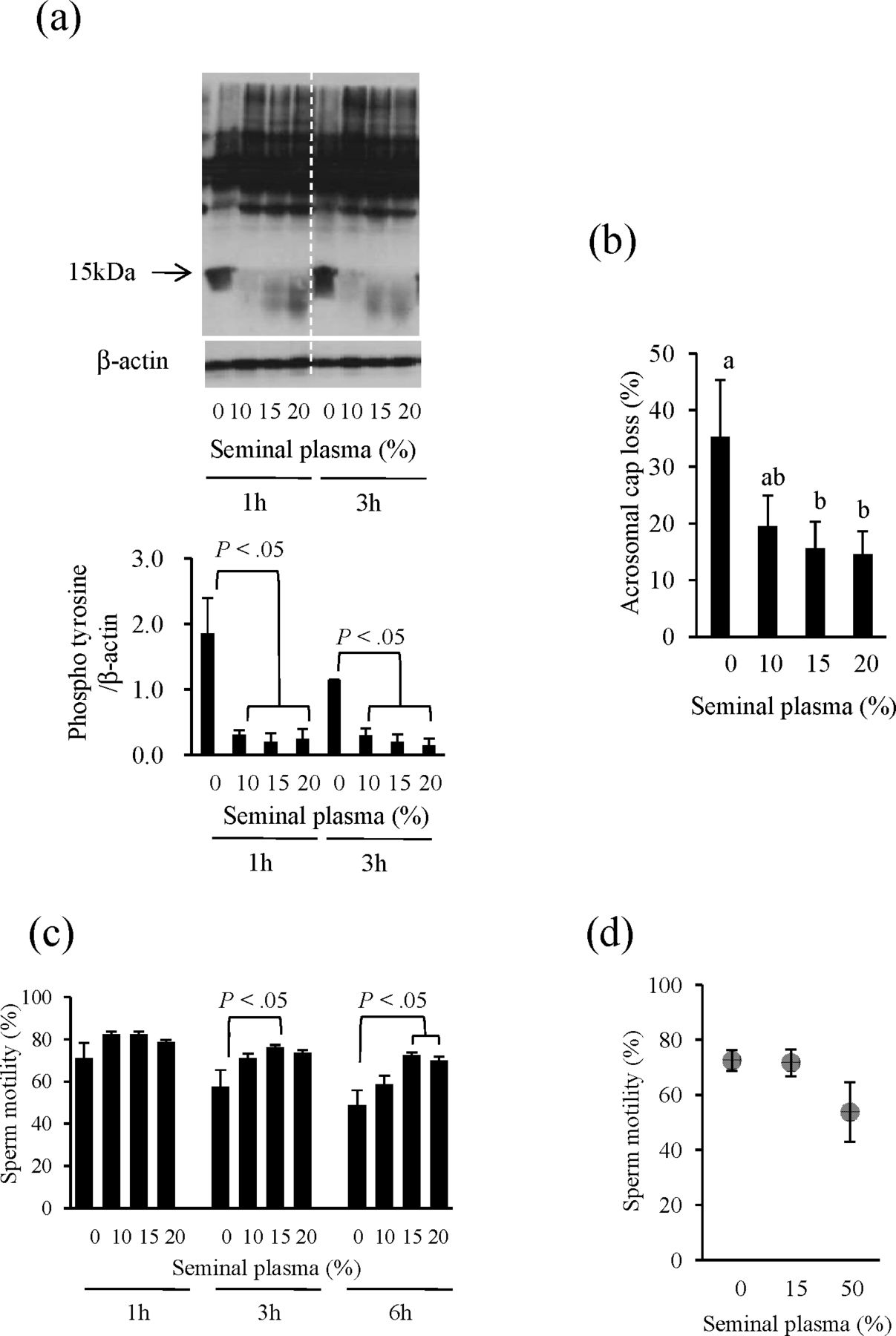
The effects of seminal plasma on early capacitation, acrosomal integrity, and motility of postthawed epididymal spermatozoa. To assess the effects of seminal plasma on postthaw epididymal sperm function, various concentrations of seminal plasma (0, 10%, 15%, or 20% [vol/vol]) were added to the thawing solution and then (a) the phosphorylation of tyrosine residues, (b) acrosomal status (3 hours), and (c) motility of the sperm were analyzed. (d) The effect of seminal plasma addition before freezing on postthaw sperm motility following a 3-hour incubation was also investigated. Values are means ± SEM calculated on the basis of 3 experiments. Significant difference (P < .05) is indicated with different letters.
On the other hand, the treatment of epididymal sperm with seminal plasma before freezing (experiment 3.2) did not produce the positive effects observed when it was added during thawing. When spermatozoa flushed from the epididymis were incubated with 15% or 50% (vol/vol) seminal plasma during cooling time (2 hours) and then subjected to the freezing process, the percentage of motile sperm after thawing without seminal plasma was not improved in the 15% treatment group and was lower in the 50% group as compared with the control (spermatozoa without seminal plasma treatment) (control vs 15% seminal plasma vs 50% seminal plasma, 72.5% vs 71.6% vs 53.8%, respectively; Figure 3d).
AI Performance of Frozen-Thawed Epididymal Spermatozoa
Finally, we investigated the effects of adding seminal plasma during the thawing process on reproductive performance using AI (experiment 4). Frozen epididymal spermatozoa were thawed either in a 15% (vol/vol) seminal plasma—containing solution or one without seminal plasma (control), and then 32 swine (control, 20; 15% [vol/vol] seminal plasma, 12) were inseminated. The conception rate (11 of 12 [92%]) was significantly higher in the seminal plasma treatment group than that in the control group (11 of 20 [55%]) (P < .05; Table). When the control frozen-thawed sperm were artificially inseminated, the mean litter size was only 5.5 ± 0.6 piglets, whereas the seminal plasma treatment significantly increased the litter size to 8.6 ± 1.4 piglets (P < .05).
| Treatment | No. of Inseminated Sows | Conception Rate, % | Litter Size |
|---|---|---|---|
| Control (0% [vol/vol] seminal plasma) | 20 | 55a | 5.5 ± 0.6a |
| 15% (vol/vol) seminal plasma | 12 | 92b | 8.6 ± 1.4b |
- a The values shown are means ± SEM. Different letters in the same column indicate significant differences (P < .05).
Discussion
It is well known that seminal plasma suppresses capacitation in fresh sperm (Davis and Niwa, 1974; Cross, 1993). We previously showed that the addition of seminal plasma to the thawing solution reduced the tyrosine phosphorylation of sperm proteins in frozenthawed ejaculated spermatozoa (Okazaki et al, 2009b). It has been reported that the induction of tyrosine phosphorylation is involved in sperm capacitation, and the presence of seminal plasma suppresses this phosphorylation after the thawing process (Okazaki et al, 2009b). Additionally, epididymal spermatozoa are not exposed to seminal plasma, and as a result, the capacitation process is easily induced (Matás et al, 2010). From these reports, we hypothesized that the treatment of boar epididymal spermatozoa with seminal plasma before freezing or after thawing would improve their motility and fertilizing ability. In the present study, the addition of seminal plasma to the thawing solution significantly increased the total motility of sperm, acrosomal integrity, and conception rate by AI. However, when epididymal spermatozoa were treated with 15% or 50% (vol/vol) seminal plasma before the freezing process, the positive effects of seminal plasma on postthaw sperm motility were not detected, suggesting that seminal plasma is required for the maintenance of epididymal sperm quality, at least after thawing.
The positive functions of seminal plasma are exerted by blocking the elevation of intracellular calcium in sperm (Lu et al, 2010; Okazaki et al, 2011). The uptake of calcium is involved in the induction of capacitation and the acrosome reaction (Arnoult et al, 1999; Baldi et al, 2000). Although capacitated sperm show a hyperactivated status, their high motility is transient and their motility decreases dramatically within a few hours (Bailey et al, 2000; Medeiros et al, 2002). From these reports, it can be concluded that frozen-thawed epididymal sperm have a highly induced capacitation status compared with that of ejaculated sperm. In this study, a specific tyrosine phosphoprotein of approximately 15 kDa was highly expressed in frozen-thawed epididymal sperm but not in ejaculated sperm. Several researchers have reported that a band of 32 kDa, detected by Western blotting using the mouse anti-phosphotyrosine monoclonal antibody clone 4G10, represents a specific marker of boar sperm capacitation or cryocapacitation (Tardif et al, 2001; Kaneto et al, 2002). In this study, we chose the P-Tyr-100 antibody to detect protein tyrosine phosphorylation because it has been used for the detection of sperm capacitation in species such as mouse and human (Shimada et al, 2008; Fujita et al, 2011). The addition of seminal plasma dramatically suppressed protein phosphorylation. Tyrosine phosphoproteins were localized at the head and tail region on sperm in the absence of seminal plasma (data not shown). On the other hand, in the seminal plasma—treated sperm, the localization was observed only in the postacrosomal region. The addition of seminal plasma to the thawing solution at 15% (vol/vol) also positively affected acrosomal integrity and motility maintenance in the frozen-thawed epididymal sperm. In ejaculated sperm, the maximum effects of seminal plasma on postthaw sperm functions were observed at 10% (vol/vol; Okazaki et al, 2009b). Therefore, it would seem that a much higher dose of seminal plasma is required to protect frozen-thawed epididymal sperm from spontaneous capacitation.
In our previous study, a high level of reproductive performance was achieved by AI using frozen-thawed spermatozoa with 10% (vol/vol) seminal plasma (Okazaki et al, 2009b). Garcia et al (2010) also reported that the addition of seminal plasma to the thawing solution significantly improved conception rates and litter sizes by AI using frozen-thawed boar semen. In this study, we used AI to evaluate frozen-thawed epididymal spermatozoa. The conception rate and mean litter size of swine inseminated with seminal plasma—treated doses was significantly increased as compared with the control (without seminal plasma).
Rozeboom et al (1999, 2000, 2001) reported that placing seminal plasma in the female genital tract suppressed polymorphonuclear neutrophilic granulocytes in the uterus (uterine clearance) and enhanced the rate of disappearance of uterine inflammation. Moreover, the differentiation of endometrial cells has been induced by seminal plasma, and these cells express cytokine and chemokine mRNAs required for embryo development and implantation preparation in pigs (O'Leary et al, 2004). Thus, it is possible that the addition of seminal plasma contributes to making the uterine environment conducive to the success of pregnancy, as well as improving sperm motility in AI.
When spermatozoa are flushed from caudal epididymides in mature boars, the total number of collected spermatozoa is approximately 5 × 1010. If 1 × 1010 spermatozoa are artificially inseminated (5 × 109 spermatozoa 2 times per sow), only 5 females can be inseminated per donor boar. Because the numbers of epididymal spermatozoa that can be recovered are dependent on individuals, it is important to develop methods that reduce the number of spermatozoa required for AI. Techniques such as deep intrauterine insemination (Roca et al, 2003, 2006) or estrus synchronization (Noguchi et al, 2010) are promising in this respect. Our findings suggest that the use of seminal plasma in the thawing solution may represent an important means to reduce the number of spermatozoa required for AI.
From these results, we concluded that the addition of 15% (vol/vol) seminal plasma to the thawing solution is beneficial when using frozen-thawed epididymal spermatozoa for AI in the pig.




