Normoxic Expression of Hypoxia-Inducible Factor 1 in Rat Leydig Cells In Vivo and In Vitro
Abstract
ABSTRACT: Hypoxia-inducible factors (HIF) are transcription factors that serve essential regulatory roles in cellular and molecular responses to oxygen debt. HIFs are composed of hypoxia-dependent α subunits (1α, 2α, 3α) and an oxygen-independent β subunit. Previously we demonstrated that HIF-1, the master regulator of hypoxic responses, is expressed in the adult rat testis. We hypothesized that HIF-1 is involved in regulating responses to oxygen tension in the testis. Goals of this study were to determine if HIF-2α and HIF-3α are expressed in rat testis, identify testis cell types that express HIF-1α, and examine patterns of testicular HIF-1α protein expression under conditions of ischemia and hypoxia in vivo and in vitro. Reverse transcriptase polymerase chain reaction revealed that mRNA for Hif-1α, Hif-2α, and Hif-3α is expressed in the testis. The HIF-1α protein is the predominant subunit in testis. HIF-1α protein was abundant in normoxic testis, and its levels remained unchanged following ischemia created by surgically induced testicular torsion and reperfusion. Immunoblot and immunocytochemical experiments demonstrated that Leydig cells are the major source of HIF-1α in normoxic and hypoxic testes. To examine potential mechanisms of testicular HIF-1 stabilization, nuclear proteins from Leydig cells cultured in 5% or 21% oxygen, or cells cultured with H2O2, were analyzed by immunoblotting. Levels of HIF-1α were significantly diminished in 5% or 21% oxygen cultures compared with freshly isolated cells. Treating Leydig cells with H2O2 as a source of reactive oxygen species did not affect HIF-1α levels. High levels of constitutively expressed HIF-1α in normoxic Leydig cells suggest potentially unique roles for HIF-1 in Leydig cell responsiveness to oxygen.
Mammalian cells are extremely sensitive to changes in oxygen tension, particularly decreases in local oxygen supply (hypoxia). When subjected to hypoxia, most cells rapidly increase transcription of specific genes involved in oxygen homeostasis in an effort to cope with oxygen debt (Semenza, 1999, 2001; Maxwell, 2005). Hypoxia-inducible factor 1 (HIF-1) is considered the master regulator of the response to hypoxia (Semenza, 2000a; Wenger, 2000). HIF-1 is a basic helix-loop-helix transcription factor that functions to restore oxygen homeostasis by activating more than 100 different hypoxia-sensitive genes involved in cellular processes such as angiogenesis, erythropoiesis, glucose transport, anaerobic glycolysis, vasodilation, and antiapoptotic and proapoptotic responses to ischemia and hypoxia (Keith and Simon, 2007; Kenneth and Rocha, 2008).
HIF-1 is known to have bimodal effects on cell physiology because it can activate either cell survival or cell death genes depending on the extent and duration of oxygen debt (Piret et al, 2002; Wiesener and Maxwell, 2003; Wang et al, 2004). Many investigators are studying functions of HIF-1 under ischemic and hypoxic conditions that occur during embryogenesis, tumor vascularization and progression, stroke, myocardial infarction, and a number of other hypoxic and ischemic processes of clinical importance (Wiesener and Maxwell, 2003; Calvert et al, 2006; Maynard and Ohh, 2007).
Active HIF-1 is a heterodimer consisting of an oxygen-dependent α subunit and a constitutively expressed β subunit, also called arylhydrocarbon receptor nuclear translocator, that is unregulated by oxygen tension. HIF-1 subunits are widely expressed in mammalian tissues from virtually all organisms studied to date (Semenza, 2001; Wiesener and Maxwell, 2003). In addition to HIF-1α, 2 other HIF-α subunits have been identified, HIF-2α and HIF-3α (Gu et al, 1998; Kietzmann et al, 2001). Both subunits form heterodimers with HIF-1β. Yet, compared with HIF-1α, relatively little is known about the functions of HIF-2α and HIF-3α, although they appear to have both redundant and nonredundant roles (Rocha, 2007).
Under normoxic conditions, HIF-1α subunits are rapidly degraded via a mechanism that involves hydroxylation of HIF-1α subunits by oxygen-dependent prolyl hydroxylases (Bruick and McKnight, 2001; Berra et al, 2006). Hydroxylated HIF-1α is then recognized by the von Hippel-Lindau (VHL) tumor suppressor protein, a ubiquitin ligase that causes polyubiquitination of HIF-1α and its subsequent degradation in the proteasome (Maxwell et al, 1999). Interactions of HIF-1α with small ubiquitin-related modifier (SUMO) protein and deSUMOytion events also regulate HIF-1α stability (Cheng et al, 2007). When intracellular oxygen reaches a critically low threshold, HIF-1α subunits are rapidly protected from proteasomal degradation, stabilizing HIF-1α and allowing HIF-1α and HIF-1β subunits to associate and form active HIF-1. Active HIF-1 interacts with a consensus hypoxia response element in the promoter region of target genes to stimulate transcription of HIF-activated genes.
Maintaining properly oxygenated microenvironments in the testis and epididymis is essential for protecting developing spermatozoa from oxidative and hypoxic damage (Hinton et al, 1996; Aitken, 2002; Hermo and Robaire, 2002). Historically, the testis has also been described as a tissue on the “brink of hypoxia” (Setchell, 1978). In addition, clinical conditions such as testicular torsion and varicocele affect oxygen levels in the testis. Testicular torsion, or torsion of the spermatic cord, is a urologic medical emergency that primarily affects adolescent boys (Pentyala et al, 2001; Ben-Meir et al, 2006; Ringdahl and Teague, 2006). Torsion can obstruct arterial and venous pathways, resulting in alterations in blood flow, ischemia, and hypoxia and causing acute or chronic cellular and molecular damage depending on the degree of cord rotation, duration of torsion, and subsequent tissue reperfusion that may occur following intervention (Pentyala et al, 2001; Ringdahl and Teague, 2006). Cellular and molecular mechanisms responsible for posttorsion tissue damage following torsion and reperfusion are complex (Lysiak et al, 2000b). It is well known that ischemia and reperfusion of the testis causes lipid peroxidation damage (Dokmeci et al, 2007), germ cell death and aspermatogenesis (Steinberger and Tjioe, 1969; Turner and Brown, 1993; Turner and Miller, 1997; Filho et al, 2004), alterations in testis protein profiles (Turner, 1997; Turner et al, 2006), oxidative stress (Turner and Lysiak, 2008), and activation of inflammatory pathways (Lysiak et al, 2003, 2005).
Previously we demonstrated that HIF-1α mRNA and protein are expressed in the rat testis (Powell et al, 2002). HIF-1 has also been studied in the murine and human testis. A testis-specific HIF-1 isoform, Hif-1α1.1, is expressed in murine germ cells (Marti et al, 2002), and a dominant-negative role for this isoform in the human testis has been proposed (Depping et al, 2004). Lysiak et al (2009) demonstrated that murine HIF-1α is expressed in normoxic Leydig cells and may play a role in regulating expression of the 3β-hydroxysteroid dehydrogenase type I gene.
We hypothesized that HIF-1 plays a key role in regulating oxygen homeostasis in the rat testis and that HIF-1 may mediate proapoptotic or antiapoptotic responses in the ischemic testis depending on the duration and extent of ischemia and hypoxia. The purpose of this study was to determine if HIF-2α and HIF-3α are expressed in the rat testis, examine the effects of ischemia and ischemia/reperfusion on testicular HIF-1α, determine which cell type(s) in the rat testis express HIF-1α, and examine HIF-1α protein expression under conditions of normoxia and hypoxia in vivo and in vitro.
Materials and Methods
Animals
Adult male, retired-breeder Sprague-Dawley rats (375–450 g; 9–12 months) were purchased from Charles River Laboratories (Stone Ridge, New York). Animals were housed 1 per cage at Monmouth University under controlled light (12:12-hour light/dark cycle) and temperature with free access to food and water. All aspects of animal handling and surgery were conducted in accordance with appropriate animal welfare criteria established by the National Research Council's publication Guide for Care and Use of Laboratory Animals, and animal research protocols were reviewed and approved by the Monmouth University Institutional Animal Care and Use Committee.
Surgical Manipulations
Animals were anesthetized by intraperitoneal injection of sodium pentobarbital (75 mg/kg body weight; Sigma-Aldrich, St Louis, Missouri), and surgeries were performed via a midline laparotomy using sterile procedures. Experimental testicular torsion surgeries were carried out as described by Turner et al (1997) and as previously reported (Powell et al, 2002). The following surgical treatment groups were used for this study: 1 hour of ischemia (I), 1 hour of ischemia followed by 4 hours of reperfusion (1h/4h I/R), 1h/24h I/R, 1h/1week I/R, and 6h I. The testis and epididymis were exposed through the laparotomy incision, connective tissue holding the testis to the epididymis was separated, and the testis was rotated 720° counterclockwise. The testis and epididymis were returned to the scrotum, the laparotomy incision was closed, and the testis was maintained in torsion. For reperfusion studies, the testicular and scrotal stumps of the divided gubernaculum were sutured together, and the testis and epididymis were returned to the scrotum for specific times. Alternating side surgeries were carried out, and contralateral testes served as sham-operated controls. Control studies were also carried out with normoxic, unoperated testes. Animals were maintained under anesthesia for the duration of the surgical treatment before being sacrificed. Rats were euthanized with carbon dioxide, and testes were excised, frozen in liquid nitrogen, and stored at 270°C before RNA isolation or freshly excised unfrozen tissues were used for nuclear protein extraction.
RNA Isolation and Reverse Transcriptase Polymerase Chain Reaction Analysis of HIF mRNA Expression
Total RNA was isolated from frozen tissues using TRIReagent according to the manufacturer's instructions (Molecular Research Center Inc, Cincinnati, Ohio). Primers were designed using Primer3 software (Rozen and Skaletsky, 2000) and synthesized by MWG-Biotech (High Point, North Carolina). Hif-1α forward (5′-TGCTTGGTGCTGATTTGTGA-3′; nucleotides [nt] 681–700) and reverse (5′-GGTCAGATGATCAGAGTCCA-3′; nt 871–890) primers amplify a 209-bp fragment of rat HIF-1α cDNA (AF057308; Zou et al, 2001). Hif-2α forward (5′-GGCCAAACATGGAGGATATG-3′; nt 1010–1029) and reverse (5′-GGGTGTGGCTTGAACAAGAT-3′; nt 1160–1179) primers amplify a 170-bp fragment of the rat HIF-2α cDNA (AJ277828; Kietzmann et al, 2001). Hif-3α forward (5′-ACCAAGACAGGTCGAACACC-3′; nt 94–113) and reverse (5′-TTTTCCACCTGGTTCCACTC-5′; nt 316–335) primers amplify a 223-bp fragment of the mouse HIF-3α cDNA (NM_016868; Heidbreder et al, 2003).
HIF polymerase chain reaction (PCR) products were coamplified by multiplex relative reverse transcriptase PCR (RT-PCR) analysis with mouse β-actin (Stratagene, La Jolla, California) primers as internal controls. Primer concentrations were optimized to amplify β-actin PCR products of 514 bp in the same linear range as HIF amplicons. Independent amplifications with different concentrations of HIF primers and amplification cycles with uniform amounts of total RNA (1 μL) were separated by agarose gel electrophoresis and amplicon amounts were quantitated using Quantity One software (version 4.4; Bio-Rad Laboratorie Inc, Hercules, California) to produce standard curves ensuring linear ranges of amplification for each primer pair under the cycling conditions used for these experiments.
Amplifications were carried out in a Bio-Rad MyCycler thermal cycler. One microgram of total RNA was reverse transcribed and amplified by the 1-step AccessQuick RT-PCR procedure (Promega Corp, Madison, Wisconsin) in a 50-μL reaction volume containing 50 pmol of each forward and reverse HIF primer and 6.25 pmol of each β-actin primer in the presence of avian myeloblastosis virus RT and Tfl DNA polymerase. Reverse transcription was carried out at 48°C for 45 minutes; followed by denaturation at 94°C for 2 minutes; and 40 cycles of PCR with a denaturing step at 94°C for 30 seconds, an annealing step at 60°C for 1 minutes, an elongation step at 68°C for 2 minutes, and a final extension at 68°C for 7 minutes. Eight-microliter aliquots of PCR products were electrophoresed through 2% agarose gels in 1× Tris-acetate EDTA buffer, and gels were stained with ethidium bromide. Gel images were captured with a ChemiDoc gel documentation system (Bio-Rad). RT-PCR controls included a single primer pair positive control amplification and no RT negative controls. PCR products were cloned into pGEM-T Easy plasmid vectors (Promega), and sequence identity of RT-PCR products was confirmed by cycle sequencing using a LI-COR 4300L DNA sequencer (LI-COR Biosciences, Lincoln, Nebraska) and BLAST analysis (Altschul et al, 1990).
Nuclear Protein Extraction
Freshly excised tissues were homogenized on ice in 3 volumes of lysis buffer (10 mM Tris-HCl [pH 7.5], 1.5 mM MgCl2, 1 mM dithiothreitol, 1 mM Na3VO4) containing protease inhibitor cocktail (P8340; Sigma-Aldrich). The homogenate was centrifuged for 5 minutes at 5000 ×g at 4°C, and the supernatant was removed for cytoplasmic proteins. Nuclei were resuspended in 3 volumes of 0.42 M KCl, 20 mM Tris-HCl (pH 7.5), 1.5 mM MgCl2, 20% glycerol; mixed for 30 minutes at 4°C; and centrifuged at 10 000 ×g for 30 minutes at 4°C; the supernatant was saved as the nuclear extract. Cytoplasmic and nuclear protein extracts were stored at 280°C. Protein concentrations were determined by the Bradford assay (Bio-Rad) using bovine serum albumin (BSA) as the standard.
Purification of Testis Cell Types
Sertoli cells, peritubular cells, spermatogonia, pachytene spermatocytes, early spermatids, and residual bodies were provided by Charles Pineau (Université de Rennes, Bretagne, France). These cell populations were isolated and purified by centrifugal elutriation as previously described by Pineau et al (1993). The purity of Sertoli cell cultures was approximately 96%, and the purity of other isolated cell types was approximately 90%.
Purification and Culture of Rat Leydig Cells
The Hardy laboratory (Population Council, Center for Biomedical Research, New York, New York) provided purified Leydig cells for culture under normoxic or hypoxic conditions. Adult Leydig cells (ALC) were purified from 325- to 350-g Sprague-Dawley rats as previously described (Salva et al, 2001). Cell yields were estimated using a hemocytometer, and percentage of purity was assessed by histochemical staining for 3β-hydroxysteroid dehydrogenase using etiocholan-3β-ol-17-one as the enzyme substrate (Payne et al, 1980). ALC preparations used for the oxygen-dependent culture experiments were more than 95% pure. Cells were evenly split into T-25 flasks and cultured in buffered Dulbecco modified Eagle medium (DMEM): F12 culture medium supplemented with ovine luteinizing hormone (0.1 ng/mL) and lipoproteins (Lipimate; Hyclone, Logan, Utah) (Klinefelter et al, 1993). The cells were cultured at 34°C in an incubator containing either a 5% O2/5% CO2 atmosphere or a 21% O2/5% CO2 atmosphere. After 20 to 24 hours, the spent media was collected for measurement of testosterone production by a testosterone radioimmunoassay (RIA) used to assess steroidogenic activities and viability of the cultured Leydig cells (Cochran et al, 1981). The ALC were collected from the flasks using trypsin, centrifuged, and frozen in liquid nitrogen as cell pellets for later HIF protein estimation. This experiment was then repeated with additional treatment groups cultured in the presence of 250 μM H2O2 with 0.1 ng/mL luteinizing hormone and lipoproteins. Again, the flasks were cultured in either a 5% O2/5% CO2 or 21% O2/5% CO2 environment. After 20 to 24 hours, spent media was saved, and cells were harvested for HIF extraction.
The Hales laboratory provided purified Leydig cells used to determine if H2O2 as a source of reactive oxygen species (ROS) could induce HIF-1α expression under normoxic conditions. To obtain enriched preparations of Leydig cells, testes were decapsulated as described (Ogilvie et al, 1999). To decapsulate the testes, the capsule opposite the testicular vein was cut with a razor and the testicular contents removed from the tunica. Both testes from each animal were pooled in one dissociation tube containing 18 mL of M199 complete media, 2 mL of 10% BSA, and 100 mL of collagenase (200 mg/mL; CLS4; Worthington Biochemical, Lakewood, New Jersey). Dissociation tubes were incubated at 37°C for 10 minutes with shaking to separate interstitial cells from the seminiferous tubules. After incubation, 30 mL of M199 complete media was added to each tube, and the tubes were inverted several times and then chilled on ice for 2 minutes. The resultant supernatant was collected, filtered through organza fabric, and centrifuged to pellet cells. Cells were resuspended in M199 and subjected to a density gradient centrifugation on 55% Percoll (Pharmacia Biotech, Piscataway, New Jersey), in Dulbecco phosphate-buffered saline (PBS; Sigma-Aldrich) and 1% BSA (Salva et al, 2001), at 13.2 × g for 15 minutes. In a separate tube, an identical Percoll gradient containing Percoll density gradient beads (Pharmacia Biotech) was centrifuged in parallel. After centrifugation, the upper layer of the gradient was removed, and the Leydig cell layer was recovered from the gradient tube from just below the red (density, 1.064) and above the blue (density, 1.075) Percoll density gradient beads.
Percoll was removed by dilution in M199 complete medium, and the fractions were centrifuged at low speed (300 × g) and resuspended in 5 mL of M199 complete medium. Cells were counted with a hemocytometer; plated in 100-mm culture dishes at a density of 2.5 × 105 cells/cm2; and incubated overnight at 34°C in DMEM/F12 media supplemented with 0.5 ng/mL bovine insulin (Sigma-Aldrich), 500 units/mL penicillin/streptomycin (Invitrogen, Carlsbad, California), and 0.1% BSA (fraction V; Sigma-Aldrich). Leydig cell–enriched cultures were shown to be approximately 85% pure by histochemical staining for 3β-hydroxysteroid dehydrogenase as described (Allen et al, 2006). Cells were incubated for 6 hours in control media or media that contained 250 μM H2O2 (Sigma-Aldrich) as described (Diemer et al, 2003). After the incubation period, cells were removed by scraping and collected by centrifugation; the cell pellet was frozen at 280°C.
Immunoblot Analysis
Nuclear proteins were separated by denaturing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) through 7.5% PAGEr Duramide polyacrylamide gels (Lonza, Rockland, Maine). Seventy-five-microgram aliquots of hypoxic PC-12 pheochromocytoma nuclear extracts (Active Motif, Carlsbad, California) or COS-7 simian virus 40–transformed kidney cell nuclear extracts (Active Motif) were included as positive controls for HIF subunits. Proteins were electroblotted onto Trans-Blot nitrocellulose (Bio-Rad), and blots were stained with Ponceau S (0.005% in 1% acetic acid) to confirm protein transfer. Blots were blocked in 1×Western wash (50 mM Tris, 30 mM NaCl, 0.001% Tween 20 [pH 7.6]) containing 5% nonfat dry milk for 30 minutes at room temperature with gentle agitation. Blots were incubated in primary antibody overnight at 4°C in 1× Western wash, 5% nonfat dry milk.
Primary antibody dilutions for HIFs were as follows: 0.5 μg/mL HIF-1α mouse monoclonal antibody (AF1935; R&D Systems, Minneapolis, Minnesota), 1:1000 dilution of HIF-2α rabbit polyclonal antibody (NB 100-122; Novus Biological Inc, Littleton, Colorado), 1:100 dilution of EPAS-1/HIF-2α (C-16, sc-8712; Santa Cruz Biotechnology Inc, Santa Cruz, California), and 1:100 dilution of HIF-3α goat polyclonal antibody (sc-8718; Santa Cruz Biotechnology). Activating transcription factor 2 (ATF-2) was detected on immunoblots as a hypoxia-independent loading control for protein quantitation using a 1:500 dilution of mouse polyclonal antibody (sc-6233; Santa Cruz Biotechnology). Blots were washed 3 times in 1× Western wash for 35 minutes, incubated for 1 hour in 1× Western wash, 5% nonfat dry milk containing a 1:10 000 dilution of horseradish peroxidase (HRP)–conjugated secondary antibodies, and then washed with 3 changes of 1×Western wash for 35 minutes. Blots were developed by enhanced chemiluminescence using either SuperSignal West Pico substrate or SuperSignal West Femto substrate (Pierce, Rockford, Illinois) and exposed to x-ray film (BioMax ML; Kodak, Rochester, New York).
After detection of HIF subunits, blots were stripped of bound primary antibody by incubating in Restore Western blot stripping buffer (Pierce) for 15 minutes at 37°C, washed in 1× Western wash for 5 minutes, and then blocked prior to subsequent immunoprobing for ATF-2. Negative control experiments for antibody specificity were carried out by incubating blots with preimmune sera followed by incubation with secondary antibodies.
Immunoprecipitation of HIF-1α and Ubiquitin
Immunoprecipitation was carried out using 325-μg aliquots of nuclear extracts from sham-operated, 1 hour ischemic, and 1h/4h I/R testes. Nuclear proteins were incubated overnight at 4°C with 2 μg of goat anti–HIF-1α antibody (AF1935; R&D Systems) and protein A/G PLUS-agarose (Santa Cruz Biotechnology). Antibody-protein complexes were centrifuged at 500 × g, washed 3 times with 1× PBS, and then subjected to SDS-PAGE. Duplicate aliquots of proteins were separated on polyacrylamide gels and blotted. Nitrocellulose membranes were then cut in half. One membrane was probed with 0.5 μg/mL goat anti–HIF-1α antibody (AF1935; R&D Systems), and the other half was probed with a 1:1000 dilution of anti-ubiquitin rabbit antibody (Santa Cruz Biotechnology). Blots were incubated with HRP-conjugated secondary antibodies, and the signal was detected by chemiluminescence as described above.
Immunocytochemistry
Adult rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (Somnitol) and perfused through the abdominal aorta with Bouin fixative. Testes were sliced longitudinally and immersed in Bouin fixative or 10% (vol/vol) neutral buffered formalin (Richard-Allan Scientific, Kalamazoo, Michigan) overnight. Following fixation, tissues were dehydrated in 3 changes of 70% ethanol, eventually dehydrated in a series of graded ethanol solutions, and then embedded in paraffin. Five-micrometer thick sections were cut, mounted on glass slides, and processed for light microscopic immunocytochemical analysis.
The following primary antibodies were used at a 1:150 dilution for peroxidase immunostaining: polyclonal goat anti-human HIF-1α (Santa Cruz Biotechnology), monoclonal mouse anti-human HIF-1α (Novus Biologicals), and polyclonal rabbit anti-human HIF-1β (Novus Biologicals). Negative control experiments were performed on adjacent sections by substituting 1× Tris-buffered saline (TBS) for the primary antibody and by incubating primary antibody with excess HIF-1α peptide before incubation with tissue sections.
Paraffin sections were deparaffinized with Histoclear and hydrated in a series of graded ethanol solutions. Endogenous peroxidase activity was inactivated with 70% ethanol containing 1% (vol/vol) H2O2, and residual picric acid was neutralized in 70% alcohol containing 1% lithium carbonate. After hydration, the tissues were washed in distilled water containing 300 mM glycine to block free aldehyde groups. Before immunostaining, the sections were blocked for 15 minutes with 10% goat serum in TBS. Tissue sections were incubated at 37°C in a humidified chamber for 90 minutes with 100 μL of diluted primary antibody. Following several washes in TBS containing 0.1% Tween-20, the sections were blocked with 10% goat serum for 15 minutes to prevent nonspecific binding of the secondary antibody.
Secondary antibody incubation was performed at 37°C with anti-rabbit IgG conjugated to HFP at a dilution of 1:250 with TBS. All sections were washed and incubated with peroxidase substrate: 0.05% 3,3′-diaminobenzidine tetrahydrochloride and 0.03% H2O2 in TBS. The sections were counterstained with 0.1% methylene blue and dehydrated in a graded series of ethanol solutions and Histoclear. Cover slips were mounted onto glass slides with Permount.
Terminal Deoxyribonucleotidyltransferase Mediated dUTP-Biotin Nick End Labeling Assays for Detecting Apoptotic Cells
To verify that our experimental conditions of I and I/R were creating germ cell apoptosis, a hallmark characteristic of cell damage following torsion that has been well documented and previously reported by many other investigators (Turner et al, 1997; Lysiak et al, 2000a; Chaki et al, 2003; Sukhotnik et al, 2008), terminal deoxyribonucleotidyltransferase mediated dUTP-biotin nick end labeling (TUNEL) assays were carried out on testis sections from control and surgically treated testes to assay for germ cell–specific damage caused by experimental testicular torsion surgeries. Testes from normoxic and surgically treated rats were sliced longitudinally and immersed in 10% (vol/vol) neutral buffered formalin (Richard-Allan Scientific) overnight. Following fixation, tissues were dehydrated and embedded in paraffin. Five-micrometer sections were mounted on glass slides, and apoptosis was detected using the ApopTag Plus peroxidase in situ apoptosis detection kit (Chemicon International, Temecula, California) according to the manufacturer's instructions. Positive control experiments were carried out on mouse mammary tissue sections, and negative control experiments were performed on adjacent sections of testis or mouse mammary gland by substituting 1× TBS buffer for the terminal deoxyribonucleotidyltransferase enzyme. Sections were counterstained with hematoxylin. An apoptotic index was determined by scoring a minimum of 30 seminiferous tubules for each time point, chosen randomly at ×400 magnification, and the number of TUNEL-positive apoptotic cells per tubule cross-section were determined. These experiments demonstrated an increase in germ cell–specific apoptosis following I and I/R, with no statistically significant changes in apoptosis of Leydig cells following I and I/R (see Supplemental Table 1, available online at http:www.andrologyjournal.org).
Quantitation of Results and Statistical Analysis
RT-PCR gel images were captured using a ChemiDoc gel documentation system, and quantitation of results was carried out with Quantity One software (version 4.4; Bio-Rad). Integrated peak areas for HIF PCR products were normalized to integrated peak areas for β-actin PCR products. HIF protein levels were normalized to ATF-2 as a loading control. RT-PCR and immunoblot data were analyzed by 1-way analysis of variance, and results were considered significantly different at P < .05.
Results
Expression of Hif-1α, Hif-2α, and Hif-3α mRNA in Normoxic and Ischemic Testis
Previously we demonstrated that Hif-1α mRNA is expressed in the normoxic adult rat testis and is unregulated by hypoxia (Powell et al, 2002). After this initial study, 2 other HIFα subunits, HIF-2α and HIF-3α, were identified (Heidbreder et al, 2003; Wiesener et al, 2003). Given the fundamental importance of HIFs in regulating oxygen homeostasis, we wanted to determine if Hif-2α and Hif-3α mRNA is also expressed in the rat testis. Total RNA from normoxic and 1 hour ischemic testes was analyzed by RT-PCR (Figure 1A). mRNA for both Hif-2α and Hif-3α subunits was detected in the normoxic testis. Quantitation of RT-PCR data showed that expression of these mRNAs was unaffected by 1 hour of ischemia compared with sham-operated controls (n = 5; P < .05).
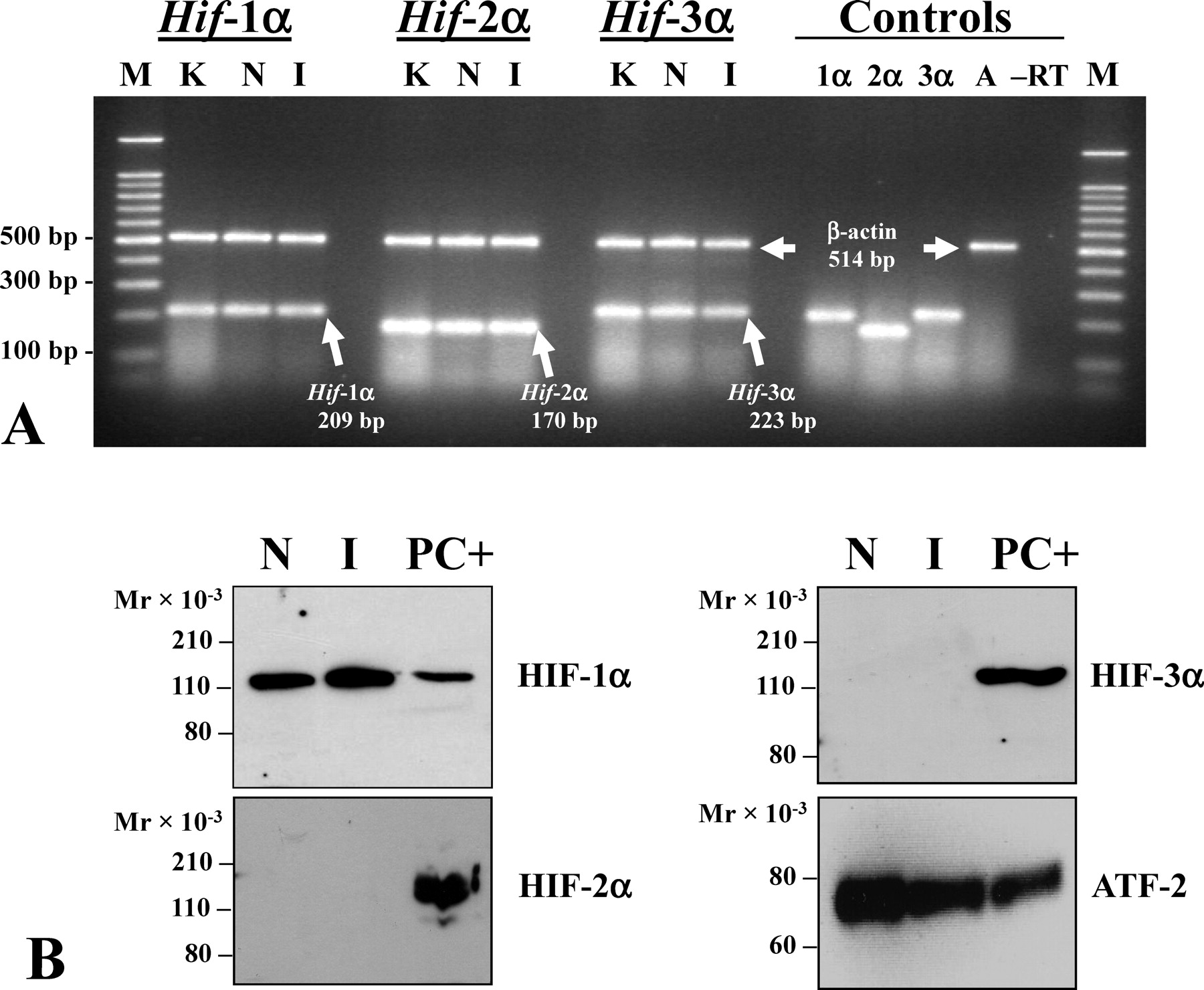
. Expression of hypoxia-inducible factor (HIF)-1α, HIF-2α, and HIF-3α mRNA and protein in the normoxic and ischemic rat testis. (A) Reverse transcriptase polymerase chain reaction analysis of total RNA to detect Hif-1α, Hif-2α, and Hif-3α expression in the normoxic, sham-operated and 1-hour ischemic (I) rat testis. RNA was coamplified with primers for each HIF gene together with β-actin primers. Controls included single primer set positive controls to detect Hif-1α, Hif-2α, Hif-3α, and β-actin (A), and a no reverse transcriptase negative control (–RT). A representative gel is shown (n = 5). (B) 100-μg aliquots of nuclear protein extracts from normoxic (N) and I testes were subjected to immunoblot analysis with antibodies for HIF-1α, HIF-2α, HIF-3α, and activating transcription factor 2 (ATF-2) as a loading control. Representative blots are shown (n = 3–5). K indicates 1-hour normoxic kidney positive control; M, 100-bp DNA size markers; PC+, hypoxic PC-12 nuclear extract included as a positive control for detecting HIFs.
HIF-1α Is Not Affected by Ischemia and Ischemia/Reperfusion of the Rat Testis
To determine if HIF-2α and HIF-3α proteins are present in the testis in addition to HIF-1α, nuclear proteins were isolated from both normoxic and I testes and immunoblot analysis was carried out with commercially available antibodies to each subunit. HIF-1α was abundant in nuclear protein extracts from both normoxic testes and I testes (Figure 1B), a result we had reported previously (Powell et al, 2002) using an antibody from a different manufacturer. In those studies, we also detected doublets for HIF-1α, which may represent the 2 isoforms detected in the mouse (Marti et al, 2002); however, these isoforms have not been definitively identified in the rat, and newer commercially available antibodies that we have used detect a single band for HIF-1α at approximately 120 kd.
HIF-1 appears to be the predominant HIF present in the rat testis. HIF-2α and HIF-3α proteins were not detected in normoxic or ischemic testes even when the blots were incubated with the more sensitive SuperSignal West Femto instead of the less sensitive SuperSignal West Pico substrate used to detect HIF-1α (Figure 1B). The apparent absence of HIF-2α in the rat testis was a consistent result observed with 2 different commercially available antibodies for HIF-2α.
Other investigators have demonstrated that HIF-1 has both proapoptotic and antiapoptotic roles (Minet et al, 2000; Piret et al, 2002). It is well known that germ cell apoptosis is a hallmark characteristic of cell damage created by testicular torsion following 1h/4h I/R (Lysiak et al, 2000b; Filho et al, 2004). To examine potential effects of ischemia and reperfusion on HIF-1α in the testis, nuclear protein extracts from 1h/4h I/R testes were examined at different time points (Figure 2A). Surgical time points were chosen to provide a range of time points representing early-stage ischemia, ischemia/reperfusion known to result in germ cell apoptosis, and long-term treatments. Quantitation of HIF-1α immunoblots revealed no statistically significant differences (P < .05) in the relative abundance of steady-state levels of HIF-1α protein in the testis at all time points of ischemia and reperfusion (Figure 2B).
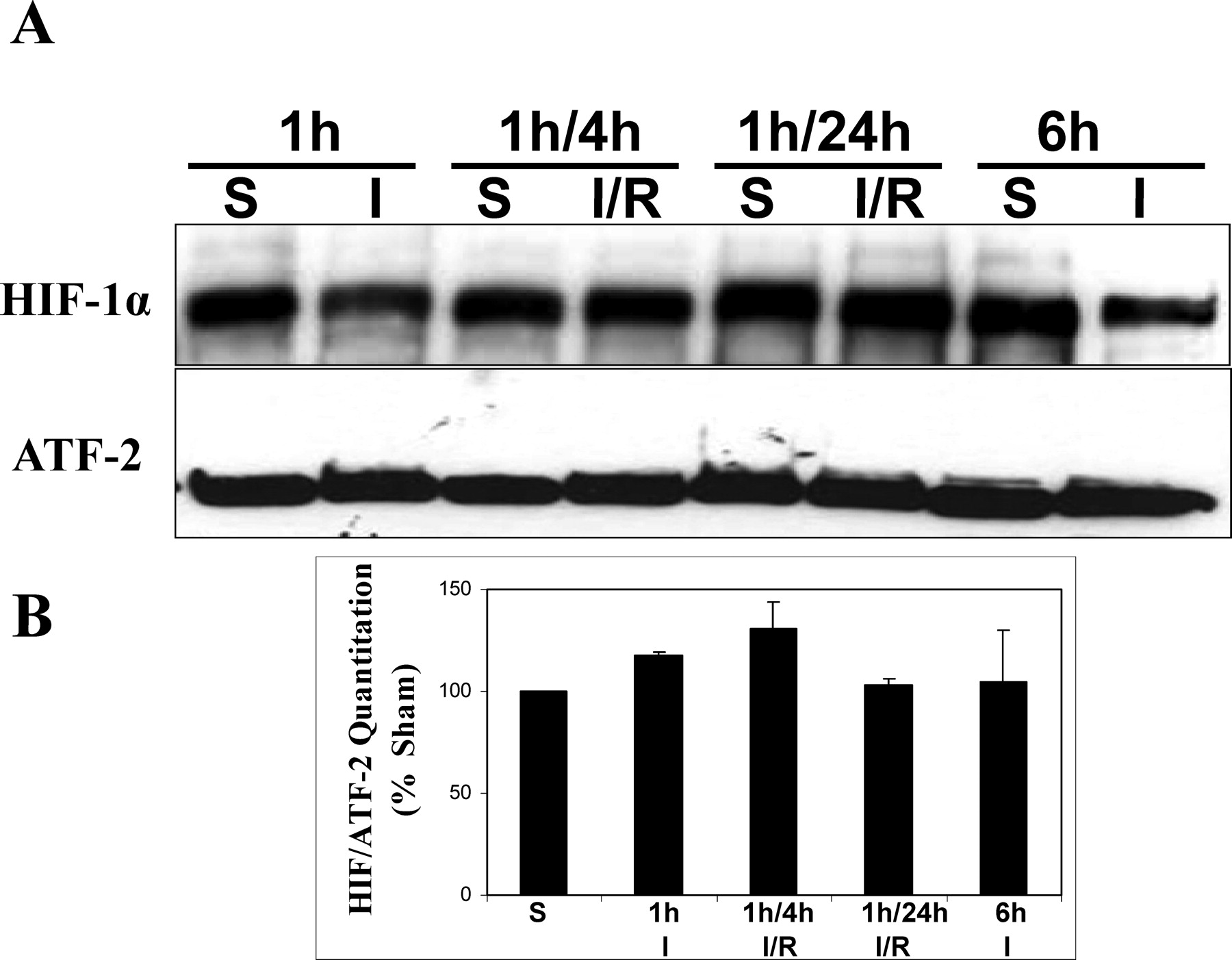
. Immunoblot analysis of nuclear proteins isolated from testes subjected to ischemia (I) or ischemia/reperfusion (I/R). (A) Immunoblot detecting hypoxia-inducible factor (HIF)-1α. Activating transcription factor 2 (ATF-2) was used as a loading control. Time points included ischemia for 1 or 6 hours, and I/R for 1/4 hours or 1/24 hours. (B) Histogram shows HIF-1α normalized to ATF-2 levels expressed as a percent of sham (S) controls. Representative results are shown. No statistically significant differences were observed between sham and ischemic treatments (n = 3; P < .001).
Experiments were also carried out to examine potential delayed effects of ischemia/reperfusion on testicular HIF-1α. Testes from 1h/3d I/R and 1h/1wk experiments showed no statistically significant changes in HIF-1α (data not shown). Cytoplasmic extracts from I and I/R time points were also analyzed by immunoblotting to determine if differences in HIF-1 could be reflected of import from the cytoplasm to the nucleus. No statistically significant differences in cytoplasmic levels of HIF-1α were detected for any of the I or I/R time points examined (data not shown).
HIF-1α in the Normoxic Testis is Primarily Present as a Nonubiquitinated Protein
In most tissues, the HIF-1α subunit is an oxygen-dependent subunit that is targeted for ubiquitination and rapidly degraded under normoxic conditions. To determine if the abundance of HIF-1α in the normoxic testis was due to detectable differences in ubiquitination and stability of testicular HIF-1α in normoxic tissues and after I or I/R, nuclear proteins from sham testes and testes subjected to I or I/R were immunoprecipitated with HIF-1α antibodies, immunoblotted, and probed for HIF-1α and ubiquitin (Figure 3). HIF-1α at approximately 120 kd, corresponding to the full-length protein, from both sham-operated and I or I/R testis did not cross-react with anti-ubiquitin antibodies; however, partially degraded HIF-1 could be detected using both anti–HIF-1α and anti-ubiquitin antibodies. Blots probed for ubiquitin were stripped and reprobed with anti–HIF-1α antibodies to demonstrate that full-length HIF-1 was present in these extracts (Figure 3). These results indicated that HIF-1α in both the normoxic and ischemic testis exists primarily in a nonubiquitinated state, suggesting functional activity for testicular HIF-1.
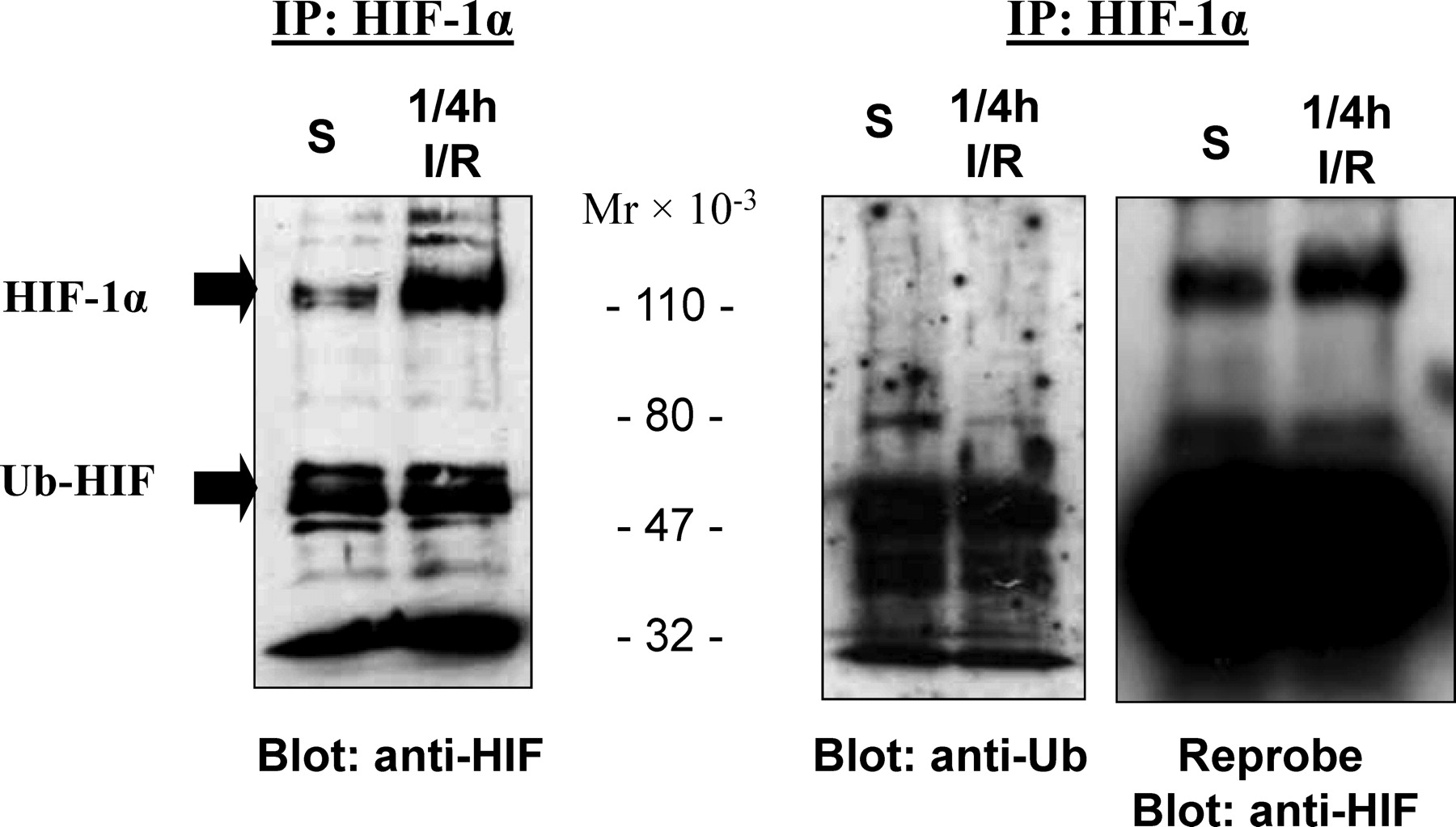
. Testicular hypoxia-inducible factor (HIF)-1α is primarily a nonubiquitinated protein. HIF-1α was immunoprecipitated (IP) using anti–HIF-1α antibodies and subjected to immunoblot analysis with antibodies for HIF-1α or ubiquitin. S indicates sham; IR, ischemia/reperfusion.
Localization of HIF-1α in Rat Leydig Cells
To determine which cell types in the testis express HIF-1α subunits, immunocytochemical studies were carried out on sections of normoxic and ischemic rat testis. HIF-1α immunoreactivity was intensely localized to Leydig cells (Figure 4a–d, arrows). Immunoreactivity was apparent in both the cytoplasm and nucleus of Leydig cells. No differences in the cellular localization of HIF-1α were apparent when normoxic and ischemic sections were analyzed. Immunoreactivity for HIF-1α was not observed in control sections incubated without primary antibody or after preincubation of primary antibody with excess HIF-1 peptide (Figure 4e and f).
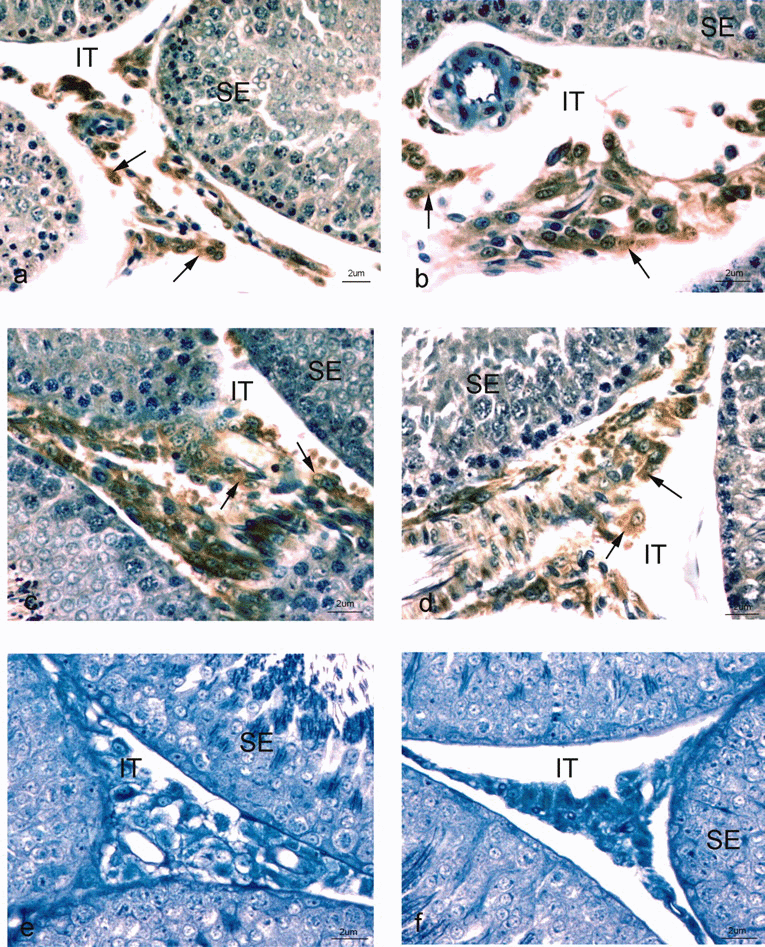
. Immunocytochemical localization of hypoxia-inducible factor (HIF)-1α in Leydig cells of the rat testis. (A–D) Photographs of the seminiferous epithelium (SE) and interstitial spaces (IT) of the normoxic testis immunostained with anti–HIF-1α primary antibody and goat anti-rabbit-horseradish peroxidase secondary antibody. An immunoperoxidase reaction (arrows) is noted over the nucleus and cytoplasm of Leydig cells. (E and F) Negative control sections of portions of SE of the testis and IT immunostained with secondary antibody in the absence of HIF-1α primary antibody or preincubation of primary antibody with blocking peptide revealed the absence of any reaction over Leydig cells. Magnification is indicated by scale bars at the lower right corner of each figure. Sections were counterstained with methylene blue.
As additional confirmation of the cell types expressing HIF-1α in the testis, specific cell types were isolated and purified from adult rat testis. Nuclear extracts from these cells were subjected to immunoblot analysis with antibodies for each HIF. Cell types analyzed included Leydig cells, Sertoli cells, peritubular cells, spermatogonia, pachytene spermatocytes, early spermatids, and residual bodies. A strong signal for HIF-1α was detected in Leydig cells, with a less intense signal in peritubular cells (Figure 5). The signal in peritubular cells may reflect coisolated Leydig cells in these preparations. Sertoli cells, spermatogonia, pachytene spermatocytes, early spermatids, and residual bodies did not show immunoreactivity for HIF-1α (Figure 5). All cell types, as well as residual bodies, contained HIF-1β subunits, which was not surprising because HIF-1β is constitutively expressed in most cells (Figure 5). HIF-2α and HIF-3α were not detected in any isolated cell types following immunoblot analysis with commercially available antibodies for these subunits. These results confirmed Leydig cells as the primary source of HIF-1α in the rat testis.
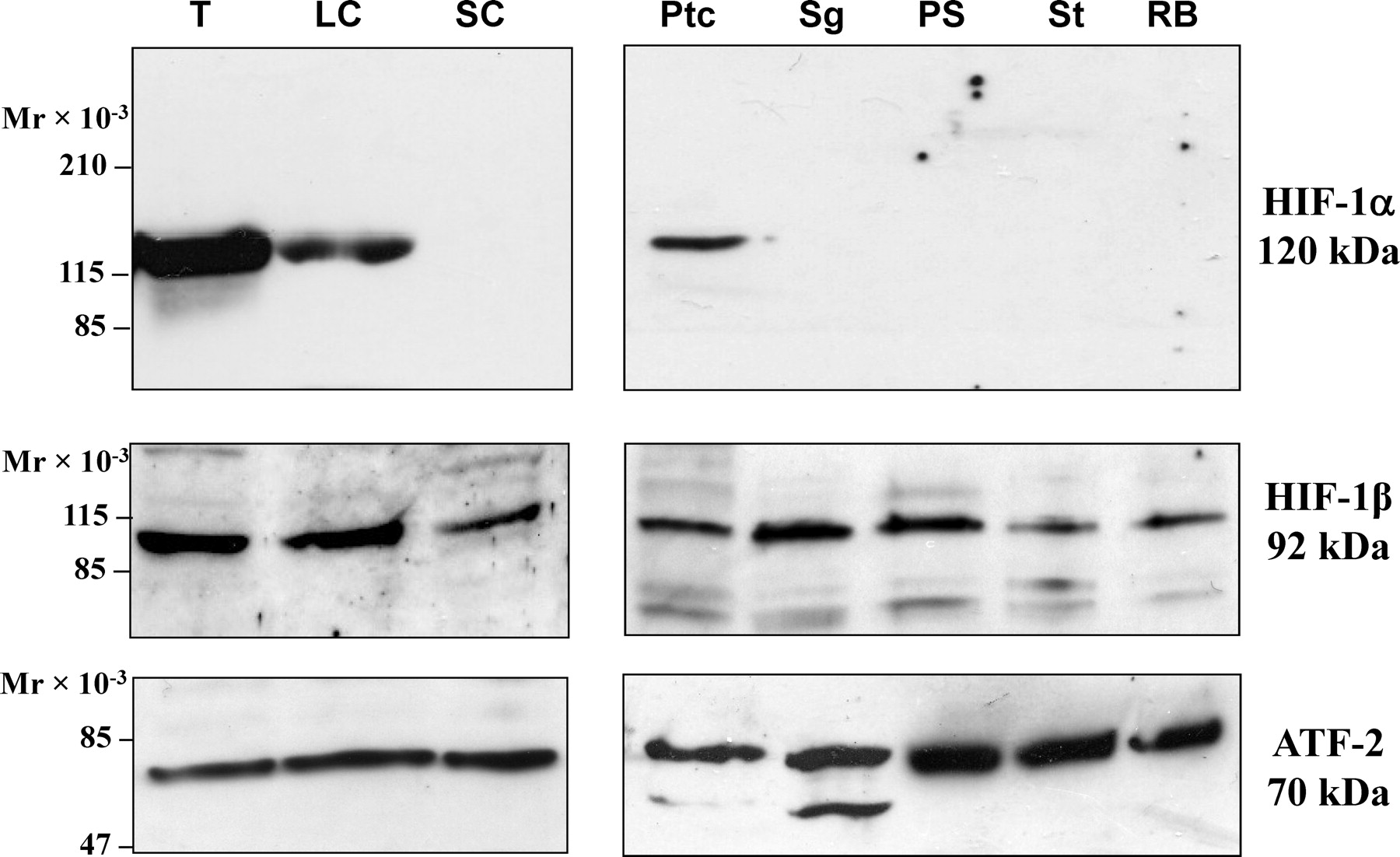
. Immunoblot analysis of hypoxia-inducible factor 1 (HIF-1) in isolated cell types of the testis. Nuclear protein extracts from whole testes (T), Leydig cells (LC), Sertoli cells (SC), peritubular cells (Ptc), spermatogonia (Sg), pachytene spermatocytes (PS), early spermatids (St), and residual bodies (RB) subjected to immunoblot analysis to detect HIF-1α, HIF-1β, and activating transcription factor 2 (ATF-2) as a loading control.
Immunoblot Analysis of HIF-1 Protein in Cultured Leydig Cells Under Normoxic and Hypoxic Conditions
Having established that Leydig cells were the source of HIF-1α in the normoxic and ischemic testis, we wanted to begin to identify mechanisms responsible for stabilizing HIF-1 in the normoxic testis. The constitutive expression and abundance of HIF-1α in the normoxic testis in vivo suggests that testicular HIF-1α may not be stabilized and activated through the traditional hypoxia-dependent prolyl hydroxlases-pVHL-ubiquitin pathway that has been so well defined in other tissues. In addition, some investigators have suggested that the testis may be a hypoxic tissue, and Leydig cells are often cultured under hypoxic conditions in vitro to maintain oxidation-reduction (REDOX) conditions required for steroidogenesis. To examine the effect of hypoxic and normoxic conditions on Leydig cells in vitro, purified Leydig cells were cultured for 20 hours in either 5% oxygen (hypoxia) or 21% oxygen; then nuclear proteins were isolated and used for immunoblot analysis. These experiments showed a statistically significant reduction of HIF-1α in Leydig cells cultured at both 5% and 21% oxygen when compared with freshly isolated Leydig cells (Figure 6). Based on RT-PCR experiments to analyze Hif-1α mRNA expression in these cultured cells, the observed reduction in HIF-1α protein levels is due to decreased steady-state levels of Hif-1α mRNA (data not shown). These experiments also consistently showed a doublet for HIF-1α, but we do not know the reason(s) why we detected 2 bands for HIF-1 from cultured Leydig cells under these conditions; these doublets were not observed under the culture conditions used for the experiments described in Figure 7.
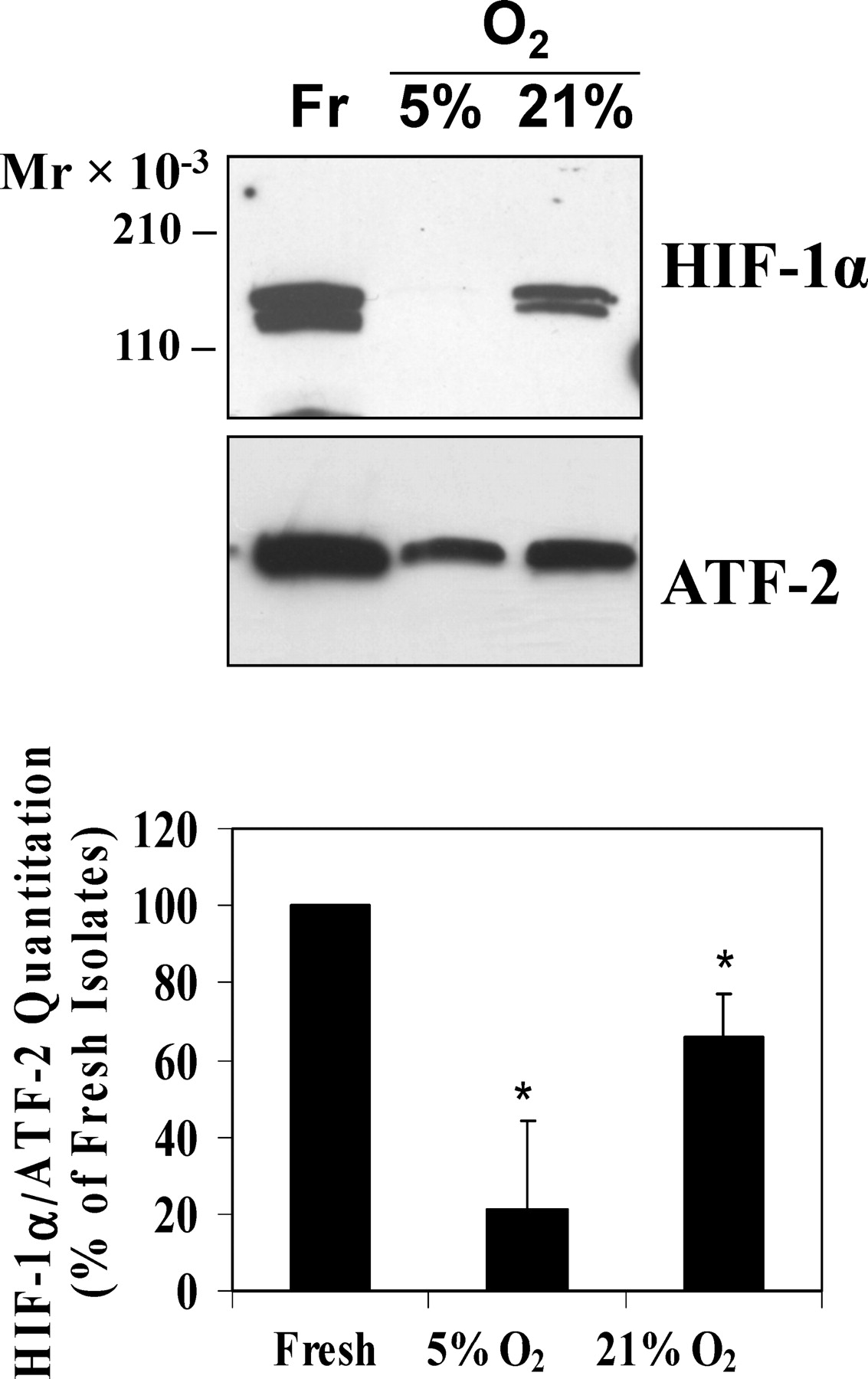
. Hypoxia-inducible factor (HIF)-1α in Leydig cells is down-regulated by hypoxic and normoxic culture conditions. Leydig cells (LC) from adult rat testes were cultured under 5% oxygen (hypoxia) or 21% oxygen (normoxia) for 20 hours, and 1 million cells were used for nuclear protein isolation and immunoblot analysis for HIF-1α. The histogram shows HIF-1α normalized to activating transcription factor 2 (ATF-2) levels expressed as a percent of HIF-1α in freshly isolated cells (Fr). * indicates significant difference compared with fresh isolates (analysis of variance; n = 6–9; P < .05).
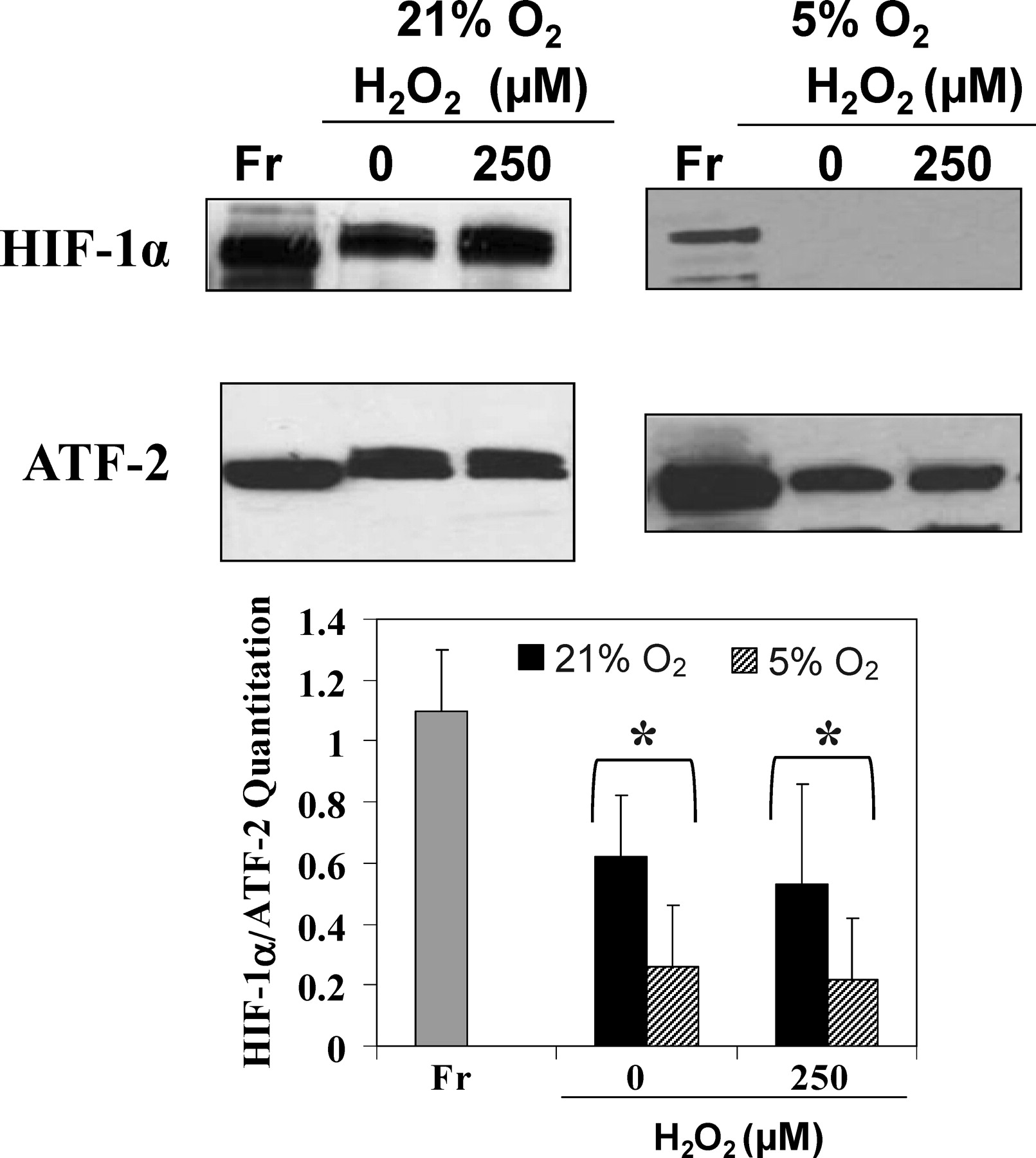
. Reactive oxygen species do not stabilize hypoxia-inducible factor (HIF)-1α in normoxic or hypoxic Leydig cells in vitro. Immunoblot analysis of fresh Leydig cells (Fr) and cells cultured at 21% oxygen or 5% oxygen for 6 hours in the presence or absence of H2O2. The histogram shows HIF-1α normalized to activating transcription factor 2 (ATF-2) levels expressed as a percentage of HIF-1α in cells cultured without H2O2. No statistically significant differences were observed between cells cultured with or without H2O2 (analysis of variance; n = 3; P < .05). * indicates significant difference of both 21% and 5% cultures compared with fresh isolates.
ROS Do Not Stabilize Leydig Cell Testicular HIF-1 In Vitro
Recently a number of investigators have demonstrated that ROS coupled to REDOX processes such as aerobic cellular respiration can stabilize HIF-1α under normoxic conditions (Pouyssegur and Mechta-Grigoriou, 2006). In addition, Leydig cells generate ROS as a result of steroidogenesis, Leydig cells closely associate with ROS-generating testicular macrophages in the interstitial spaces of the testis, and it is well established that ROS are implicated in many conditions of male infertility and testicular dysfunction (Turner and Lysiak, 2008). Because of this, we wanted to consider the possibility that ROS stabilize HIF-1α in Leydig cells under normoxic conditions. To determine if ROS has a stabilizing effect on HIF-1α in Leydig cells, isolated Leydig cells were cultured at 21% oxygen for 6 hours with 250 μM H2O2, and nuclear proteins were examined by immunoblot analysis. Results from these experiments showed no statistically significant differences in the levels of HIF-1α in normoxic Leydig cells (21% O2) following H2O2 treatment compared with HIF-1α levels in normoxic cells, which were not treated with H2O2. Further, we considered the possibility that perhaps a combination of hypoxic conditions coupled with ROS is required for maintaining HIF-1α expression. To explore this possibility, Leydig cells were cultured for 6 hours under presumptive hypoxic conditions (5% O2) in the presence or absence of H2O2 (Figure 7). Even under these conditions, the levels of HIF-1α in Leydig cells cultured under hypoxia were unaffected by H2O2. These results suggest that ROS do not stabilize testicular HIF-1α under normoxic or hypoxic conditions.
Discussion
We hypothesized that HIFs, particularly HIF-1, play important roles in molecular responses to oxygen changes in the testis. In this study, we showed that HIF-1α is the predominant HIF expressed in the rat testis and that Leydig cells are the primary cell type producing HIF-1. We did not detect HIF-2α or HIF-3α proteins in the normoxic or ischemic testis, suggesting that these HIFs likely do not have a significant role in oxygen regulation and the response to hypoxia in the testis. Although mRNAs are expressed for Hif-2α and Hif-3α, we do not know the mechanism responsible for suppressing protein production of these subunits in the testis; therefore, we cannot rule out the possibility that there may be conditions of hypoxia or anoxia that result in expression of HIF-2α or HIF-3α proteins in the testis. It has been shown that Hif-2α mRNA is expressed in many tissues even when steady-state levels of the protein cannot be detected. Although the mechanisms involved in this regulation are thought to involve destabilization and degradation of HIF-2α, this has not been clearly discerned (Wiesener et al, 2003).
HIF-2α in humans and rats is primarily present in highly vascularized tissues such as the brain, lung, heart, and liver, and is induced by hypoxia (Kietzmann et al, 2001; Wiesener et al, 2003). HIF-3α has been detected in a number of cell lines and mouse tissues including brain, heart, kidney, thymus, and lung (Gu et al, 1998), but HIF-3α protein is primarily localized to the perivenous zone of liver acini in rats (Kietzmann et al, 2001) and its function remains largely unknown. Initially it was suggested that HIF-2α and HIF-3α have redundant roles relative to HIF-1α, but HIF-2α may have differential roles in ischemic responses by activating subunit-specific target genes (Fedele et al, 2002; Hu et al, 2003; Sowter et al, 2003). Indeed, HIF-1 and HIF-2 are thought to have nonredundant roles, based in part on phenotypes observed in HIF-2α2/2 mice phenotypes (Rocha, 2007).
Other investigators have detected HIF-1α in the murine testis, which expresses an oxygen-independent germ cell–specific splice variant (HIF-1α1.1) in elongated spermatids and a hypoxia-induced ubiquitously expressed variant (HIF-1α1.2) (Marti et al, 2002). In our work, both immunocytochemistry studies and immunoblotting results using proteins from purified populations of testis cell types have demonstrated that Leydig cells are the primary source of HIF-1 in the testis. We have not definitively detected HIF-1α in spermatogenic cells.
Although in most tissues, HIF-1α is an oxygen-dependent subunit, HIF-1α protein is constitutively present in the normoxic rat testis and is unaffected by ischemia and reperfusion. It has been well established that the hypoxic up-regulation of HIF-1α protein and rapid degradation under normoxia is controlled through changes in protein stability (Semenza, 2000b; Wenger, 2000). The VHL tumor suppressor protein binds to the protein stabilization domain of HIF-1α to assemble a complex with E3 ubiquitin ligase, which targets HIF for polyubiquitination and subsequent proteasomal degradation (Ivan et al, 2001; Jaakkola et al, 2001). However, our immunoprecipitation experiments support the idea that testicular HIF-1α exists primarily in a nonubiquitinated state and suggest that it is likely to be an active protein.
Although the dogma on HIF-1α is degradation under normoxic conditions and stabilization following hypoxia, there is evidence of HIF activation under normoxic conditions. Studies by Lysiak et al (2009) reported normoxic expression of HIF-1α protein in murine Leydig cells with no increase in HIF-1α protein levels following ischemia up to 4 hours. We have previously also shown that HIF-1α is present in the normoxic epididymis (Palladino et al, 2004). Normoxic expression of HIF-1α has been reported in normoxic mouse brain and kidney (Stroka et al, 2001), rat kidney medulla (Bianciardi et al, 2006), cultured pulmonary artery smooth muscle cells (BelAiba et al, 2004), vascular smooth muscle cells (Page et al, 2008), and normoxic cytotrophoblast cells (Qian et al, 2004). In addition, nucleus pulposus cells of the intervertebral discs of several species that reside in a tissue with a limited vascular supply and primarily rely on anaerobic glycolysis to generate energy express high levels of HIF-1 (Risbud et al, 2006), as do chondrocytes, although cartilage is generally considered to a hypoxic tissue (Schipani et al, 2001).
In our initial studies on HIF-1 in the rat testis, we detected faint amounts of HIF-1α protein in the normoxic testis (Powell et al, 2002) using a different commercially available antibody, which detected a doublet at a lower molecular weight (∼90 kd) than expected. In those studies, we also saw a slight but statistically significant increase in HIF-1α protein following ischemic injury created by suturing the spermatic artery or by I/R surgery. In those studies, we did not examine as complete a range of time points for I or I/R as was carried out in this work. In addition, our previous studies were carried out using the volatile anesthetic halothane. We believe that halothane use may have contributed to systemic hypoxia and altered levels of HIF-1α protein in those experiments. Halothane has been shown to inhibit HIF-1α activation under hypoxic conditions in vitro and in vivo, which can lead to altered stability of HIF-1 protein and either abnormal accumulation or accelerated degradation of HIF-1α, depending on the degree of hypoxia preconditioning or anesthesia preconditioning prior to hypoxia (Itoh et al, 2001; Grimm et al, 2005). Because of this, we changed to a different anesthesia, sodium pentobarbital, which has not been reported to affect HIF-1α stability, for the studies described in this article.
The significance of constitutive expression of HIF-1α, and presumably active HIF-1, in the normoxic testis is not clear. But these results suggest fundamental differences in how the testis responds to changes in oxygen tension compared with other tissues. Historically, the testis has been described as a tissue on the “brink of hypoxia” (Setchell, 1978). This description is based on several anatomic and physiologic considerations, including studies demonstrating that testicular blood flow is comparatively low in relation to the metabolic requirements of the tissue (Setchell, 1964), the appreciable metabolic activity and oxygen consumption ability of developing spermatozoa, decreased oxygen tension inside the tubules compared with the interstitium, long diffusion distances between the seminiferous epithelium and the location of capillaries in interstitial spaces, and testicular venous blood contains less oxygen than the blood in most veins (Setchell et al, 1964; Free et al, 1976; Max, 1992; Wenger and Katschinski, 2005). It is likely that the avascular nature of the testis sets it apart from other more highly vascularized organs such as the kidney, liver, heart, and brain, with respect to oxygen tension and the molecular mechanisms that regulate oxygen homeostasis and compensatory responses to ischemia and hypoxia.
Pulsatile delivery of blood flow in testicular interstitial capillaries has also been observed (Damber et al, 1986; Hinton and Turner, 1993), coupled with cyclical variations in interstitial oxygen tension (Lysiak et al, 2000a), suggesting that local control (autoregulation) of blood flow is important for regulating oxygen concentration in the testis. In vitro studies have provided evidence that decreased oxygen tension is important for maintaining aspects of testis physiology. For example, it is known that reduced oxygen tension (1%–5% O2) is required for expression and activity of steroidogenic enzymes such as the mitochondrial cholesterol side-chain cleavage (P450scc) enzyme and P45017α-hydroxylase in mouse and rat Leydig cells (Perkins et al, 1988; Payne and Youngblood, 1995; Diemer et al, 2003).
To begin to examine the effects of oxygen tension potentially being responsible for stabilizing the HIF-1α protein in Leydig cells in vivo, we cultured Leydig cells under conditions of 5% (presumptive hypoxia) or 21% oxygen (ambient oxygen, presumptive normoxia) and then analyzed HIF-1α levels by immunoblotting. Surprisingly, the levels of HIF-1α were significantly reduced by both conditions. Several factors may explain these results. It is possible that culturing Leydig cells in the absence of other cells types such as Sertoli cells, spermatogenetic cells, and macrophages influences the signaling pathway controlling HIF-1 stabilization. In vivo perhaps the metabolic demands of Leydig cells or the metabolic demands of spermatozoa affect oxygen microenvironments affecting Leydig cell production of HIF. For example, sperm mitochondrial consumption of oxygen may be a factor influencing HIF activity. If in fact the testis is a hypoxic tissue in vivo, then culturing Leydig cells under presumptive conditions of normoxia (21%) may expose Leydig cells to hyperoxic conditions. This possibility has also been noted by other investigators (Lysiak et al, 2009). Indeed, a number of studies designed to measure physiologic oxygen tension in the testis have indicated that testicular pO2 levels are likely low, bordering hypoxia (Free et al, 1976; Lysiak et al, 2000a).
We do not yet have an explanation for the mechanisms responsible for constitutive levels of HIF-1α in the testis in vivo or the reduction of HIF-1α protein observed when Leydig cells were cultured at 5% or 21% oxygen. Changes in Hif-1α mRNA expression do not account for changes in the levels of HIF-1α protein in cultured cells. It is likely that HIF-1α protein levels are controlled via mechanisms that influence protein stability, but translational regulation of HIF-1α cannot be excluded as a potential regulatory mechanism.
HIF-1α protein can be stabilized and activated by nonhypoxic conditions, including oxidative phosphorylation and other metabolic pathways that release ROS (Lopez-Lazaro, 2006). ROS in particular are known to stabilize HIF-1α (Pouyssegur and Mechta-Grigoriou, 2006), and there is considerable evidence mounting that oxygen consumption, increases in oxygen metabolism, and ROS generation are also key regulatory pathways that affect HIF stability. It has been demonstrated that ROS can regulate HIF-1 stability and transcriptional activity in normoxic tissues (Pouyssegur and Mechta-Grigoriou, 2006). For example, in vitro H2O2 has been shown to contribute to a pro-oxidant state that promotes increases levels of HIF-1α in pulmonary smooth muscle cells (BelAiba and Gorlach, 2003).
Studies such as these led us to consider that ROS may be an important regulator of HIF-1α protein in vivo and in vitro in Leydig cells in part because ROS are known to influence Leydig cell physiology. For example, ROS generated by ambient oxygen (19%–21%) can cause free radical damage of cultured Leydig cells, leading to reductions in P450scc, P-45017α, and steroidogenic acute regulatory protein (Quinn and Payne, 1985; Diemer et al, 2003). Steroidogenic enzymes in Leydig cells generate ROS, and Leydig cells are susceptible to damage by ROS (Zirkin and Chen, 2000). Leydig cells are in close proximity to ROS-generating interstitial macrophages normally and during ischemic injury; yet Leydig cells do not undergo apoptosis following torsion, and they appear to be unaffected and perhaps protected from ischemic injury (Baker and Turner, 1995).
For these reasons, we hypothesized that ROS may activate HIF-1 in Leydig cells; however, when purified Leydig cells were cultured at 21% or 5% oxygen in the presence of 250 μM H2O2, there was no change in HIF-1α levels compared with cells cultured without exogenous H2O2. Studies by BelAiba and Gorlach (2003) have demonstrated that 10 to 50 μM H2O2 increased HIF-1α protein in cultured smooth muscle cells, whereas 100 μM H2O2 decreased HIF-1α levels in these cells. Nitric oxide (NO) has also been shown to induce stabilization of HIF-1α under both normoxic and hypoxic conditions in microvascular endothelial cells (Natarajan et al, 2005) and human oral squamous carcinomas (Quintero et al, 2006). Leydig cells are responsive to NO (Weissman et al, 2005), and NO has a number of roles in testis physiology; therefore, we cannot exclude the possibility that this free radical may be involved in stabilizing testicular HIF-1α.
The mechanisms involved in stabilizing HIF-1α in the testis are likely multifactorial and complex, and current studies in our laboratory are focused on understanding how the HIF-1α protein is stabilized and regulated in vivo and in vitro.
The physiologic relevance of constitutively expressed HIF-1α in the testis warrants further investigation. Studies are underway in our laboratory to determine if HIF-1 is active in the normoxic and hypoxic testis and to identify HIF-1 target genes to better understand function(s) of testicular HIF-1. Previously, we reported that in silico analysis of potential HIF-1 target genes in the testis revealed several potential HIF-1 target genes in germ cells, Sertoli cells, and Leydig cells, including genes involved in ROS metabolism (eg, superoxide dismutase), proapoptotic pathways, and antiapoptotic pathways (Powell et al, 2002).
Because germ cell apoptosis is a hallmark aspect of cell damage following ischemia and reperfusion in the testis and because several of the proapoptotic HIF-1 target genes were expressed in germ cells, we initially proposed a potential proapoptotic role for HIF-1 in ischemic and reperfusion injury. However, now that we have localized HIF-1 to Leydig cells, we believe that a more likely possibility is that HIF-1 may activate antiapoptotic target genes to protect Leydig cells from apoptosis during ischemia and reperfusion. It is well known that Leydig cells are not susceptible to apoptosis following ischemia and reperfusion. HIF-1 may also have other fundamentally important roles in Leydig cell physiology involving potentially novel mechanisms of HIF regulation and target gene activation.
In conclusion, the abundance of constitutively expressed HIF-1 in Leydig cells of the normoxic testis suggests a role for HIF-1 and Leydig cell in cellular and molecular responses to ischemia and hypoxia in the testis. Further examination of HIF-1 target genes in the normoxic and ischemic testis will be relevant to understanding the role of HIF-1 in Leydig cells and provide fundamental insight on the role of testicular HIF-1 in normal and pathophysiologic conditions of the testis such as testicular ischemic injury.
Acknowledgment
We dedicate this work to Dr Matthew P. Hardy, our dear friend and colleague who passed away while experiments were being completed for this study and while this manuscript was under preparation. We acknowledge Dr Charles Pineau (Group d'Etude de la Reproduction chez le Male-Institut National de la Santé et de la Recherche Médicale, Université de Rennes, Bretagne, France) for generously providing purified testicular cell types.




