Development, reliability, and validity of a diagnostic algorithm for sarcopenic dysphagia
Abstract
Background
Sarcopenic dysphagia is characterized by difficulty swallowing due to loss of whole-body skeletal and swallowing muscle mass and function. Despite multiple reports regarding sarcopenic dysphagia, no verified diagnostic methods exist. The purpose of this study was to develop a diagnostic algorithm for sarcopenic dysphagia and verify its reliability and validity.
Methods
First, our research group, the Working Group on Sarcopenic Dysphagia, developed a diagnostic algorithm for sarcopenic dysphagia. Patients 65 years and older who could follow commands were eligible for assessment using the algorithm. Patients without whole-body sarcopenia, with normal swallowing function, and with a disease that was an obvious cause of their dysphagia were considered not to have sarcopenic dysphagia. Then, swallowing muscle strength was assessed by tongue pressure. Those with poor swallowing muscle strength were deemed to be at probable risk for sarcopenic dysphagia and those with normal swallowing muscle strength to be at possible risk for sarcopenic dysphagia. Second, we applied the algorithm to inpatients 65 years and older and investigated their characteristics, muscle mass, muscle strength, motor function, swallowing muscle strength, swallowing function, and nutritional status. We investigated the reliability of the algorithm by analyzing intra- and inter-class correlation coefficients using kappa statistics. Third, we investigated the validity of the algorithm by analyzing the difference in the proportion of patients with sarcopenic dysphagia between the no malnutrition and malnutrition groups and investigated the risk factors for sarcopenic dysphagia using logistic regression analysis.
Results
A total of 119 patients participated in this study. Their mean age was 86.1 years, and 55 (46%) were men. Among the 119 patients, 32 were categorized as having possible sarcopenic dysphagia, 18 probable sarcopenic dysphagia, and 69 no sarcopenic dysphagia. The intra-class coefficient for the algorithm was 0.87 (95% confidence interval [CI]: 0.73-1.01), and the inter-class coefficient was 0.98 (95% CI: 0.92-1.02), indicating high intra- and inter-rater reliabilities. In the investigation of the algorithm's validity, 67 patients were analyzed. The proportion of patients with sarcopenic dysphagia was significantly higher in the malnutrition than in the no malnutrition group (P = 0.028). Malnutrition and age were independently associated with sarcopenic dysphagia (P = 0.013 and 0.003, respectively).
Conclusions
A diagnostic algorithm for sarcopenic dysphagia was developed, and its reliability and validity were verified. All older patients with sarcopenia or dysphagia should be assessed for sarcopenic dysphagia, which requires treatment involving not only rehabilitation for dysphagia but also nutritional improvement.
Introduction
Sarcopenic dysphagia is a novel concept characterized by difficulty swallowing because of the loss of whole-body skeletal and swallowing muscle mass and function [1–3]. The presence of dysphagia and whole-body sarcopenia are necessary diagnostic criteria for sarcopenic dysphagia, but these are not sufficient criteria (Table 1) [1]. Most individuals with dysphagia and loss of swallowing muscle mass and function have whole-body sarcopenia and thus may be diagnosed with sarcopenic dysphagia. In contrast, those with loss of swallowing muscle mass or muscle power without whole-body sarcopenia are not diagnosed with sarcopenic dysphagia, because this condition occurs only in patients with other neuromuscular diseases such as myositis and amyotrophic lateral sclerosis. Causes of sarcopenia are age, inactivity, malnutrition, and diseases including invasion (acute inflammation), cachexia (chronic inflammation), and neuromuscular diseases [4]. We determined that neuromuscular disease-related sarcopenia is not included among the causes of sarcopenic dysphagia, because dysphagia due to neuromuscular diseases appears to be a separate category from sarcopenic dysphagia. Dysphagic patients with age-, activity-, nutrition-, invasion-, cachexia-related sarcopenia of the whole-body and swallowing muscle, in whom the main cause of dysphagia is sarcopenia, but not neuromuscular diseases, are diagnosed with sarcopenic dysphagia.
| 1) Presence of dysphagia. |
| 2) Presence of whole-body sarcopenia (generalized loss of skeletal muscle mass and strength). |
| 3) The results of imaging tests (computed tomography, magnetic resonance imaging, ultrasonography) are consistent with a loss of swallowing muscle mass. |
| 4) The causes of dysphagia are excluded except for sarcopenia. |
| 5) The main cause of dysphagia is considered to be sarcopenia (if other causes of dysphagia such as stroke, brain injury, neuromuscular diseases, head and neck cancer, and connective tissue diseases exist). |
| Definite diagnosis: 1, 2, 3, 4 |
| Probable diagnosis: 1, 2, 4 |
| Possible diagnosis: 1, 2, 5 |
Whole-body sarcopenia is likely to occur prior to sarcopenic dysphagia. Maeda et al [5] reported that among patients without dysphagia, 68.8% have sarcopenia with a mean appendicular skeletal muscle index lower than the cutoff value reported by the Asian Working Group for Sarcopenia (AWGS) [6]. In contrast, 95.5% of patients with dysphagia have sarcopenia [5], suggesting that whole-body sarcopenia precedes sarcopenic dysphagia. Moreover, a patient with sarcopenic dysphagia after lung cancer surgery exhibited restored normal swallowing function, while whole-body sarcopenia persisted [7]. Therefore, whole-body sarcopenia should be included among the diagnostic criteria for sarcopenic dysphagia.
Sarcopenia and dysphagia are common in older people, especially in those over the age of 75 years. According to the International Sarcopenia Initiative, the prevalence of sarcopenia was 1%–33% in older individuals [8]. The prevalence of dysphagia has been reported to range between 11%–68% in older individuals [2]. Older patients with oropharyngeal dysphagia often have whole-body sarcopenia [9]. Diminished chewing ability and dysphagia are common among older Asian patients with sarcopenia [10]. The diagnosis of sarcopenic dysphagia is important because dysphagia increases the risk of related complications such as aspiration pneumonia, choking, dehydration, and malnutrition. Pneumonia, mainly aspiration pneumonia, in older adults is the third leading cause of death in Japan [11] following cancers and heart diseases. Moreover, sarcopenic dysphagia is both the cause and the result of aspiration pneumonia [1]. Furthermore, people with severe dysphagia who are not able to manage the oral intake of food have a lower quality of life attributable to loss of the pleasure of eating. Therefore, the management of sarcopenic dysphagia is an important current and future public health issue, and further advances in this area are required [12].
The association between sarcopenia and dysphagia has been investigated. Age-related loss of the muscle mass involved in swallowing, such as a decrease in tongue thickness [13], the geniohyoid muscle [14], and the pharyngeal lumen size [15] has been reported. Decreased tongue pressure [16–18] and head lifting strength [19] are associated with dysphagia. Loss of whole-body muscle mass [20–24] and hand grip strength [25, 26] are associated with dysphagia. Furthermore, whole-body sarcopenia is a risk factor for dysphagia in older individuals [5, 27, 28]. Moreover, sarcopenic dysphagia may occur in stroke patients with a severe risk of malnutrition, independently of neurological deficits [29]. These results indicate the importance of sarcopenic dysphagia, although evidence for this condition is limited.
The prevalence of sarcopenic dysphagia has not been reported, because no firm diagnostic criteria exist for this condition. At the symposium of the 19th Annual Meeting of the Japanese Society of Dysphagia Rehabilitation, a consensus was reached on the proposed diagnostic criteria for sarcopenic dysphagia (Table 1) [1]. Some case reports concerning treatment of sarcopenic dysphagia have used these diagnostic criteria [7, 30]; however, the reliability and validity have not been verified. Developing reliable and valid diagnostic criteria for sarcopenic dysphagia is fundamental to conducting research and improving the management of this condition in clinical practice.
The purpose of this study was to develop a diagnostic algorithm for sarcopenic dysphagia and verify its reliability and validity.
Methods
A cross-sectional study was performed in a convenient sample of patients who had been admitted to acute-care hospitals, convalescent rehabilitation hospitals, and long-term care hospitals between May 2015 and January 2016. Eligible patients were aged 65 years and older, recommended to undergo a dysphagia assessment or rehabilitation, able to answer the questionnaire, and provided written informed consent. Excluded patients were those for whom participation was deemed inappropriate by doctors because of aggravated general conditions, consciousness disturbance, and/or severe dementia. The Ethics Committee of the Hamamatsu City Rehabilitation Hospital approved the study. All patients provided written informed consent prior to enrollment.
A diagnostic algorithm for sarcopenic dysphagia (Figure 1) was developed by the Working Group on Sarcopenic Dysphagia, which is comprised of multidisciplinary experts and researchers of dysphagia and sarcopenia, including doctors specialized in rehabilitation, neurology, geriatrics, and dentistry, speech therapists, dieticians and physical therapists. Several meetings were conducted from September 2013 to February 2016. We referenced the Consensus Diagnostic Criteria for Sarcopenic Dysphagia [1], the Consensus Report of the AWGS [6], and the European Working Group on Sarcopenia in Older People (EWGSOP) criteria [4] when developing a diagnostic algorithm for sarcopenic dysphagia. We divided the diagnosis of sarcopenic dysphagia into three categories as follows: probable sarcopenic dysphagia, possible sarcopenic dysphagia, and no sarcopenic dysphagia. We did not include the category of definite sarcopenic dysphagia, because of difficulties encountered with measuring swallowing muscle mass and determining a cutoff value of swallowing muscle mass, which had not been established by the time of the study. Diagnosing definite sarcopenic dysphagia without assessing the swallowing muscle mass should be avoided.
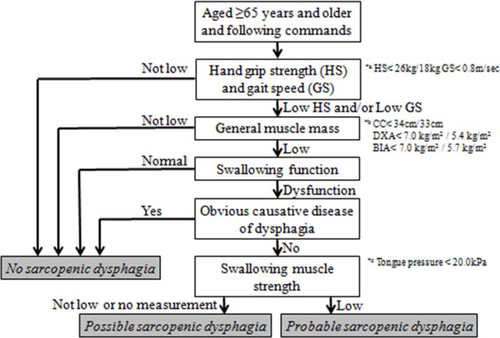
Diagnostic algorithm for sarcopenic dysphagia
- a) Cutoff values for hand grip strength (26 kg for men and 18 kg for women) and usual gait speed (0.8 m/s).
- b) Cutoff values for muscle mass: calf circumference, 34 cm for men and 33 cm for women; general muscle mass (measured by DXA), 7.0 kg/m2 for men and 5.4 kg/m2 for women; general muscle mass (measured by BIA), 7.0 kg/m2 for men and 5.7 kg/m2 for women
- c) Cutoff values for swallowing muscle strength: 20 kPa tongue pressure.
Patients 65 years and older who could follow commands were eligible to be assessed using the algorithm because the assessment of sarcopenia using the EWGSOP criteria was limited to patients aged 65 years and older [4] and following commands is necessary when using the algorithm. People without whole-body sarcopenia assessed using the AWGS criteria and who had normal swallowing function were judged as not having sarcopenic dysphagia. We used cutoff values for muscle strength measurements when assessing hand grip strength (<26 kg for men and <18 kg for women) and for usual gait speed (<0.8 m/s), both of which were values recommended by the AWGS criteria [6]. Appendicular muscle mass divided by height squared (dual X-ray absorptiometry [DXA]: 7.0 kg/m2 for men and 5.4 kg/m2 for women; bioimpedance analysis [BIA]: 7.0 kg/m2 for men and 5.7 kg/m2 for women) was used for assessing low general muscle mass [6]. In cases when DXA and BIA were not available, we used calf circumference for general muscle mass measurements with cutoff values (<34 cm for men and <33 cm for women) based on previous studies [31, 32].
Patients who had a disease that was the obvious cause of the dysphagia were excluded. However, patients with stroke, brain injury, neuromuscular disease, head and neck cancer, or connective tissue disease in whom the main cause of dysphagia was considered to be age-, activity-, nutrition-, invasion-, or cachexia-related sarcopenia were not excluded.
Finally, swallowing muscle strength was assessed by tongue pressure using a maximum tongue pressure measurement instrument (JMS, Hiroshima, Japan); this is a feasible method in clinical practice, and decreased tongue pressure is thought to be associated with sarcopenia and sarcopenic dysphagia [18]. Tongue pressure measurements using a balloon are becoming common in the clinical setting. Tongue pressure measurements are now covered by medical fees in Japan. Furthermore, several instruments used to measure tongue pressure, such as the JMS and Iowa Oral Performance Instrument, are available. Swallowing pressure should be measured primarily to assess the strength of the swallowing muscle. Measurement of swallowing pressure by manometry is available, but it requires invasive testing. Therefore, we recommend measurement of tongue pressure instead of swallowing pressure. Indeed, whole-body sarcopenia affects tongue pressure [33].
We measured tongue pressure using a balloon between the front of the palate and the tongue. Measurements were performed once calibration of the inner-balloon pressure stabilized to 19.6 kPa. This calibration was automatically performed by the instrument, and the display screen showed 0.0 kPa if the instrument was calibrated successfully. During the procedure, the patient compresses a balloon attached to the tip of the probe between the tongue and front of the hard palate with maximum voluntary effort. Tongue pressure was measured three times, and the maximum data were recorded. We set a cutoff value for low swallowing muscle strength of less than 20 kPa tongue pressure, because the mean tongue pressures reported in older individuals with and without dysphagia are 14.7 kPa and 25.3 kPa, respectively [18]. People with low swallowing muscle strength are judged as being at probable risk for sarcopenic dysphagia, and people with normal swallowing muscle strength are judged as being at a possible risk for sarcopenic dysphagia, because they have dysphagia without an obvious causative disease.
The patients' characteristics, including age, sex, disease history, severity of dysphagia, nutritional status, and results from the diagnostic algorithm for sarcopenic dysphagia were examined. The severity of dysphagia was assessed using the Food Intake Level Scale [34], which is a 10-point observer-rated scale that measures the severity of dysphagia, from most severe (Level 1) to least severe (normal oral intake; Level 10). More specifically, Levels 1 to 3 relate to various degrees of non-oral feeding, Levels 4 to 6 pertain to various degrees of oral food intake and alternative nutrition such as enteral and parenteral nutrition, and Levels 7 to 9 refer to various degrees of oral intake alone. The reliability and validity of the Food Intake Level Scale have been previously established [34]. Nutritional status was assessed using the Mini Nutritional Assessment-short form (MNA-SF) [35–37]. The MNA-SF is comprised of six questions addressing 1) decreased food intake over the past 3 months, 2) weight loss over the past 3 months, 3) mobility, 4) psychological stress or acute disease within the past 3 months, 5) neuropsychological problems, and 6) body mass index.
Approximately one-third of the participants were used to evaluate intra-rater reliability, and two-thirds of the participants were used to evaluate inter-rater reliability. We did not assign the participants to the intra- and inter-rater reliability evaluations randomly. For inter-rater reliability, patients were assessed twice by a different rater each time. For intra-rater reliability, patients were assessed twice by the same rater, with a 1-week interval between tests.
We analyzed the difference in the MNA-SF score among the no, possible, and probable sarcopenic dysphagia groups. The patients were divided into two groups based on their MNA-SF score: the no malnutrition group (MNA-SF score: 8–14) and malnutrition group (MNA-SF score: 0–7). Statistical analyses were performed using EZR [38] software version 1.31, which was developed from the open-source statistical software R (The R Foundation for Statistical Computing, Vienna, Austria) [39]. Continuous data were presented as the mean ± standard deviation (SD). Nonparametric data were expressed as the median and the interquartile range (IQR). Categorical data were described using a percentage. Intra-class and inter-class correlation coefficients were analyzed using Kappa statistics. The difference in the MNA-SF score between the no sarcopenic dysphagia and sarcopenic dysphagia groups was analyzed by Student's t-test and Wilcoxon signed rank test. The difference in the prevalence rate of sarcopenic dysphagia between the no malnutrition and malnutrition groups was investigated using the chi-squared test. The risk factors for sarcopenic dysphagia were analyzed by logistic regression analysis, using sarcopenic dysphagia as the dependent variable and malnutrition, age, and sex as the independent variables.
Results
A total of 119 patients participated in this study, and their characteristics are summarized in Table 2. Among the 119 patients, the mean age was 86.1 ± 7.7 years, and 55 (46%) were men. The majority of the causative diseases at admission were hip fractures (24) and pneumonia (21). The median of the Food Intake Level Scale was 8 (IQR: 7-10).
| Characteristics | Mean ± SDa |
|---|---|
| Total number of patients | 119 |
| Age, years | 86.1 ± 7.7 |
| Male gender, n (%) | 55 (46) |
| BMI, kg/m2 | 20.3 ± 4.4 |
| CC, cm | 29.2 ± 3.9 |
| Hand grip (right), kg | 15.2 ± 7.6 |
| Hand grip (left), kg | 14.5 ± 7.2 |
| 0.42 | |
| Gait speedb, m/s | (0-0.9) |
| FILSc | 8 (7-10) |
| 23.3 | |
| MTPd, kPa | (15.6-30.1) |
| Sarcopenia, n (%) | 105 (88) |
| Dysphagia, n (%) | 83 (70) |
| Diseases causing hospital | |
| admission, n | |
| Hip fracture | 24 |
| Pneumonia | 21 |
| Aortic aneurysm | 6 |
| Cerebral infarction | 6 |
| Head and neck cancer | 4 |
| Osteoarthritis of the knee | 4 |
| Lumbar compression | |
| fracture | 4 |
| Spinal cord injury | 4 |
| Vertebral fracture | 2 |
| ANCA-associated vasculitis | 2 |
| Ileus | 2 |
| Malignant lymphoma | 2 |
| Rotator cuff tear | 2 |
| Lumbar spinal canal stenosis | 2 |
| Colorectal cancer | 2 |
| Brain tumor | 2 |
| Other diseases | 30 |
- ANCA, antineutrophil cytoplasmic antibody; BMI, body mass index; CC, calf circumference; FILS, Food Intake LEVEL Scale; IQR, interquartile range; MTP, maximum tongue pressure
- a All values are the mean ± standard deviation (SD) unless otherwise indicated
- b Values represent the median (IQR) for n=104
- c Values represent the median (IQR)
- d Values represent the median (IQR) for n=76
In the diagnostic algorithm used for the assessment of sarcopenic dysphagia, 105 patients had sarcopenia, 73 of whom had dysphagia with concurrent sarcopenia. Among the 73 dysphagia patients, 23 were excluded because they had dysphagia caused by another disease. The remaining 50 patients were divided into two groups: 32 patients were categorized as having possible sarcopenic dysphagia and 18 patients were categorized as having probable sarcopenic dysphagia. Among all of the patients, 69 had no sarcopenic dysphagia (The intra-rater reliability was assessed in 41 patients and the inter-rater reliability was assessed in 78 patients. The intra-class coefficient was 0.87 (95% confidence interval [CI]: 0.73-1.01), and the inter-class coefficient was 0.98 (95% CI: 0.92-1.02), indicating high intra-rater and inter-rater reliability.
Of the 119 total patients, 90 were administered the MNA-SF. Analysis of the algorithm's validity was performed in 67 patients who had no obvious diagnosis for dysphagia (Table 3). There were 22 patients in the no sarcopenic dysphagia group and 45 in the sarcopenic dysphagia (28 and 17 in the possible and probable risk subgroups). The prevalences of sarcopenic dysphagia in the no malnutrition and malnutrition groups were 45.5%, and 76.0%, respectively. The proportion of patients with sarcopenic dysphagia was significantly higher in the malnutrition group than in the no malnutrition group (P = 0.028). In the logistic regression analysis, malnutrition and age were independently associated with sarcopenic dysphagia (P = 0.013 and 0.003, respectively).
| Characteristics | Total (n=67) | Malnutrition (n=46) | No malnutrition (n=21) | P value |
|---|---|---|---|---|
| Age, years a | 82.5 ± 6.7 | 82.5 ± 6.5 | 82.5 ± 6.7 | 0.445 c |
| Male gender, n (%) | 32 (47.8) | 22 (47.8) | 10 (47.6) | 0.934 e |
| BMI, kg/m2a | 20.4 ± 4.3 | 19.4 ± 3.67 | 22.6 ± 4.8 | 0.007 c |
| CC, cm a | 28.8 ± 4.1 | 28.0 ± 3.6 | 30.6 ± 4.6 | 0.021 c |
| Hand grip (right), kg a | 13.9 ± 6.5 | 13.3 ± 6.6 | 15.4 ± 6.2 | 0.111 c |
| Hand grip (left), kg a | 13.3 ± 6.1 | 13.1 ± 6.4 | 13.7 ± 5.4 | 0.359 c |
| Gait speed, m/s a | 1.32 ± 3.3 | 0.9 ± 2.5 | 2.3 ± 4.6 | 0.127 c |
| FILS b | 8 (6-9) | 8 (4-9) | 8 (7-10) | 0.199 d |
| MNA-SF (score) b | 7 (4-8) | 5 (4-7) | 9 (8-10) | <0.001 d |
| MTP, kPa a | 22.4 ± 13.4 | 22.3 ± 14.8 | 22.6 ± 4.76 | 0.437 c |
| Sarcopenia, n (%) | 57 (85.1) | 40 (87.0) | 17 (81.0) | 0.713e |
| Dysphagia, n (%) | 57 (85.1) | 42 (91.3) | 15 (71.4) | 0.060e |
| Sarcopenic dysphagia | ||||
| Probable and Possible, n (%) | 45 (67.2) | 35 (76.1) | 10 (47.6) | 0.028 e |
| Probable, n (%) | 17 (25.3) | 14 (30.4) | 3 (14.3) | |
| Possible, n (%) | 28 (41.8) | 21 (45.6) | 7 (33.3) | |
| No sarcopenic dysphagia, n (%) | 22 (32.8) | 11 (23.9) | 11 (52.4) |
- BMI, body mass index; CC, calf circumference; FILS, Food Intake LEVEL Scale; MNA-SF, Mini Nutritional Assessment Short-Form; MTP, maximum tongue pressure
- a Mean ± standard deviation
- b Median (interquartile range)
- c Student's t test
- d Wilcoxon signed rank test
- e chi-squared test
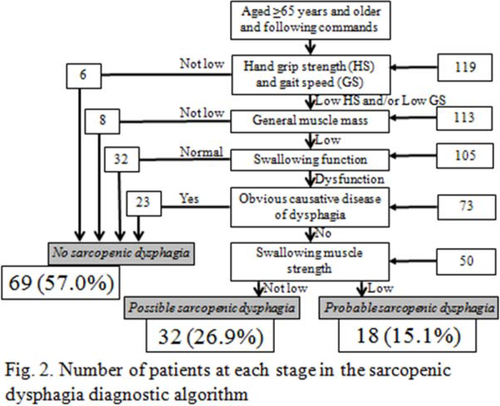
Distribution of patients according to the risk of sarcopenic dysphagia using the diagnostic algorithm
A total of 105 patients had sarcopenia, 73 of whom had dysphagia. There were 32 patients with possible sarcopenic dysphagia, 18 patients with probable sarcopenic dysphagia, and 69 patients with no sarcopenic dysphagia.
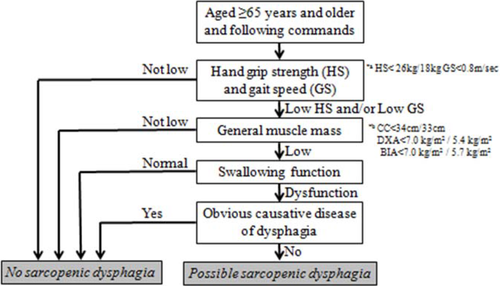
Abridged version of the diagnostic algorithm
Patients with whole-body sarcopenia, swallowing dysfunction, and no obvious causative disease of dysphagia can be diagnosed with possible sarcopenic dysphagia without measuring tongue pressure.
- a) Cutoff values for hand grip strength (26 kg for men and 18 kg for women) and usual gait speed (0.8 m/s).
- b) Cutoff values for muscle mass: calf circumference, 34 cm for men and 33 cm for women; general muscle mass (measured by DXA), 7.0 kg/m2 for men and 5.4 kg/m2 for women; general muscle mass (measured by BIA), 7.0 kg/m2 for men and 5.7 kg/m2 for women
Discussion
We performed a cross-sectional study and made three important clinical advancements. First, we developed a diagnostic algorithm for sarcopenic dysphagia. Second, we verified the reliability of a diagnostic algorithm for sarcopenic dysphagia by assessing inter- and intra-rater reliabilities. Third, we verified the validity of the diagnostic algorithm by evaluating the association between sarcopenic dysphagia and malnutrition.
We have developed a diagnostic algorithm specifically to identify sarcopenic dysphagia. The strength of the diagnostic algorithm is that it confers the ability to diagnose probable or possible sarcopenic dysphagia without assessing swallowing muscle mass. The measurement of swallowing muscle mass is more difficult to achieve than is the measurement of whole-body sarcopenia and tongue pressure. Further, setting a cutoff value for swallowing muscle mass to enable the diagnosis of sarcopenic dysphagia is also quite difficult. Therefore, the improved feasibility of diagnosing sarcopenic dysphagia using the diagnostic algorithm can promote its research and management in clinical practice.
We verified the reliability of the diagnostic algorithm for sarcopenic dysphagia. The kappa coefficients for the intra- and inter-class reliabilities were 0.87 and 0.98, respectively, indicating that this diagnostic algorithm is highly reliable; therefore, all qualified medical staff will be able to utilize this tool.
We also verified the validity of the diagnostic algorithm for sarcopenic dysphagia. Malnutrition was independently associated with sarcopenic dysphagia. Malnutrition is an independent risk factor for sarcopenic dysphagia in hospitalized older patients [40]. Furthermore, nutrition improvement seems to be necessary for patients with sarcopenic dysphagia to improve swallowing function [7, 41, 42]. Therefore, an association between malnutrition and sarcopenic dysphagia is reasonable.
Not every examiner has an instrument to measure tongue pressure. When tongue pressure measurements are not available, we suggest two alternatives. First, we propose using an abridged version of the diagnostic algorithm for cases in which tongue pressure measurement is difficult. Patients with whole-body sarcopenia, swallowing dysfunction, and no obvious causative disease of dysphagia can be diagnosed with possible sarcopenic dysphagia without measuring tongue pressure (Figure 3). Second, we suggest assessing head lifting strength, which does not require any instruments. The ability to lift the head reflects the strength of the suprahyoid muscles, and individuals who cannot lift their head in the supine position tend to have dysphagia [19]. However, we recommend tongue pressure measurements to assess the strength of the swallowing muscle.
In patients with severe cognitive impairment, measurements of muscle strength and physical function are difficult. Diagnosis of sarcopenic dysphagia in patients with cognitive impairment is important, because cognitive impairment is associated with sarcopenia [43], and low skeletal muscle mass was associated with poor swallowing function in patients with Alzheimer's disease [24]. Using the diagnostic algorithm for sarcopenic dysphagia may be difficult in patients with cognitive impairment. However, measurement of muscle mass and assessments of activities of daily living, presence of dysphagia, and obvious conditions considered to be the main cause of dysphagia are possible. In our personal opinion, cognitively impaired patients with low whole muscle mass, low activities of daily living, swallowing dysfunction, and no obvious condition considered to be the main cause of dysphagia can be diagnosed with possible sarcopenic dysphagia without assessing muscle strength or physical functioning. However the reliability and validity of this opinion were not verified.
Therapy for sarcopenic dysphagia includes rehabilitation for the dysphagia and treatment for the sarcopenia [1], both of which require a multidisciplinary medical team. Regardless of whether there is a possible or probable risk of sarcopenic dysphagia, it is essential to treat sarcopenic dysphagia using a combination of nutritional management to increase muscle mass and early dysphagia rehabilitation [1, 2]. Resistance training for the swallowing muscles, such as head-raising exercises and tongue-strengthening exercises, should be included in dysphagia rehabilitation, especially in cases of probable sarcopenic dysphagia. Early oral intake has been shown to promote an early hospital discharge in older patients with pneumonia [44, 45]. Furthermore, early physical therapy decreases in-hospital mortality after aspiration pneumonia [46]. The improvement of nutrition and early dysphagia rehabilitation may prevent and treat sarcopenic dysphagia, by improving activity- and nutrition-related sarcopenia.
This study has some limitations. First, the sample size of the study was small, and we did not include healthy older individuals. While investigations of healthy older individuals would be useful, diagnosis and treatment of sarcopenic dysphagia in hospitalized patients are clinically urgent. Second, selection bias possibly exists because consecutive sampling was not used in this study. Therefore, the proportion of general sarcopenia among all patients may be not typical. Third, we used calf circumference for general muscle mass measurements, with cutoff values of <34 cm for men and <33 cm for women. DXA or BIA are more appropriate tools for measuring muscle mass, and a different cutoff value for calf circumference (<33 cm for men) has been reported previously [47, 48]. Finally, the investigation of validity was performed in only 67 patients, because no MNA-SF data were available in the remaining patients.
In conclusion, we have developed and verified the reliability and validity of a diagnostic algorithm for sarcopenic dysphagia. All older patients with sarcopenia or dysphagia should be assessed for sarcopenic dysphagia to see if it also exists, as it can occur and requires treatment that involves not only dysphagia rehabilitation but also nutritional improvement.
Acknowledgment
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle - Clinical Reports (von Haehling S, Ebner N, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle - Clinical Reports. J Cachexia Sarcopenia Muscle Clinical Reports 2016;1;e28:1-2). This work was supported by a research Grant-in-Aid for Scientific Research C (no. 16K01460) from the Ministry of Education, Science, Culture, Sports, Science, and Technology of Japan. Hidetaka Wakabayashi received reimbursement for travel expenses from Nestlé Health Sciences. Takashi Mori, Ichiro Fujishima, Fumiko Oshima, Masataka Itoda, Kenjiro Kunieda, Jun Kayashita, Shinta Nishioka, Akiko Sonoda, Yoshitosi Kuroda, Minoru Yamada, Sumito Ogawa declare no conflicts of interest. The study protocol was approved by the ethics committee of Hamamatsu City Rehabilitation Hospital. This study has been performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki and later amendments.




