Littoral Fish Community Response to Smallmouth Bass Removal from an Adirondack Lake
Present address: Center for Limnology, University of Wisconsin, Madison, Wisconsin 53706, USA.
Abstract
Large-scale observational studies in eastern Canada and the northeastern USA have concluded that introduced littoral predators are responsible for reductions in native fish diversity and abundance. To determine whether nonnative predator removal could increase native littoral fish abundance, we removed 47,682 smallmouth bass Micropterus dolomieu from a 271-ha Adirondack lake during a 6-year period. Two years after removal began, habitat-stratified snorkel surveys indicated a greater than 90% reduction in smallmouth bass abundance. The relative abundances of six native littoral species increased (4-90 times preremoval abundances) within 2 years of smallmouth bass removal. Decreased relative predation risk during the experiment reflected the reduction in littoral predators and identified seasonal differences in nearshore predation risk. The smallmouth bass population was resilient to removal, producing strong year-classes throughout the experiment. Mechanical removal was successful at decreasing smallmouth bass abundance and increasing native fish abundance, but removal must be conducted on a yearly basis to maintain low smallmouth bass population abundance. Our results provide experimental evidence regarding the need to prevent littoral predator introductions in Adirondack waters and offer support for nonnative control wherever native fish species conservation is a management priority.
Despite frequent observations that nonnative piscivore introductions into freshwater lakes have had severe negative effects on native fish communities (Zaret and Paine 1973; Okemwa 1981), few published studies have reported results from removal efforts intended to reverse the effects of these introductions. In this study, we report the response of a native littoral fish community to large-scale removal of a nonnative littoral piscivore that has had widespread, negative effects on both native prey fish and sport fish populations in north temperate lakes (Chapleau et al. 1997; Vander Zanden et al. 1999; Whittier and Kincaid 1999). Previous observational studies have shown that introductions of smallmouth bass Micropterus dolomieu, largemouth bass M. salmoides, and northern pike Esox lucius have been associated with extirpations and declines of small-bodied, nearshore native fishes (Chapleau et al. 1997; Whittier and Kincaid 1999). In Ontario lakes, black bass introductions alter the food web and reduce growth of the native apex predator, lake trout Salvelinus namaycush, by reducing littoral prey fish abundance (Vander Zanden et al. 1999; Pazzia et al. 2002). Historically, littoral predator introductions resulted from resource management agency efforts to diversify sport fisheries, but more recent introductions have been attributed to natural dispersal and unauthorized introductions (Jackson 2002; Warner 2005).
Vander Zanden et al. (2004) suggested that future conservation of native littoral fishes in north temperate lakes should focus on preventing further introductions by identifying lakes where native species are vulnerable and the potential for introduction is great. While such prevention efforts are vital for maintaining current species diversity, they do not provide for conserving or restoring native species in waters where nonnative predators have established abundant populations. Whole-lake chemical piscicide applications have helped restore fish communities disturbed by nonnative fish introductions, but this method is limited by lake size and public concerns about the health risks of chemical use (Harig and Bain 1998; Cailteux et al. 2001). Mechanical removal of nonnative fishes provides an alternative approach to chemical reclamation, yet formal evaluations of the effectiveness of mechanical removal are scarce and are often summarized in government reports (Meronek et al. 1996). Fisheries managers are apparently repeating a pattern described for eradication efforts targeting nuisance mammals that plague islands (e.g., goats, rats), in that such studies are usually summarized in non-peer-reviewed literature and lack sufficient detail to evaluate the methods, timing, or success of eradication (Campbell and Donlan 2005). This observation led Donlan et al. (2003) to call for the publication of more results from terrestrial invader eradication efforts instead of allowing the literature to be dominated by studies of invasive species' impacts and population biology.
Experimental manipulations of piscivore abundance have provided important insights regarding ecological interactions between trophic levels in lake systems and have provided a rationale for large-scale predator removal as a fisheries management tool to increase the abundance of prey fishes (Beamesderfer et al. 1996). However, it is important to contrast the scale of predator removal from waters where trophic ecology experiments were conducted with the challenge of removing established predators from large aquatic systems. Studies that conclusively established a causal link between piscivore reductions and increases in prey fish abundance were conducted in small ponds less than 11 ha in surface area (Swingle 1946; Carpenter and Kitchell 1993; Mittelbach et al. 1995). In contrast, the refereed journal literature is silent about the success of targeted programs to remove nonnative piscivorous fish from large waters of anthropogenic interest. Such efforts include widely publicized attempts to remove lake trout from Yellowstone Lake, northern pike from Lake Davis, California, and a series of predators from the Colorado River (news media accounts provide the most comprehensive record of such efforts, but also see Osmundson 2003 and Mueller 2005).
Tonn and Magnuson (1982) showed a correlation between presence of minnows and the absence of littoral predators in small northern Wisconsin lakes, after which a causal mechanism for this observation was strengthened by an experiment in which introduced piscivores caused sharp declines in minnow abundance (He and Kitchell 1990). Similarly, observational studies of lakes across eastern Canada and the northeastern United States have shown a negative correlation between native littoral fish diversity or abundance and the presence of introduced littoral predators (Whittier and Kincaid 1999; Findlay et al. 2000; MacRae and Jackson 2001). These studies offer strong observational evidence for the effect of introduced predators on native littoral fishes, but no experiments have previously been conducted to evaluate these observations and the extent to which they can be reversed.
With this in mind, our study was designed to determine whether native littoral fish abundances could be increased by reducing the nonnative smallmouth bass population in an Adirondack lake. The study objectives were to determine (1) whether mechanical removal could be used to reduce smallmouth bass abundance, (2) whether native littoral fish abundances would increase in response to decreased smallmouth bass abundance, and (3) whether declining prey fish predation risk could be quantified after the removal.
Methods
Study site
Little Moose Lake, located on a private preserve within the Adirondack Park of New York, has a surface area of 271 ha, a maximum depth of 44 m, a mean summer total phosphorus level of approximately 4 μg/L, and a mean summer Secchi depth of 7.3 m; the lake is fed by five first-order tributaries. Human disturbance to the littoral zone is minimal; approximately 20 boathouses are located along its shoreline. The lake supports three self-sustaining salmonid populations: lake trout, brook trout Salvelinus fontinalis, and round whitefish Prosopium cylindraceum. The littoral fish community consists of creek chub Semotilus atromaculatus, pumpkinseeds Lepomis gibbosus, white suckers Catostomus commersonii, common shiners Luxilus cornutus, brown bullheads Ameiurus nebulosus, and central mudminnow Umbra limi. Juvenile brook trout are also considered a littoral species in our analyses (Biro 1998). Smallmouth bass were introduced into the lake during the 1940s and were briefly described in management reports as “abundant” by 1955 (Warner 1952; Webster 1955). The establishment of a smallmouth bass population in the 1950s coincided with local observations that “minnows” had disappeared from the lake. Studies conducted in 1996 and 1997 concluded that smallmouth bass were the dominant fish in the littoral zone during spring and summer (Brown et al. 2000). A diet study found that large smallmouth bass consumed slimy sculpin Cottus cognatus, but the study failed to document smallmouth bass predation on littoral prey fish (Weidel et al. 2000).
The experimental design for the fish community manipulation originally included a reference lake that would have allowed us to employ a before–after control–impact design to analyze study results. However, smallmouth bass abundance in the reference lake was severely reduced due to handling and tagging mortality during the initial year of premanipulation data collection. Because the magnitude of the mortality in the reference lake was unknown, the experimental design was modified to only evaluate responses in the manipulated lake and to do so over a longer time period. The before–after design cannot statistically differentiate between stochastic variation in native fish populations and changes due to the manipulation; therefore, our results and conclusions focus on describing differences in the fish populations before and after the removal rather than conclusively stating that observed differences were due to smallmouth bass removal.
Removal and evaluation
Boat electrofishing was used to assess littoral zone fish abundances and to remove smallmouth bass. The electrofishing boat was equipped with a Smith-Root Type VI-A pulsator (set at 120 pulses/s; 1,016-V DC) and a 6,500-W Honda generator. Pulse width was adjusted to produce an output of approximately 5–6 A. Littoral zone assessment electrofishing was conducted by successively pulling in perpendicular to the shoreline for the entire length of a delineated shoreline section. This technique was effective at capturing all species present in the littoral zone from a depth of approximately 2 m to the edge of the water. All fish collections were made using one of two boat operators and an experienced netting crew.
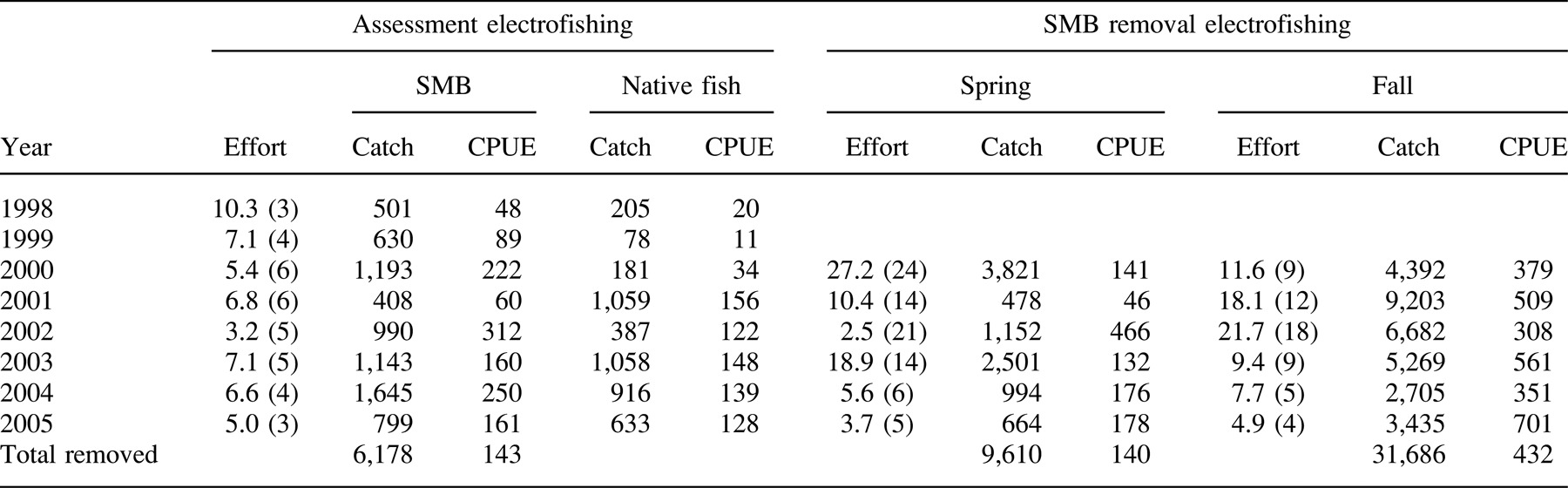
Assessment electrofishing to determine littoral zone fish abundances was conducted each spring (between May 15 and June 15) from 1998 to 2005, during which time the entire lake shoreline was sampled in three to five successive nights. Captured fish were identified, measured, and then released in the shoreline section where they were captured. Electrofishing catch per unit effort (CPUE) of native littoral species was calculated using counts of prey fish that were of a length vulnerable to smallmouth bass predation (Demers et al. 2001). Previous laboratory experiments determined that the largest smallmouth bass in the population (∼380 mm) could consume deep-bodied fish (e.g., pumpkinseeds) less than 110 mm in length and shallow-bodied fish (e.g., creek chub) less than 215 mm (authors' unpublished data). Smallmouth bass CPUE was also determined using the yearly assessment electrofishing samples. In 2001 and 2002, a limited number of smallmouth bass were removed before assessment electrofishing, although the number removed was less than 5% of the total removed each spring.
Smallmouth bass removal began in spring 2000 and continued through fall 2005; the total removal effort varied each year. Day and night smallmouth bass removal electrofishing was employed during May–June and September–November (outboard motor use on the study lake was prohibited during July and August). Total length (TL) was measured for all smallmouth bass removed from the lake. Angling and gill nets were used to remove a limited number of smallmouth bass during July and August.
Smallmouth bass were counted via line-transect snorkel surveys from 1999 to 2001 to provide a habitat-specific measure of smallmouth bass relative abundance independent of the electrofishing removal effort. The littoral zone was classified into four habitat types based on substrate type and presence or absence of woody cover. We classified substrate as either rock (particle size > 2.0 mm) or fine sediment (particle size < 2.0 mm), and woody cover was defined as the presence of at least three downed trees (basal diameter > 25 cm) per 20 m of shoreline. Eight transects (20 m long × 4 m wide) were placed in each of the four littoral habitats (rock, rock–wood, fine sediment, fine sediment–wood). Transect locations were randomly chosen and permanently delineated, parallel to shore, and the transect midline was located at the 2-m depth. All 32 transects were snorkeled in a single day (0900–1500 hours) every 2–3 weeks during mid-May through September. The number of snorkeling occasions varied annually (8, 10, and 6 for 1999, 2000, and 2001, respectively). Smallmouth bass were counted, and length was estimated to the nearest 50-mm size increment. Size reference markers (painted alternately red and white at 50-mm increments) were anchored along the sampling transects to provide a reference for size estimates. The average Secchi disk transparency was greater than 7 m during the summer and was never less than 5 m for any sampling date throughout the experiment. Snorkel observations were also used to describe smallmouth bass habitat use relative to the presence or absence of wood habitat in the littoral zone.
Prey fish predation risk was measured for 3 study years (1999–2001) using tethered creek chub. Tethering provides an index of relative predation risk, measured as the proportion of prey attacked by predators from a standard number of tethers set for a fixed time interval (Post et al. 1998). Two tether lengths, 1.5 and 10.0 m, were constructed of 22.6-kg-test monofilament line that was vertically hung between a float and anchor. Juvenile creek chub (60–100 mm TL) were anesthetized and tied to a 0.4-m length of 0.9-kg-test monofilament. Each tethered fish was attached to the vertical line 0.5 m from the anchor, allowing the creek chub to swim in a 0.4-m radius around the tether but not to reach the lake bottom. All tethered creek chub survived and were retained after 6 h (n = 10) during initial tests of tethers in a 3,200-L tank. In the field, a tethered fish was considered to be attacked by a predator if the fish was missing, the monofilament loop was pulled tightly around the head, or the scales had been scraped from the fish (Post et al. 1998). Tethering trials were conducted at a consistent location with 25 or 50 tethers set concurrently at two depths (1.5 and 10 m) for 6 h. Day (900-1500 hours) and night (2200–400 hours) trials were conducted 2–5 d apart in July and again in August of 1999–2001 (total tethers set: 250 in 1999, 300 in 2000, and 200 in 2001). Three additional fish were tethered with hooks at both depths during all July trials (n = 18) to identify predators of tethered fish (e.g., Post et al. 1998).
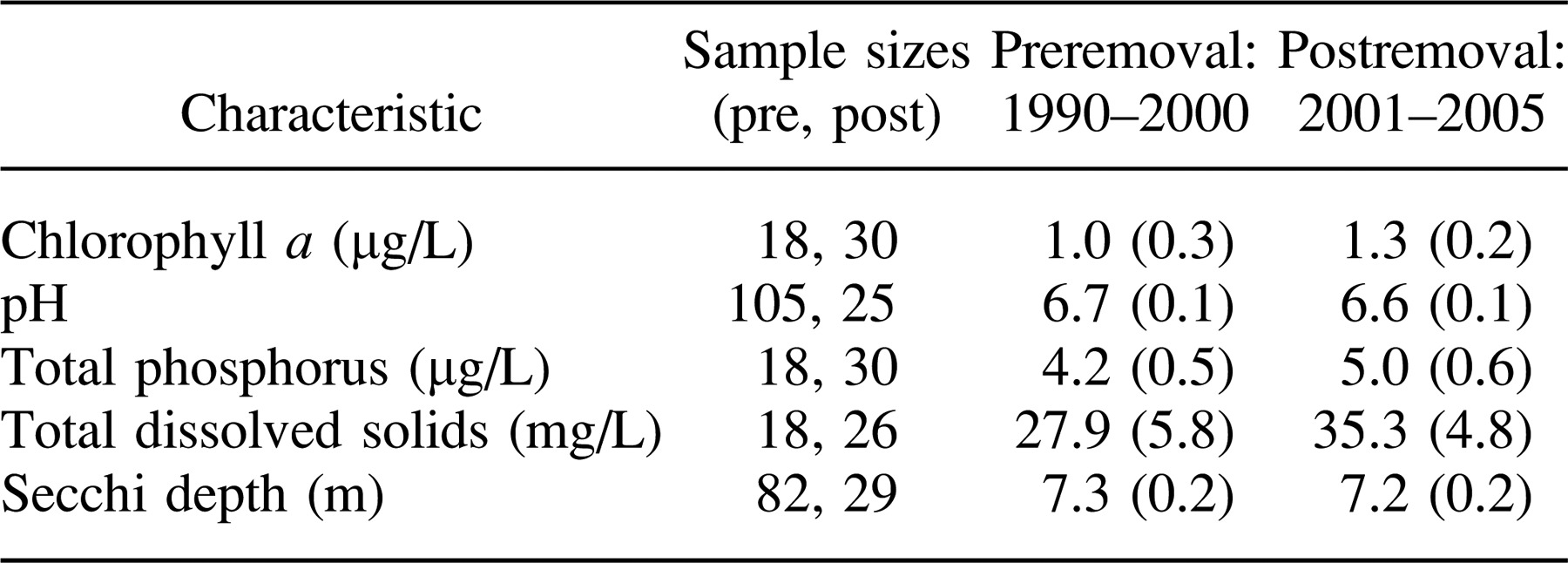
Data from routine chemical and limnological monitoring were collected throughout the study to confirm that abiotic conditions within the lake were consistent between the preremoval and postremoval time periods. Total phosphorus, total dissolved solids, pH, and chlorophyll a were measured from depth-integrated epilimnetic samples collected each summer during the preremoval (1990–2000) and postremoval (2001–2005) periods.
Data analyses
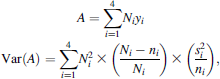
Catch rates for three size-classes of smallmouth bass from assessment electrofishing were calculated to show the relative abundance of smallmouth bass each spring before removal efforts for that year. To address our second objective, cumulative spring electrofishing catch rates from preremoval years were pooled (n = 3 years), and a t-test assuming unequal variances (α = 0.05) was used to compare preremoval data with the pooled postremoval data (n = 5 years) for seven littoral species. Cumulative spring catch rates were calculated as the total number of fish caught during the whole-lake assessment divided by the total hours of effort. We also estimated changes in the size structure of native fish populations from spring assessment electrofishing catches. We compared the mean length of fish between the preremoval and postremoval periods using a t-test assuming unequal variances (α = 0.05), while only considering native fishes of a size vulnerable to smallmouth bass predation.
To determine whether predation risk changed during the manipulation, a logistic regression model was used to evaluate the tethering results (S-Plus software, version 6.0). The tethered fish's probability of being attacked (dependent variable = 1 for attacked or 0 for not attacked) was calculated based on four main independent factors: depth (1.5 or 10.0 m), day or night, season (July or August), and year (1999, 2000, or 2001). All two-way interactions for combinations of depth, season, and year were included in the model. Differences between day and night trials were expected to be consistent across years, seasons, and depths (Emery 1973). Therefore, day–night interactions were excluded from the model. The significance of two factors—year and season—was assessed based on a partial F-test statistic that compared the model's deviance when the factor was excluded with the full model's deviance.
Results
We removed 47,682 smallmouth bass from Little Moose Lake by electrofishing, gillnetting, and angling during spring 2000 through fall 2005. Of that total, 47,474 smallmouth bass (99.5%) were caught by boat electrofishing and the remaining 208 fish were removed by gillnetting and angling. Electrofishing was the most efficient removal method: the average electrofishing catch was 274 smallmouth bass/trip, whereas the average for gillnetting was 9 fish/trip (9 trips) and the average for angling was 12 fish/trip. Removal electrofishing effort was highest during the first 4 years of the experiment (23–39 trips/year) but decreased to approximately 10 trips/year in 2004 and 2005 (Table 1). Broad size ranges of smallmouth bass were captured during spring, including spawning adults; fall samples were dominated by age-0 and juvenile smallmouth bass (Figure 1).
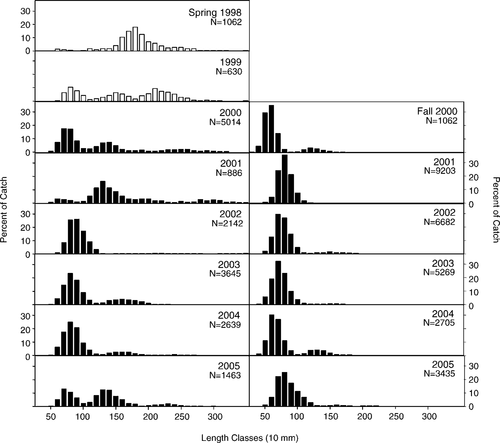
Length frequency histograms of smallmouth bass caught by boat electrofishing in Little Moose Lake, New York (1998–2005). Open bars correspond to initial preremoval surveys, when captured smallmouth bass were returned to the lake (1998 and 1999); filled bars represent the period in which smallmouth bass were removed (2000–2005). For each panel, N represents the total number caught for that season and year.
Line-transect snorkel surveys indicated that abundance of smallmouth bass larger than 50 mm was reduced by 88% within the first year and remained low through 2001 (Figure 2). Yearly smallmouth bass abundance estimates from 2000 and 2001 were significantly lower than the 1999 estimate for the total smallmouth bass population (F2,21 = 39.58, P < 0.001) and the adult (TL > 200 mm) population (F2,21 = 25.85, P < 0.001). Reductions from 2000 to 2001 were not significant (P = 0.235) for the total or adult smallmouth bass population. Overall yearly means indicated an 88% reduction in the smallmouth bass population from 1999 to 2000, a 43% reduction from 2000 to 2001, and a 92% reduction from 1999 to 2001 (Figure 2). Snorkel transects containing woody cover contained the highest proportion of smallmouth bass relative to those without wood for all sizes of smallmouth bass (76, 85, and 73% for 1999, 2000, and 2001, respectively) and for those greater than 200 mm (95, 95, and 100% for 1999, 2000, and 2001, respectively).
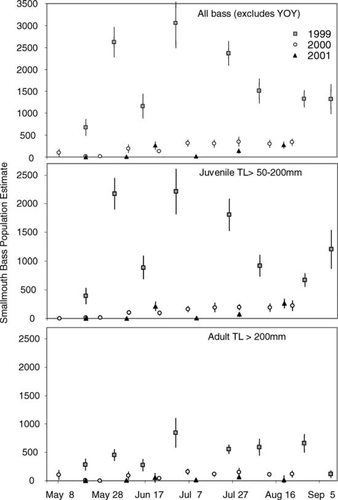
Smallmouth bass population estimates (±SE) from line-transect snorkel surveys in Little Moose Lake, New York, 1999–2001. Seasonal abundance of all smallmouth bass (TL > 50 mm) is shown in top panel; juvenile (TL = 50–200 mm) abundance is shown in middle panel; and adult (TL > 200 mm) abundance is shown in bottom panel.
Smallmouth bass catch rates from assessment electrofishing indicated that early spring relative abundances did not decline after removal for two of the three size-classes evaluated (<100 and 100–200 mm) (Figure 3). Catch rates represent smallmouth bass relative abundance before the removal effort began in any given year. Catch rates of smallmouth bass greater than 200 mm were lower in the postremoval period than in the preremoval period.
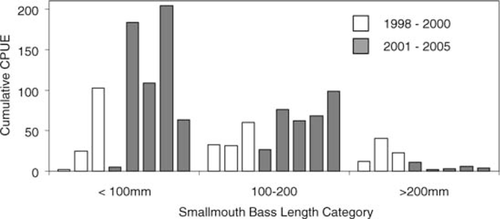
Smallmouth bass CPUE (fish/h) from spring assessment electrofishing in Little Moose Lake, New York. Open bars represent the preremoval period (1998–2000); shaded bars represent the period in which smallmouth bass were removed (2001–2005). Removal began in 2000, and data from that year are considered to be preremoval. Each catch rate represents the total number caught divided by the total effort for one complete sample of the lake shoreline. Total effort varied (3.2–10.3 h) by year.
Relative abundances significantly increased for the six of the seven native littoral species after smallmouth bass removal: pumpkinseeds (t = 4.50, df = 6, P < 0.01), creek chub (t = 7.04, df = 6, P < 0.01), common shiners (t = 4.65, df = 6, P < 0.01), white suckers (t = 3.13, df = 6, P < 0.05), brook trout (t = 5.68, df = 6, P < 0.01), and central mudminnow (t = 4.5, df = 6, P < 0.01) (Figure 4). Brown bullhead abundance increased sharply after removal, but the increase was not sustained (t = 2.038, df = 6, P = 0.11). Although we also observed increased CPUE for slimy sculpin (preremoval mean = 0.48 fish/h, postremoval mean = 8.0 fish/h), this species was excluded from further analysis due to a lack of consistent catchability across habitat types. Mean length of fish considered vulnerable to smallmouth bass predation was significantly smaller in postremoval catches than in preremoval catches for pumpkinseeds (t = −15.72, df = 396, P < 0.001), common shiners (t = −3.19, df = 22, P < 0.01), white suckers (t = −6.36, df = 54, P < 0.001), brook trout (t = −2.3, df = 24, P < 0.05), and brown bullheads (t = −6.01, df = 78, P < 0.001). Mean length did not significantly change for creek chub (t = −0.71, df = 10, P = 0.49) or central mudminnow (t = 0.28, df = 15, P = 0.78) after smallmouth bass removal.
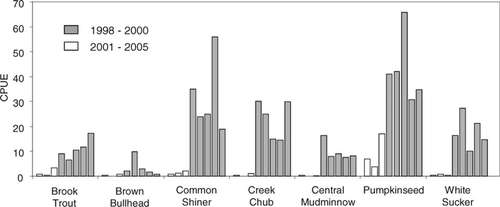
Native littoral species CPUE (fish/h) from spring assessment electrofishing in Little Moose Lake, New York. Open bars represent the period prior to removal of nonnative smallmouth bass (1998–2000); shaded bars represent the removal period (2001–2005). Removal began in 2000, and data from that year are considered to be preremoval. Each catch rate represents the total number of fish caught divided by the total effort for one complete sample of the lake shoreline. Total effort varied (3.5–9.0 h) by year.
Relative predation risk assessed by tethering of creek chub decreased in the littoral zone (1.5 m) after smallmouth bass removal, but no trends were observed at deeper (10 m) locations (Figure 5). Year and season were both significant factors in the logistic regression model (year: F6,739 = 7.35, P < 0.001; season: F6,743 = 18.58, P < 0.001). The coefficient for year was negative, indicating that a tethered fish's probability of being attacked by a predator declined as the removal progressed. Season had a positive coefficient, which reflected an increase in the probability of attack between the July and August sampling periods. Smallmouth bass (n = 5) were the only predators captured on the shallow hooked tethers; three lake trout were captured on deep hooked tethers. Smallmouth bass were observed consuming creek chub from hooked tethers without being captured, which probably accounted for our low catch rate of predators on the hooked tethers.
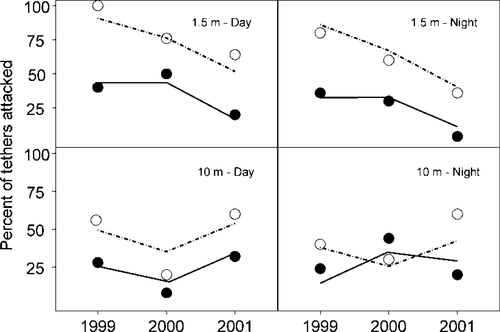
Predicted and observed levels of relative risk of smallmouth bass predation at various depths, times, and years, as measured using tethered creek chub in Little Moose Lake, New York. Solid lines represent mid-July logistic model predictions; dashed lines represent mid-August model predictions. Open circles indicate the observed proportion of tethered fish attacked by a predator.
No changes were observed in lake chemical or limnological parameters between the preremoval and postremoval periods (Table 2).
Discussion
Response of the Fish Community to Smallmouth Bass Removal
Before the removal effort, both electrofishing and snorkeling data indicated that the littoral zone fish community in the experimental lake was dominated by 100–200-mm smallmouth bass and had low abundances of native littoral fishes (this study; also Brown et al. 2000). Subsequently, native littoral fish relative abundances increased within 1 year of the start of smallmouth bass removal and were maintained throughout the 6-year removal effort. Although species introductions and biotic homogenization have been viewed as inevitable (Rahel 2000; Simberloff 2001), our results demonstrate that mechanical removal of nonnative smallmouth bass can be used to increase native littoral fish abundance on a short timescale. Our results also suggest that ongoing removal efforts would be required to maintain the reduced abundance of large smallmouth bass and high native fish abundances. To our knowledge, this is the first example where nonnative littoral piscivore removal has been shown to increase native fish abundances.
Overall, the removal effort shifted the smallmouth bass population size structure toward smaller individuals, consistently reducing the abundance of smallmouth bass greater than 200 mm in both electrofishing and snorkel surveys. We also observed a consistent and strong compensatory recruitment response within the smallmouth bass population, evident in the large proportion of age-0 smallmouth bass (50–100 mm) in fall length-frequency distributions and similar-sized age-1 smallmouth bass in subsequent spring surveys. Although electrofishing catch rates indicated that smallmouth bass smaller than 100 mm were always abundant during the removal period, the fact that these fish were removed as part of the surveys probably accounted for the lower observed abundance of all smallmouth bass larger than 50 mm during the late-spring through early fall snorkel surveys. The consistently higher native fish catch rates in the smallmouth bass removal period relative to the preremoval period suggest that the compensatory response of small smallmouth bass had relatively little effect on native fish abundances. Instead, the consistently lower abundance of smallmouth bass larger than 200 mm suggests that these larger predators had the greatest negative effect on native fish abundances. Before removal, fish were an important component of the diet for 250–300-mm smallmouth bass, but smaller fish relied on benthic invertebrates and crayfish (Weidel et al. 2000). The shift in the smallmouth bass population size structure toward smaller individuals probably reduced predation on native littoral fishes despite the increase in abundance of small smallmouth bass.
The size of our study lake (271 ha) and the presence of a single dominant nonnative predator probably contributed to our ability to successfully increase native fish abundances by removing predators. Little Moose Lake is one to two orders of magnitude larger than the small lakes where predator removal experiments established the causal link between piscivore reductions and prey fish increases (Swingle 1946; Carpenter and Kitchell 1993; Mittelbach et al. 1995); however, the lake is two orders of magnitude smaller than Yellowstone Lake (34,000 ha), where an extensive lake trout removal effort has not been successful in increasing native fish abundance (Ruzycki et al. 2003; Koel et al. 2005). Our ability to sample the entire littoral zone many times during a season helped identify local areas of smallmouth bass aggregation and focused additional removal efforts upon those locations. Additionally, our removal targeted a single nonnative littoral predator with a well-documented life history within this and similar north temperate lakes (Ridgway et al. 1989, 1991; Brown et al. 2000). In contrast, nonnative fish removals in other systems (e.g., Colorado River) often confront predators in unfamiliar habitats with connections to other bodies of water that permit regular recolonization (Tyus and Saunders 2000; Mueller 2005). In considering the relative scale of our removal effort with regards to the potential for success in other lake systems, we note the observation by Campbell and Donlan (2005) that over a 30-year period, nuisance mammal eradication efforts became increasingly successful on larger islands as managers learned from previous eradication efforts in smaller ecosystems.
Although we lost an opportunity to improve statistical inference by inadvertently reducing smallmouth bass abundance in the intended reference lake, extending the experiment's time scale reduced the potential for factors other than the manipulation to influence native fish abundances. We also note that incorporating a true replicate system in ecosystem-scale experiments becomes more difficult when the study system is large and the fish community is relatively rare (Schindler 1998). For example, Little Moose Lake includes a relatively intact native littoral fish community and only one nonnative predator, in contrast with all neighboring Adirondack lakes of similar size (Baker et al. 1990). We acknowledge the inherent limitation of conducting an experiment without a reference; however, the timing, magnitude, and consistency of the observed littoral fish community response support our inferences and conclusions about the effect of nonnative predators and the effectiveness of mechanical removal. Measured changes in predation risk (e.g., tethering results) and the lack of changes in human disturbance or lake physical and chemical conditions between the preremoval and postremoval periods further support our interpretation of study results.
Relative Predation Risk for Prey Fish
Tethering showed a decrease in the probability of a prey fish being attacked in the littoral zone after the removal, but we expected greater reductions in predation risk based on the observed magnitude of increases in native fish abundance. Post et al. (1998) observed an exponential decline in predation risk of age-0 rainbow trout Oncorhynchus mykiss with predator reductions similar to those in this study, but prey fish survival only increased in those lakes where predation risk was extremely low (probability of attack < 0.10). These contrasting results could be a consequence of our tethers being set in ideal smallmouth bass habitat where the remaining smallmouth bass may have concentrated, therefore causing predation risk at the tethering site to decline less quickly relative to the entire lake. Our tethering technique integrates a measure of predator abundance and activity levels but does not measure indirect effects of predation, such as predator avoidance behaviors that can strongly influence prey fish abundance when predators are added to a system (He and Kitchell 1990). In a similar fashion, reductions in large smallmouth bass abundance could have also reduced indirect predation effects and thereby augmented prey fish survival.
Significant increases in predation risk from July to August probably reflected increased predator activity caused by warmer water temperature, given that water temperature at 0.5-m depth increased between the two sampling periods during all 3 years (increases of 3.0, 1.5, and 3.0°C for 1999, 2000, and 2001, respectively). This also suggests that (1) predation risk for native littoral prey fish is greatest during late summer and (2) prey fish species that move to cold, deep water at that time may find seasonal refuge from smallmouth bass predation. This is supported by the persistence of slimy sculpin in smallmouth bass diets before this study, suggesting that predation by an abundant smallmouth bass population did not reduce slimy sculpin abundance to the same degree as other native prey fish populations (Weidel et al. 2000). In contrast, prey species that occupy littoral habitat throughout the year (creek chub, pumpkinseeds, common shiners) probably remained vulnerable to intense late-summer predation from smallmouth bass and were in such low abundance before the removal that they did not appear in smallmouth bass diets (Weidel et al. 2000).
Impact of Smallmouth Bass Removal on Native Salmonid Populations
Smallmouth bass removal increased the relative abundance and altered the food web of native salmonid populations in Little Moose Lake. Lepak et al. (2006) found littoral prey fish increased significantly in lake trout diets after smallmouth bass removal and stable isotope analysis tracked the changes in the food web, as measured by higher lake trout trophic position and more littoral δ13C signatures. These results support mechanical removal of introduced predators as a potential method of restoring food web linkages in fish communities that have been altered by introductions.
Increased brook trout catch rates suggest that a high density of smallmouth bass limited the abundance of this native salmonid before the removal effort, supporting previous observations that brook trout are found less frequently in northeastern lake fish communities containing smallmouth bass (Whittier and Kincaid 1999). Age-0 and juvenile brook trout inhabit the littoral zone during spring while water temperatures are cool (Biro 1998; Borwick et al. 2006), and our electrofishing collections in late April and early May routinely found large adult smallmouth bass and juvenile brook trout in shallow water associated with woody habitat. Additionally, catch rates of juvenile round whitefish (a New York State designated endangered species) increased from 0 to 4.5 fish/h during the postremoval period. Although round whitefish are not year-round residents of the littoral zone, we regularly observed juvenile round whitefish (30–70 mm) in littoral habitats before lake stratification. The increased abundance of round whitefish after smallmouth bass removal suggests that they experienced substantial predation losses when large smallmouth bass were present in the littoral zone.
Management Implications
Removal of piscivores is an accepted practice in fisheries management to increase the growth rate or abundance of a preferred sport fish, but predator removal to increase native fish abundance is uncommon (Meronek et al. 1996). We were successful at removing enough smallmouth bass to increase native fish abundances, but further experimentation is needed to determine the amount of removal effort required to induce and maintain increases in native fish abundance. For example, we initially implemented an intense removal effort, which was then scaled back due to logistical constraints. The consistent strength of smallmouth bass year-classes in fall samples suggests that the littoral fish community would return to a smallmouth bass dominated system if our removal efforts ended. However, if native species abundance increases can consistently result from reductions in smallmouth bass population size structure, it may be possible to manage for increased native fish abundance without large-scale removal efforts by focusing on reducing the abundance of large nonnative predators.
Our approach focused on removing all size-classes of smallmouth bass, but alternative approaches might focus on removing specific size-classes to avoid a density-dependent response in the number of spawning smallmouth bass or age-0 recruitment. The juvenile transition hypothesis from Ridgway et al. (1991, 2002) suggests that a high abundance of subadult smallmouth bass limits the proportion of males that spawn in a given or subsequent year. Based on this idea, initial efforts could focus on successively preventing age-0 smallmouth bass recruitment through nest destruction and fry netting while maintaining subadult abundance to limit the total number of spawning smallmouth bass. Our results indicate that native littoral fish abundances would not increase by this approach until larger smallmouth bass are removed. Alternatively, we note the successful use of “Judas goats” in terrestrial eradications, in which animals fitted with radiotelemetry collars are released and allowed to seek out other goats that are subsequently killed (Campbell and Donlan 2005). Smallmouth bass populations are known to regularly aggregate at specific overwintering locations; identifying such areas could allow large numbers of smallmouth bass to be removed (Webster 1954; Langhurst and Schoenike 1990). Anglers could also be employed to reduce smallmouth bass abundance, as has been done with northern pikeminnow Ptychocheilus oregonensis (Columbia River) and lake trout (Yellowstone Lake) (Beamesderfer et al. 1996; Ruzycki et al. 2003), but angler harvest is often size selective and would probably not be effective in removing small fish.
While our results showed that smallmouth bass removal can increase native fish abundances, we do not advocate smallmouth bass removal from all waters where they have been introduced. Although the negative effects of bass introductions (both largemouth bass and smallmouth bass) have recently received renewed attention, warnings of these effects were first published more than 50 years ago (NYSDC 1927–1939; Catt 1949). The popularity of bass fishing has created what Ruzycki et al. (2003) described as a “clientele” for an introduced species, making it more difficult to create conservation awareness for native species. Our manipulation provides experimental evidence against the further spread of smallmouth bass into waters where they are not native and indicates the need to (1) protect these waters from reductions in native fish abundance and (2) implement and report results from fish community restoration efforts of the magnitude demonstrated in this study. We support and reiterate the importance of educating anglers and nonanglers about the effects of smallmouth bass introduction on native fish communities to limit further unauthorized introductions (Vander Zanden et al. 2004). We hope the results of our work will provide clear evidence in support of such education efforts, as well as encourage further studies to formally evaluate and report results from efforts to eradicate nonnative piscivores.
Acknowledgments
We thank A. Barbato, T. Brooking, B. Gardner, C. Krueger, J. Lepak, C. Stieber, and J. Robinson for invaluable assistance and advice at many stages of the research; O. Baird, H. Baird, G. Eckerlin, R. Klumb, S. Krueger, A. Millward, N. Smith, R.A. Valdez, R. Walker, S. Weidel, and J. Weidel Jr. for their field, laboratory, and editing assistance; P. Sullivan and S. Boomer for statistical assistance; and the Adirondack League Club for access to our study site. This work was funded in part by the Adirondack League Club and the Adirondack Kieckhefer Fellowship.




