Evaluation of Dairy–Yeast Prebiotic Supplementation in the Diet of Juvenile Goldfish in the Presence or Absence of Phytoplankton and Zooplankton
Abstract
Prebiotics recently have been shown to increase immune responses and disease resistance in certain fish species; therefore, the current study was conducted to evaluate the commercially available dairy–yeast prebiotic, GroBiotic-A, for use with juvenile goldfish Carassius auratus. The study consisted of two 10-week feeding trials in which juvenile goldfish were fed practical diets that were either unsupplemented or supplemented with the dairy–yeast prebiotic at 2% by dry weight. Juvenile fish were sorted by size and stocked into 12 units within each of two culture systems: one indoor system supplied with recirculated well water and one system located outdoors with a continuous flow of pond water to provide a source of phytoplankton and zooplankton. Both diets were fed to fish in six units within each system at the same fixed percentage of body weight twice daily. Culture system (i.e., presence or absence of phytoplankton and zooplankton) was the primary factor influencing (P < 0.0001) percent weight gain, feed efficiency, and survival of goldfish during the feeding trials. No dietary effect was detected, although there was a significant (P < 0.05) interaction between culture system and diet, with supplementation of the dairy–yeast prebiotic tending to improve weight gain and feed efficiency of fish in the presence of phytoplankton/zooplankton. During a controlled disease challenge with an intraperitoneally administered dose of Aeromonas hydrophila that was equivalent to a predetermined LD50 (dose lethal to 50% of test fish), average survival values ranged between 67% and 83% for fish that previously had access to phytoplankton/zooplankton compared with 17–33% for fish that had no access to phytoplankton/zooplankton. The dairy–yeast prebiotic, however, did not enhance resistance of goldfish to the bacterial pathogen and did not greatly alter microbiota of the anterior or posterior gastrointestinal tract based on denaturing gradient gel electrophoresis analysis. In conclusion, the dairy–yeast prebiotic did not improve feed efficiency in goldfish or resistance to a bacterial pathogen as previously observed in golden shiners Notemigonus crysoleucas and hybrid bass (white bass Morone chrysops × striped bass M. saxatilis).
The goldfish Carassius auratus is a prominent bait and ornamental fish produced en masse by aquaculture. In the southeastern United States, goldfish are typically raised in large outdoor ponds, where fish are exposed to seasonal photoperiods and temperature cycles; supplemental fertilization of ponds is provided to encourage growth of phytoplankton and zooplankton as primary food resources (Lochmann and Phillips 2002). In comparison, goldfish raised in indoor culture systems experience constant environmental conditions and generally lack access to supplemental nutrients via phytoplankton or zooplankton. During various production phases, especially during harvest from ponds and prior to shipment to retail locations, fish are subjected to different stressors, such as netting, crowding, air exposure, transportation, and potentially different water quality conditions (e.g., temperature and dissolved oxygen level; Barton 2002). These stressors typically make the fish more susceptible to various disease outbreaks, which can result in large numbers of losses. Members of the genus Aeromonas are common pathogens of cyprinid species and are the agents of a disease known by several names, such as red-sore disease, hemorrhagic septicemia, and ulcer disease (Swann and White 1989; Guz and Kozinska 2004; Yildiz et al. 2005). Symptoms typically include ulcers in the fish's integument, widespread septicemia, pale gills, abnormal swimming behavior, bloating, and sudden death (Swann and White 1989; Guz and Kozinska 2004). Aeromonas spp. are considered to be opportunistic pathogens, and various common stressors can therefore lead to outbreaks (Swann and White 1989; Guz and Kozinska 2004). Traditionally, medications have been used in response to disease outbreaks; however, expense and limited effectiveness have prompted interest in developing strategies to increase fish resistance to disease and thus reduce the incidence of disease.
Gastrointestinal (GI) tract microbiota play a significant role in the health and nutrition of humans and terrestrial animals. Recently, the addition of prebiotics to diets has been shown to provide some protection against various diseases (Bailey et al. 1991; Grieshop et al. 2004; Arslanoglu et al. 2007; Sink et al. 2007; Macfarlane et al. 2008). Prebiotics were originally described as “nondigestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacterial species already resident in the colon” (Gibson and Roberfroid 1995). These beneficial bacterial have typically been identified as Bifidobacteria spp. and Lactobacilli spp. (Gibson et al. 1999; Bouhnik et al. 2004). Prebiotics affect communities of microbiota already present in the gut; however, if the targeted beneficial bacteria are not present in the gut, prebiotics will not be able to provide any intended effects. Favorable results of prebiotics have been reported in humans (Manning and Gibson 2004; Rastall 2004), swine (Tzortzis et al. 2005), poultry (Patterson and Burkholder 2003; Chung and Day 2004; Chen et al. 2005), canines (Howard et al. 2000; Beynen et al. 2002), felines (Sparkes et al. 1998), and some fishes (Li and Gatlin 2004; Mahious et al. 2006; Sink and Lochmann 2008). While prebiotics research in fishes is still limited, a growing body of literature has reported various beneficial effects of prebiotic compounds, including increased growth, immunity, and disease resistance in several fish species (Tovar et al. 2002; Li and Gatlin 2003, 2004, 2005; Burr et al. 2005, 2008a, 2008b; Mahious et al. 2006; El-Dakar et al. 2007; Gatlin et al. 2007; Sealey et al. 2007; Sink et al. 2007; Abdel-Tawwab et al. 2008; Sink and Lochmann 2008) and in humans and terrestrial animals (Gibson and Roberfroid 1995; Howard et al. 2000; Beynen et al. 2002; Patterson and Burkholder 2003; Smiricky-Tjardes et al. 2003; Bouhnik et al. 2004; Chung and Day 2004). Further research is needed to gain greater understanding of the role of prebiotics in the diets of various fish species produced in aquaculture. Therefore, the present study was conducted with goldfish.
Methods
Experimental trials and systems
The study consisted of two 10-week feeding trials conducted simultaneously in which juvenile goldfish were cultivated in two different culture systems to allow the presence or absence of phytoplankton and zooplankton. In each feeding trial, juvenile fish were obtained from a local retailer, sorted by size into reasonably similar groups, and stocked into 12 units within each of the two culture systems. For each feeding trial, the total initial body weight of fish in each container was similar among all groups in each culture system. The fish were allowed 1–3 weeks for conditioning in each experimental setup before the trials began. Goldfish in one feeding trial were placed into a culture system without phytoplankton or zooplankton; this indoor system consisted of 38-L aquaria supplied with recirculated well water maintained at 26 ± 1°C throughout the trial. A low-pressure blower provided supplemental aeration. Water quality conditions were monitored throughout the length of the experiment. A HACH DR/2000 direct reading spectrophotometer was used to measure alkalinity, nitrite, and total ammonia nitrogen using the appropriate HACH reagents. Total hardness was measured via EDTA titration, and dissolved oxygen, temperature, and salinity were assessed with a YSI Model 85–25 meter. Water quality conditions were maintained at acceptable levels for warmwater fish (Stickney 2005) by biological and mechanical filtration, and water exchanges were performed as needed. A diurnal photoperiod cycle of 12 h light :12 h dark was provided via fluorescent lighting.
In the other feeding trial, an outdoor system provided phytoplankton and zooplankton to 19-L plastic containers accommodated within a 1,200-L fiberglass tank, which received a continuous supply of pond water. Water was pumped from a 0.10-ha pond with dense aquatic vegetation dominated by filamentous algae, and that pond received a continuous supply of water from a 5-ha reservoir. No mechanical filtration was provided, while biological filtration consisted of a natural nitrogen cycle provided by the pond biota. A low-pressure blower provided supplemental aeration through an air stone within the fiberglass tank. Water conditions were monitored throughout the length of the experiment and remained at acceptable levels for warmwater fish (Stickney 2005). Throughout the experiment, daytime water temperatures remained relatively constant at 29.5 ± 0.5°C due to the large volume of water that supplied the fiberglass tank. A naturally occurring diurnal photoperiod cycle gradually decreased from 13.00 to 11.25 h light over the course of the trial.
Diets
The experimental diets were composed of practical ingredients and consisted of a basal diet without supplementation and a test diet supplemented with a commercially available dairy–yeast prebiotic, GroBiotic-A, at 2% by dry weight (Table 1). GroBiotic-A is a mixture of partially autolyzed brewer's yeast Saccharomyces cerevisiae, dairy components, and fermentation products. Both diets were manufactured by a commercial feed mill (ARKAT Feeds, Dumas, Arkansas).
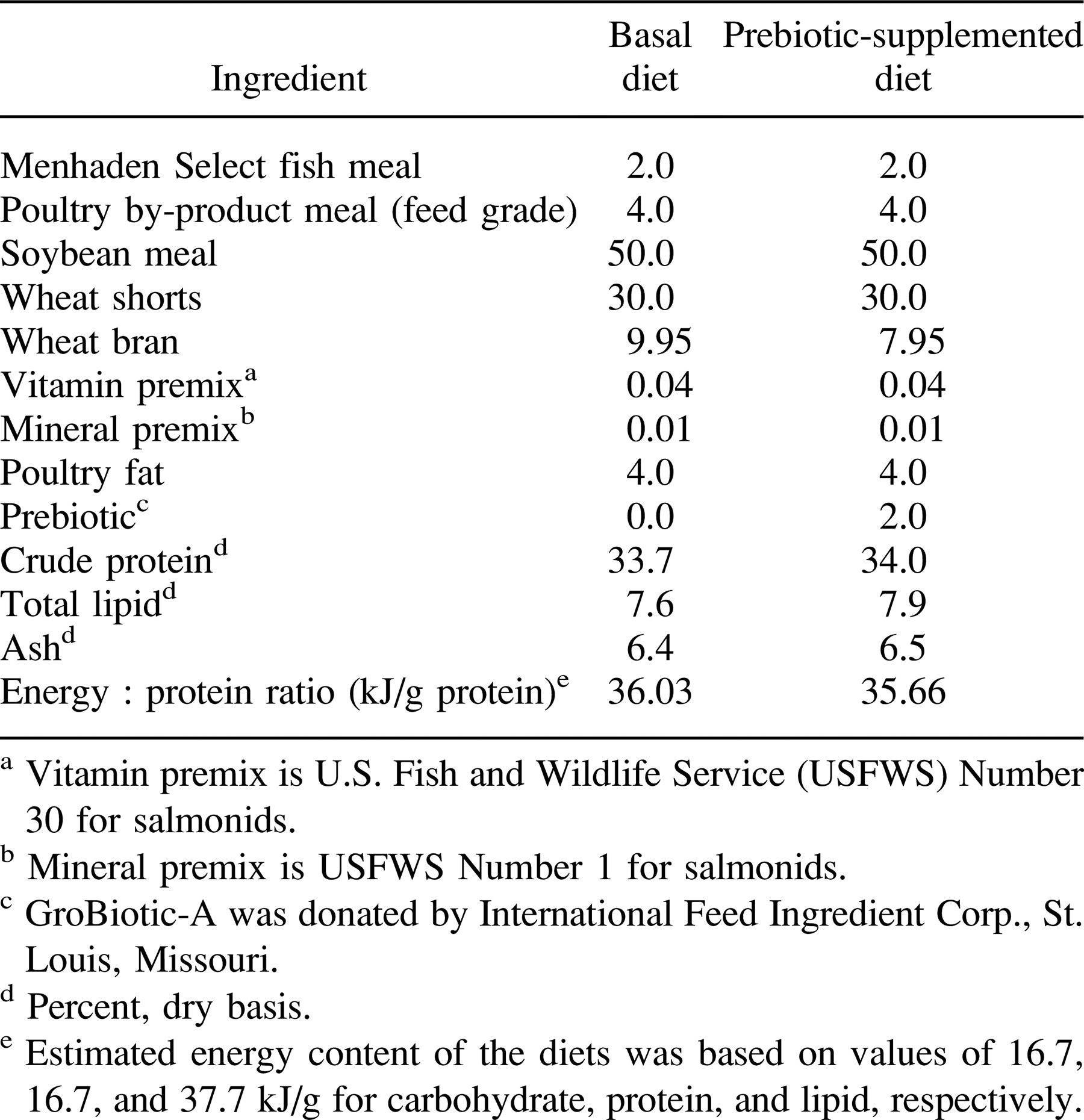
Each of the experimental diets was randomly assigned to six units within each culture system and fed to fish at a fixed percentage of body weight divided into 2 feedings/d for a total of 10 weeks. In the indoor aquarium trial, 20 fish initially averaging 1.38 g were randomly selected and stocked into each aquarium at the beginning of the conditioning period and were fed each diet initially at 3% of body weight per day for the first 3 weeks; the ration was then increased to 4% of body weight at the start of the experiment. In the outdoor trial, 10 fish initially averaging 1.74 g were stocked into each container at the beginning of the 1-week conditioning period and were fed 4% of body weight per day at the start of the experiment. Goldfish raised in the indoor experimental aquarium system were weighed once per week, whereas goldfish cultured in the outdoor system were weighed once every other week. After each weighing, the quantity of diet provided to fish in each container was adjusted according to the biomass present and established percentage of body weight per day, which was the same for each diet and culture system.
The weighing process consisted of netting the goldfish and then group-weighing by placing the fish into a tared container of water on an electronic balance; this procedure minimized handling stress. The experimental aquaria and associated equipment were located at the Texas A&M University System's Aquacultural Research and Teaching Facility in Burleson County.
Sample collection and analyses
During the course of the feeding trials, percent weight gain, feed efficiency, and percent survival were measured as response criteria. Weight gain was expressed as a percentage of initial weight, and feed efficiency was calculated based on the total wet body weight gain of fish and the total dry feed given to fish in each group. Survival was expressed as a percentage of initial number stocked.
Gut microbiota analysis
At the end of the 10-week feeding period, three goldfish were selected at random from each tank in the indoor aquarium system and were dissected to obtain digesta from anterior and posterior sections of their GI tracts, which were collected and stored at −80°C until further processing could begin. Digesta samples from the two GI tract sections of individual fish in each tank were processed for genomic DNA extraction as previously described (Burr et al. 2008b). The extracted DNA was subjected to polymerase chain reaction (PCR) using the method of Hume et al. (2003) with bacteria-specific PCR primers to conserved regions flanking the variable V3 region of 16S ribosomal DNA. Denaturing gradient gel electrophoresis (DGGE) was then performed on the isolated DNA fragments to compare microbial communities of the gut. At the time of DGGE analysis, the replicate samples processed from all fish per tank were pooled to simplify the resulting DGGE dendrogram. The DGGE analysis was performed using the method described by Hume et al. (2003) as modified from Muyzer et al. (1993); materials and fragment analysis pattern relatedness were as described by Burr et al. (2008b). The fragment analysis pattern relatedness was determined with Molecular Analysis Fingerprinting software (version 1.6; Bio-Rad Laboratories, Hercules, California). This analysis is based on the Dice similarity coefficient and the unweighted pair group method using arithmetic averages for clustering (Hume et al. 2003). Relatedness of 80% or less was required to declare microbial communities as being distinct.
Disease challenge
Remaining goldfish from each replicate container within each culture system were pooled by dietary treatment and then were randomly distributed into triplicate groups of 12 goldfish for inoculation with Aeromonas hydrophila. The bacterial sample was obtained from the Texas Veterinary Medical Diagnostic Laboratory (College Station) and was maintained in tryptic soy broth. The Aeromonas hydrophila sample was passed through goldfish in order to increase its virulence, and pretrial testing with serial dilutions of the bacteria indicated that 8.5 × 106 colony-forming units/mL was the effective dose lethal to 50% of test fish (LD50). Fish were intraperitoneally (IP) injected with 0.5 mL of the LD50, while three separate groups of 12 control fish each were injected with 0.5 mL of phosphate-buffered saline solution to ensure that no mortalities occurred due to injection method. After injection, each group of goldfish was placed in a 38-L aquarium receiving a continuous flow of well water at 24.0 ± 0.5°C; fish were monitored every 12 h, and mortalities were recorded for 168 h after injection.
Nonspecific immunity
Representative fish from each replicate container (three from the indoor system and two from the outdoor system) were exsanguinated via venipuncture of the caudal vasculature with heparinized needles approximately 12 h after their final feeding. Whole blood was analyzed for neutrophil oxidative radical production (nitroblue tetrazolium test; Siwicki et al. 1994; Li and Gatlin 2003, 2005) as a measure of nonspecific immunity.
Statistical analysis
Factorial analysis of variance (ANOVA) in a 2 × 2 arrangement was used to analyze data from the two feeding trials, and Duncan's multiple-range test was used to separate treatment means when appropriate. The main factors were diet (basal versus test) and culture system (presence or absence of natural productivity; SAS Institute 1996). Responses obtained only for fish in the indoor system were analyzed by ANOVA according to a completely random design. Statistical differences were considered significant at P-values of 0.05 or less.
Results
Fish Responses in the Feeding Trials
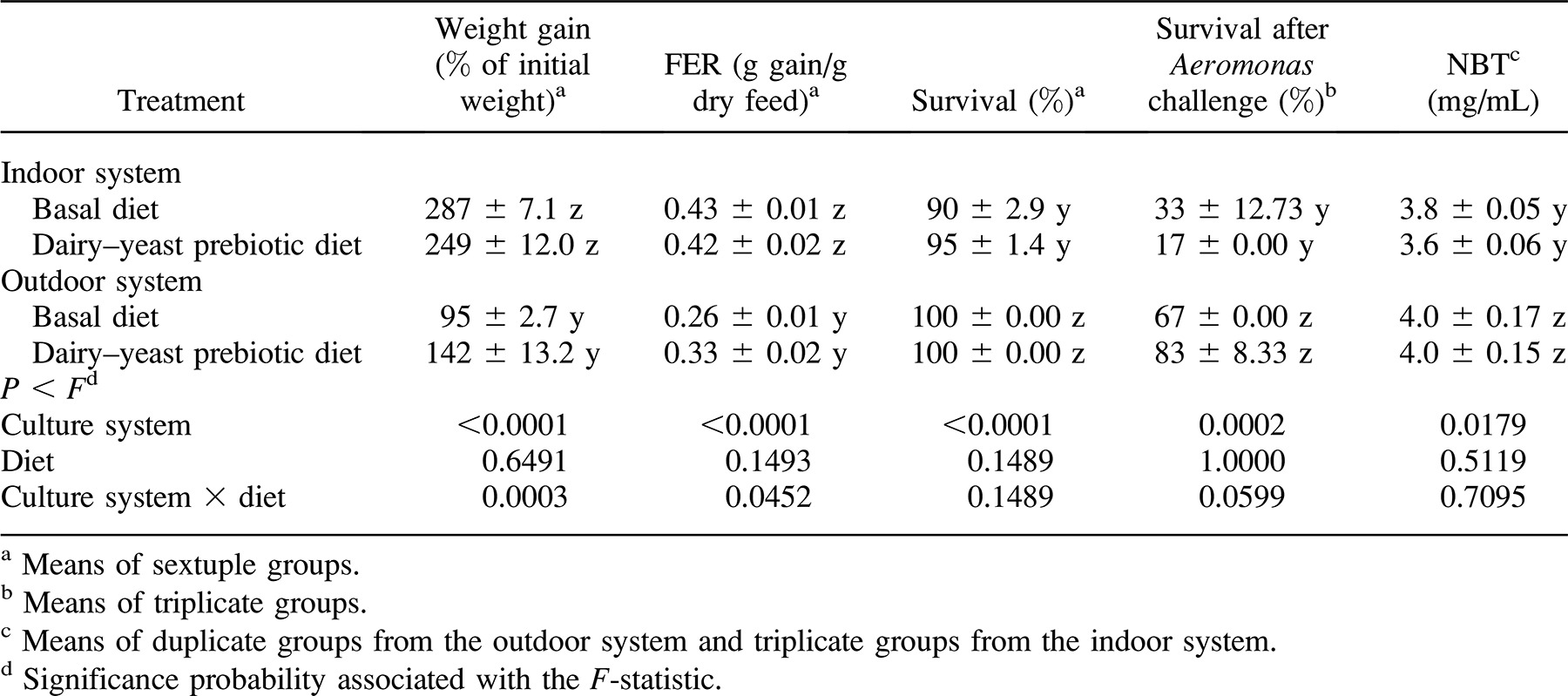
After 10 weeks of receiving the experimental diets, fish in the indoor system without access to phytoplankton or zooplankton had a greater feed efficiency ratio (FER; P < 0.0001) and enhanced weight gain (P < 0.0001; expressed as a percentage of initial weight) in comparison with fish that were raised in the outdoor system and given access to phytoplankton and zooplankton (Table 2). There was no difference in weight gain or FER values between fish fed the two diets in either system, although there were significant (P < 0.05) interactions, with the dairy–yeast prebiotic tending to improve fish performance in the outdoor culture system (Table 2). Survival during the trial was higher for fish raised in the outdoor system in the presence of phytoplankton and zooplankton than for fish raised indoors (P < 0.0001), regardless of the dietary treatment (P = 0.1489; Table 2).
Gut Microbiota Analysis
Results of DGGE of the 16S rDNA amplicons from goldfish gut microbial communities are shown in Figure 1. Analysis of the dendrogram showed high relatedness (92%) among the GI microbial communities regardless of dietary treatment, presence–absence of phytoplankton and zooplankton during rearing, or GI tract section examined (anterior or posterior).

Denaturing gradient gel electrophoresis of 16S ribosomal DNA amplicons of microbial communities from sections of the gastrointestinal (GI) tracts of goldfish cultured in an indoor aquarium system: (A) anterior GI sections of fish fed a basal diet; (B) posterior GI sections of fish fed the basal diet; (C) anterior GI sections of fish fed a dairy–yeast prebiotic-supplemented diet; and (D) posterior GI sections of fish fed the prebiotic-supplemented diet.
Disease Challenge and Nonspecific Immune Response
Fish reared in the presence of phytoplankton and zooplankton, regardless of dietary treatment, survived the Aeromonas challenge better (P = 0.0002) than fish reared in the absence of phytoplankton or zooplankton (Table 2). Average survival ranged between 67% and 83% for fish with access to phytoplankton and zooplankton, whereas survival was between 17% and 33% for fish without access to phytoplankton or zooplankton (Table 2). Dietary treatment had no effect on disease trial survivability (P = 1.0000), although the interaction between culture system and diet approached significance (P = 0.0599), with fish fed the dairy–yeast prebiotic tending to have greater survival when reared in the presence of phytoplankton and zooplankton (Table 2). Neutrophil oxidative radical production also was greater (P = 0.0179) in goldfish reared in the presence of phytoplankton and zooplankton, with no dietary effect detected (Table 2).
Discussion
The use of prebiotics in piscine diets has gained interest in recent years. GroBiotic-A, which contains partially autolyzed brewer's yeast, dairy components, and fermentation products, is a prebiotic that has been most extensively studied and has produced various beneficial effects—including increased weight gain, feed efficiency, and resistance to various pathogens—in several fish species.
Weight gain of goldfish in the current study was not influenced by the supplementation of the dairy–yeast prebiotic, although there was a tendency for improvement in the outdoor culture system. In a recent study with golden shiners Notemigonus crysoleucas, Lochmann et al. (2009) reported that the same dairy–yeast prebiotic slightly improved weight gain and feed efficiency of fish in an indoor aquarium trial but not in outdoor pools where fish had access to natural productivity. In the present study, somewhat surprisingly, the weight gain of goldfish located in the indoor system was greater than that of fish in the outdoor system with access to phytoplankton and zooplankton. This was most likely due to the difference in the fish culture systems, which resulted in differential access to the prepared diets. In the indoor system, diet that was not immediately eaten was available on the bottom of the aquaria for an extended period of time, allowing fish to continue foraging after the initial feeding. In contrast, the outdoor containers had mesh bottoms; uneaten diet slowly sank through the container and then through the grated bottom, making further foraging impossible. The amount of phytoplankton and zooplankton available to goldfish in the culture system was not measured at the time of the trial. However, during a subsequent trial in the same seasonal period 1 year later, zooplankton in the system averaged 207 organisms/L and was composed primarily of copepods and rotifers. Thus, goldfish had access to zooplankton as an additional food source, although the level was approximately one-third the amount found in the water of the supply pond (583 organisms/L); this difference was possibly due to destruction of organisms in the water pump. The level of zooplankton available to goldfish in the present study was considerably lower than that reported for fertilized ponds used for aquaculture production of koi (domesticated common carp Cyprinus carpio) and goldfish (Jha et al. 2006a, 2006b).
Unlike the results of the current study, in which dietary supplementation with the dairy–yeast prebiotic had limited effect on goldfish FER values, Li and Gatlin (2004, 2005) reported enhanced feed efficiency in hybrid bass (white bass Morone chrysops × striped bass M. saxatilis) that were fed diets supplemented with GroBiotic-A. Weight gain of hybrid bass fed GroBiotic-A in those studies also generally tended to be greater than that in fish fed the basal diet, although the differences were not always statistically significant. After 3 weeks of receiving diets supplemented with GroBiotic-A or partially autolyzed brewer's yeast, rainbow trout Oncorhynchus mykiss exhibited enhanced FER, but no differences in weight gain were observed (Sealey et al. 2007). The improved FER may be due to further enhanced nutrient digestibility, as reported in different fish species that were fed diets supplemented with prebiotics or live yeast (Tovar et al. 2002; Waché et al. 2006; Abdel-Tawwab et al. 2008). A recent study of red drum Sciaenops ocellatus (Burr et al. 2008a) also reported enhanced protein and energy digestibility coefficients for a soybean-meal-based diet supplemented with GroBiotic-A at 1% dry weight compared with the basal diet.
Feeding the dairy–yeast prebiotic to goldfish in the present study did not result in differences in the microbiota of the GI tract according to DGGE analysis of the 16S rDNA amplicons. This analysis requires relatedness to be 80% or lower for microbial communities to be considered distinctive; values greater than 80% therefore indicate similar bacterial species. The DGGE analysis of the anterior and posterior sections of goldfish GI tracts indicated that relatedness among gut microbiota was 92% overall (Figure 1), which is in contrast to results of a recent in vitro study (Burr et al. 2008a) in which incubation of red drum intestinal contents with GroBiotic-A produced a distinct microbial population. A change in microbial composition of GI tract microbiota also was recently reported (Burr 2007) for hybrid bass fed a soybean-meal-based diet supplemented with GroBiotic-A at 1% of dry weight.
Goldfish reared in the presence of phytoplankton and zooplankton in the present study expressed far greater survival during the disease challenge with IP injection of Aeromonas hydrophila, regardless of dietary treatment. The IP administration of phosphate-buffered saline to the control goldfish did not kill any of the fish, thus confirming that Aeromonas hydrophila was the cause of mortality in goldfish exposed to the pathogen. Mortality continued until 96 h after IP injection, and then no further mortality was recorded through 168 h postinjection. Due to Aeromonas spp. naturally occurring in freshwater pond systems (Swann and White 1989), it is believed that goldfish in the present study reared in the outdoor system were naturally exposed to the bacterium and thus were able to build some immunological resistance to the disease. As a result, goldfish from the outdoor system had higher survival after the disease challenge (83% survival) compared with those from the indoor system. Goldfish from the outdoor system also had higher blood neutrophil oxidative radical production, indicating elevated nonspecific immunity.
Li and Gatlin (2004, 2005) demonstrated statistically improved survival of hybrid bass fed a diet supplemented with dairy–yeast prebiotic when exposed to Streptococcus iniae and Mycobacterium sp. via immersion. GroBiotic-A also has been reported to improve survival of rainbow trout after experimental exposure to infectious hematopoietic necrosis virus (Sealey et al. 2007). Similarly, golden shiners fed GroBiotic-A experienced reduced mortality when experimentally exposed to the agent of columnaris disease, Flavobacterium columnare, which is a major pathogen in golden shiner aquaculture (Sink and Lochmann 2008). However, based on the results of the current study with goldfish, this dairy–yeast prebiotic did not improve resistance to Aeromonas hydrophila. However, the presence of phytoplankton and zooplankton conferred benefits to goldfish in terms of survival after Aeromonas hydrophila challenge.
Acknowledgments
Many thanks are expressed to the following persons: Patricia Varner at the Texas Veterinary Medical Diagnostic Laboratory for bacterial isolation and histopathology, Gary Burr for assistance with DNA extraction and DGGE analysis of bacterial samples, Alejandro Buentello for assistance with the disease trial, and graduate and undergraduate students at the Texas A&M University Aquacultural Research and Teaching Facility.




