Identification of Edwardsiella ictaluri and E. tarda by Species-Specific Polymerase Chain Reaction Targeted to the Upstream Region of the Fimbrial Gene
Abstract
Phylogenetic analysis of nine strains of Edwardsiella ictaluri and eight strains of E. tarda (six typical motile strains and two atypical nonmotile strains) isolated from diseased fish was performed using the upstream region of the fimbrial gene cluster. Strains of E. ictaluri and E. tarda were significantly clustered into separate groups. Moreover, atypical E. tarda strains were clustered into a different group from the other strains. Three polymerase chain reaction (PCR) primer sets for differential detection of E. ictaluri as well as typical and atypical E. tarda were developed from the respective characteristic sequences. Strains of E. ictaluri, typical E. tarda, and atypical E. tarda were specifically detected by PCR using each primer set. No amplifications were observed after the use of these three primer sets with 25 other bacterial species, including fish pathogens. In addition, the three primer sets were able to detect the DNA of each target species from fish kidney and liver artificially infected with E. ictaluri or E. tarda.
The genus Edwardsiella belonging to the family Enterobacteriaceae is composed of three species: E. hoshinae, E. ictaluri, and E. tarda (Sakazaki 2001). Edwardsiella ictaluri and E. tarda have often been reported as the causative agents of serious infections that are damaging to the aquaculture industry.
Edwardsiella ictaluri is known to be the causative agent of enteric septicemia in catfish and has been isolated from a number of catfish species: channel catfish Ictalurus punctatus, blue catfish I. furcatus, white catfish Ameiurus catus, brown bullhead Ameiurus nebulosus, and striped catfish Pangasius hypophthalmus (Hawke et al. 1981; Crumlish et al. 2002; Yuasa et al. 2003). Furthermore, the bacterium has been isolated from the rosy barb Puntius conchonius (Humphrey et al. 1986), Bengal danio Danio devario (Waltman et al. 1985), green knife fish Eigenmannia virescens (Kent and Lyons 1982), and rainbow trout Oncorhynchus mykiss (Keskın et al. 2004). The disease has been confirmed in the United States of America (Hawke et al. 1986), Vietnam (Ferguson et al. 2001), Thailand (Kasornchandra et al. 1987), Indonesia (Yuasa et al. 2003), and Turkey (Keskın et al. 2004). In Japan, E. ictaluri was isolated from ayu Plecoglossus altivelis in some rivers in 2007 (Sakai et al. 2008).
Edwardsiellosis due to E. tarda has had serious impacts on the aquaculture industries for the Japanese flounder Paralichthys olivaceus, red sea bream Pagrus major, and Japanese eel Anguilla japonica (Wakabayashi and Egusa 1973; Nakatsugawa 1983; Miyazaki and Kaige 1985; Kusuda and Salati 1993). Furthermore, the bacterium has been isolated from ayu and Nile tilapia Oreochromis niloticus (Yamada and Wakabayashi 1999) and from eel pond water (Wakabayashi and Egusa 1973). Two phenotypes of E. tarda have been reported: one is motile (typical), and the other is nonmotile (atypical; Yasunaga et al. 1982). The typical strains are mainly isolated from freshwater fishes and Japanese flounder, and the atypical strains are mainly isolated from marine fishes.
Edwardsiella ictaluri and E. tarda are easily isolated within 2 or 3 d using agar of tryptic soy, brain–heart infusion, or Salmonella–Shigella (SS) medium. However, identification by biochemical characterization requires much time and energy (Wyatt et al. 1979). Consequently, development of rapid identification techniques for Edwardsiella spp. is needed for the diagnosis and surveillance of epidemics. Many polymerase chain reaction (PCR) methods have been developed as rapid detection techniques for fish pathogenic bacteria (Buller 2004). Recently, Panangala et al. (2007) suggested multiplex PCR for simultaneous detection of three bacterial fish pathogens, Flavobacterium columnare, E. ictaluri, and Aeromonas hydrophila. The primers for detection of E. ictaluri were designed from the 16S ribosomal DNA (rDNA) region. The nucleotide sequence shows remarkably high homology with 16S rDNA of E. tarda in BLAST homology analysis (BLASTN program; National Center for Biotechnology Information, Bethesda, Maryland), and the differences are in only a few bases. Sakai et al. (2007) reported that four genes (etfA*, etfB*, etfC*, and etfD*) comprising a type 1 fimbrial cluster of E. tarda were detected by PCR in the fish pathogenic strains but not in the nonpathogenic strains. Recently, an E. ictaluri fimbrial gene cluster showing high homology to the etf* genes has been registered in GenBank (accession number AY626368). In this study, we performed an analysis of upstream regions of the fimbrial gene cluster among strains of E. ictaluri and E. tarda and developed species-specific PCR methods for detection of E. ictaluri and E. tarda. Furthermore, we evaluated the detection limit of each PCR using heat-extracted DNA.
Methods
Bacterial strains and DNA extractions
Strains of E. ictaluri and E. tarda and the other species used in this study are shown in Table 1. Nonmotility in E. tarda is characteristic of the typical strain (Yasunaga et al. 1982) and was confirmed by a motility test using semisolid agar containing 1% tryptone (Becton, Dickinson, and Company, USA), 0.5% NaCl, and 0.3% agar. Edwardsiella ictaluri and E. tarda were routinely cultured in tryptic soy broth (Becton, Dickinson, and Company) at 28°C for 48 and 24 h, respectively. These bacteria were stored at −80°C in Luria-Bertani broth (Becton, Dickinson, and Company) containing 10% glycerol. The DNA was isolated using a Gentra Puregene cell kit (Qiagen, Japan) according to the manufacturer's protocol.
Analysis of DNA sequences in the upstream region of the fimbrial gene
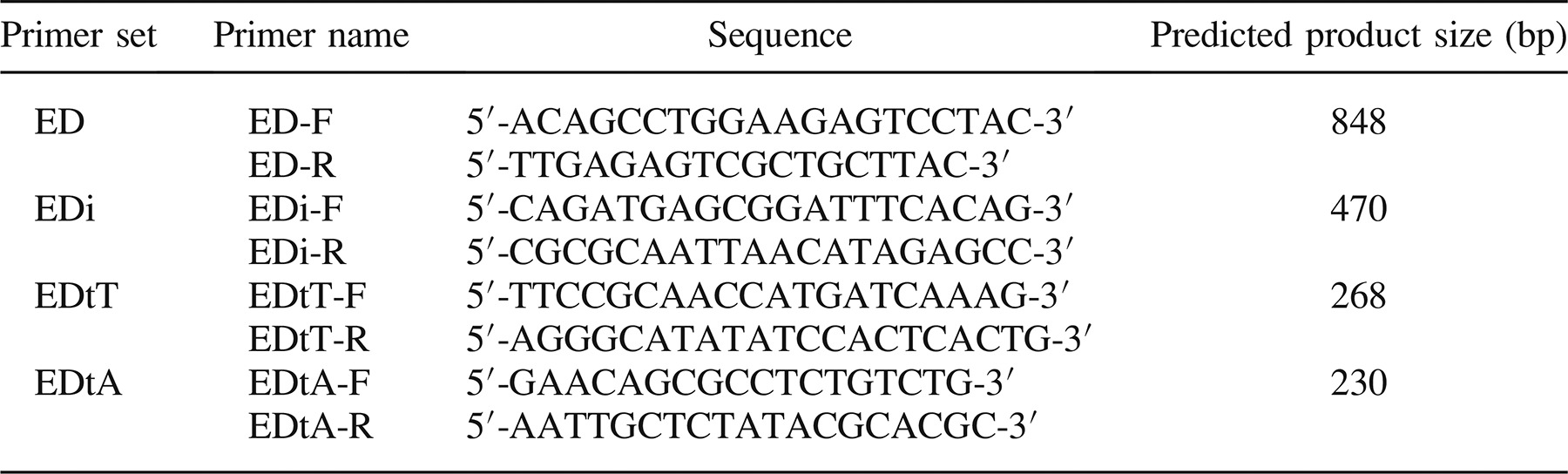
For DNA sequencing analysis of the upstream region of the fimbrial gene cluster, a primer set, ED (Table 2), was designed from consensus sequences in upstream regions of the fimbrial gene clusters of E. ictaluri (AY626368) and E. tarda strain KG8401 (AB100170). The PCR amplification was performed with TaKaRa Ex Taq (TaKaRa Bio, Japan) according to the manufacturer's instructions. One microliter of the DNA template in a total volume of 20-μL reaction mixture was reacted on an iCycler thermal cycler (Bio-Rad Laboratories). The PCR amplification consisted of an initial denaturation step of 3 min at 94°C; 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72 for 2 min; and a final extension for 5 min at 72°C. The PCR amplicon was cloned into Escherichia coli JM109 with pGEM-T Easy Vector Systems (Promega, Japan) according to the manufacturer's instructions. The DNA sequence was analyzed on an ABI 3730 genetic analyzer (Applied Biosystems, Inc., Japan) using the BigDye Terminator Cycle Sequencing kit (version 3.1; Applied Biosystems). MEGA4 software (Tamura et al. 2007) was used for the multiple sequence alignment and construction of the phylogenetic tree.
Polymerase chain reaction conditions for identification of Edwardsiella ictaluri and E. tarda
Primer sets EDi, EDtT, and EDtA (Table 2) were designed for specific detection of E. ictaluri (EDi) and typical (EDtT) and atypical (EDtA) strains of E. tarda isolated from fish. Polymerase chain reaction amplification using each primer set was performed with TaKaRa Ex Taq (TaKaRa Bio) according to the manufacturer's instructions. The PCR amplification consisted of an initial denaturation step of 3 min at 94°C; 40 cycles of 94°C for 30 s, 65°C for 30 s, and 72°C for 1 min; and a final extension for 5 min at 72°C. Polymerase chain reaction amplicons were analyzed by 2% agarose gel electrophoresis and stained with ethidium bromide at a concentration of 0.5 mg/mL.
Sensitivity of the polymerase chain reaction against easily prepared DNA template
For easy preparation of PCR template, heat shock extraction was performed as follows. Bacterial suspension in distilled water was heated at 100°C for 5 min and centrifuged at 10,000 × gravity for 1 min at 4°C. The supernatant was then removed. Serial 10-fold dilutions of E. tarda or E. ictaluri were subjected to heat extraction and PCR amplification. In addition, the colony-forming units (CFU) in the bacterial suspension were determined by culture on tryptic soy agar.
Detection from artificially infected fish
Ten individual ayu (average body weight = 2.4 g) and striped catfish (2.8 g) were intraperitoneally injected with E. ictaluri strain FPC1091 at a dose of 6.2 × 106 CFU/fish. Each fish was kept in a 30-L freshwater tank at 20°C. Also, 10 individual Japanese flounder (720 g) were injected with E. tarda typical strain NE8003 at 5.3 × 106 CFU/fish, and 10 red sea bream (75 g) were injected with E. tarda atypical strain FPC503 at the same dose. Each fish was reared in a 30-L marine water tank at 25°C. Fish used as controls received 0.1 mL of sterile phosphate-buffered saline. Kidney or liver (10 mg) was removed from three fish that had died 4–6 d postchallenge and was submitted to DNA extraction using the Gentra Puregene cell kit. A 200-pg quantity of the total extracted DNA was employed for the PCRs using primer sets EDi, EDtT, and EDtA.
Results and Discussion
For DNA sequencing analysis of the upstream region of the fimbrial gene cluster, the primers ED-F (forward) and ED-R (reverse) were designed at positions 1376–1395 and 2206–2223 of the fimbrial genes cluster of E. tarda strain KG8401. These sites were conserved in the fimbrial gene cluster of E. ictaluri pBK58LM (AY626368). An amplicon with the correct predicted size of 848 base pairs (bp) using the ED primer set was detected in all strains of E. ictaluri and E. tarda isolated from fish. The sites for the ED primer set are thus sufficiently highly conserved among fish pathogenic strains of E. ictaluri and E. tarda. The DNA sequencing analysis was performed against the nine strains of E. ictaluri and eight strains of E. tarda indicated in Table 1. The sequences were aligned by the ClustalW program (DNA Data Bank of Japan; http://clustalw.ddbj.nig.ac.jp/top-j.html), and the phylogenetic tree was then constructed by the neighbor-joining algorithm of MEGA4 (Tamura et al. 2007; Figure 1). The percent identity of nucleotides among the sequences is shown in Table 3. Nucleotide substitutions among nine strains of E. ictaluri comprised only a few bases. Furthermore, the sequences obtained from strains of E. ictaluri isolated from ayu in Japan were identical. There were many sequence differences between E. ictaluri and E. tarda strains, which clustered into separate groups on the phylogenetic tree (bootstrap values > 95). The region amplified by the ED primer set would therefore be useful for distinguishing between E. ictaluri and E. tarda isolated from diseased fish. Atypical E. tarda strains NB8030 and TC223 clustered into a different group from typical strains of E. tarda (bootstrap values > 95). Matsuyama et al. (2005) reported that typical strains were virulent in Japanese yellowtail and Japanese flounder but had weak virulence in red sea bream, while atypical strains were virulent in all three species. The region targeted by the ED primer set may be associated with the difference in virulence between the typical and atypical strains. After finding that strains of E. ictaluri and E. tarda differentiated into three phylogenetic groups, we developed three PCR primer sets—EDi, EDtT and EDtA—for species-specific detection of E. ictaluri and typical and atypical E. tarda based on characteristic sites in the ED amplicon. Sites for each PCR primer set are shown in Figure 2.
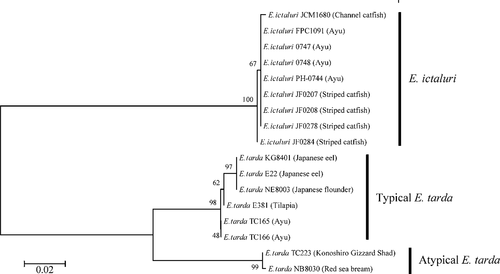
Phylogenetic tree based on the upstream region of the Edwardsiella fimbrial gene cluster. The tree was generated by the neighbor-joining method. Bootstrap values (expressed as percentage of 1,000 replicates) are shown at the tree nodes (bar = 0.02 substitutions/nucleotide position).
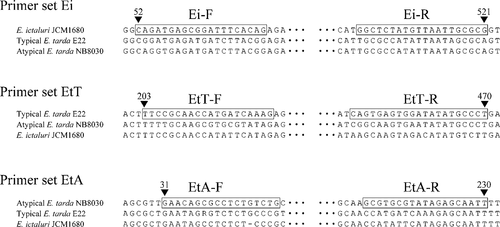
Sites for Edwardsiella fimbrial gene cluster primer sets EDi (E. ictaluri), EDtT (typical strains of E. tarda), and EDtA (atypical strains of E. tarda). Number symbols indicate positions of polymerase chain reaction products using the ED primer set. Each primer sequence is boxed.
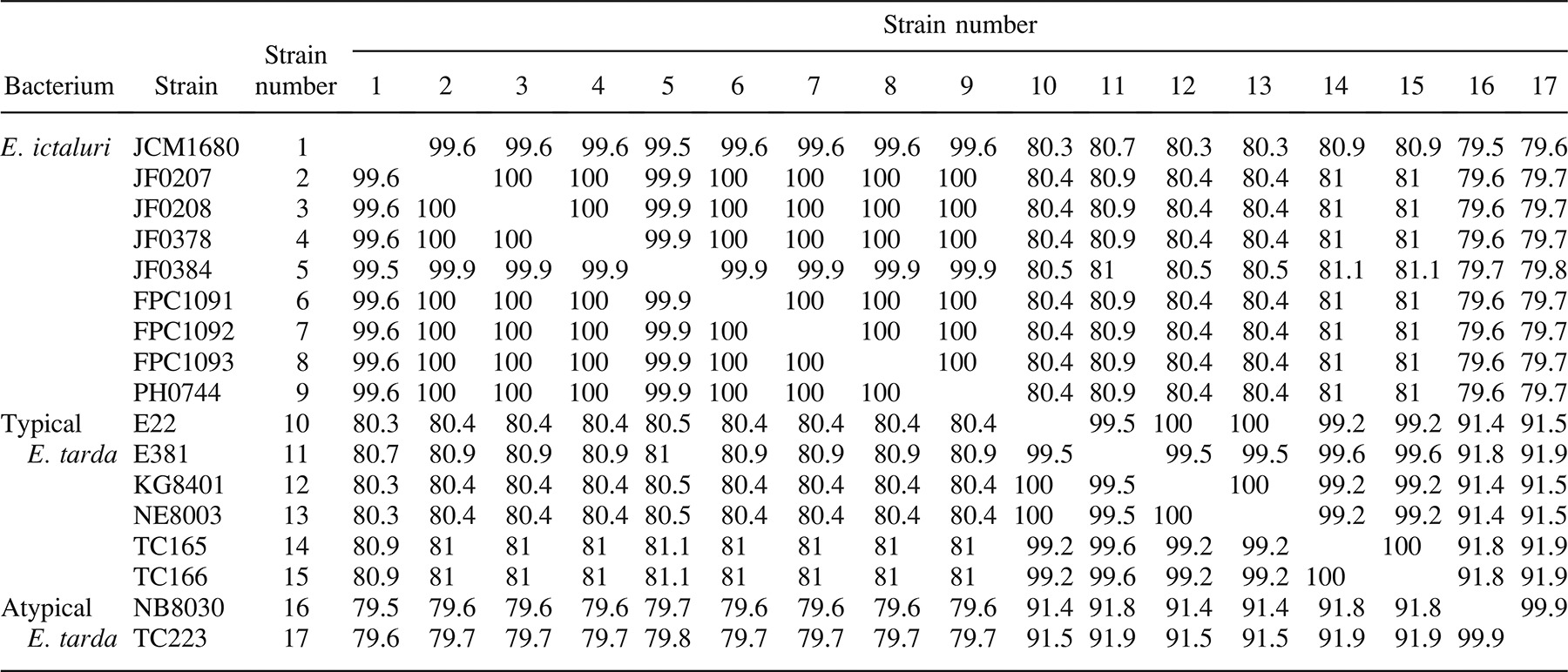
Polymerase chain reaction amplifications against genomic DNA of Edwardsiella spp. by use of EDi, EDtT, and EDtA are shown in Figure 3. The strain-specific PCR amplification products of 470, 268, and 230 bp (for EDi, EDtT, and EDtA, respectively) were obtained for E. ictaluri and typical and atypical E. tarda. Nonspecific amplicons were not observed in any PCR. Three reactions using these primer sets could specifically detect the target Edwardsiella spp. Edwardsiella ictaluri and E. tarda are differentiated biochemically in terms of the production of indole and hydrogen sulfide; E. tarda produces both, while E. ictaluri does not. However, confirmation of these characteristics takes several days. Ainsworth et al. (1986) reported an indirect fluorescent antibody technique for the rapid detection of E. ictaluri by use of monoclonal antibodies. However, specific antibodies for the diagnosis of E. tarda infection and discrimination of the typical and atypical strains have not been developed. We suggest that easy and rapid discrimination of E. ictaluri and typical and atypical E. tarda is possible using the PCR primer sets EDi, EDtT, and EDtA. These three primer sets were optimized to run under the same conditions in order to allow all the PCR amplifications to be performed at the same time on a thermal cycler.
Agarose gel showing the polymerase chain reaction amplicons (bp = base pairs) produced using three primer sets: (A) EDi against Edwardsiella ictaluri (lane 1 = strain JCM [Japan Collection of Microorganisms] 1680, 2 = JF0207, 3 = JF0208, 4 = JF0378, 5 = JF0384, 6 = FPC1091, 7 = FPC1092, 8 = FPC1093, 9 = PH0744), (B) EDtT against typical strains of E. tarda (lane 10 = strain E22, 11 = KG8401, 12 = NE8003, 13 = E381, 14 = TC165, 15 = TC166), and (C) EDtA against atypical strains of E. tarda (lane 16 = strain TC223, 17 = FPC503, 18 = NB8030, 19 = NB8031, 20 = TC158, 21 = TC159, 22 = TC161). Lane 23 represents E. hoshinae strain JCM 1679.
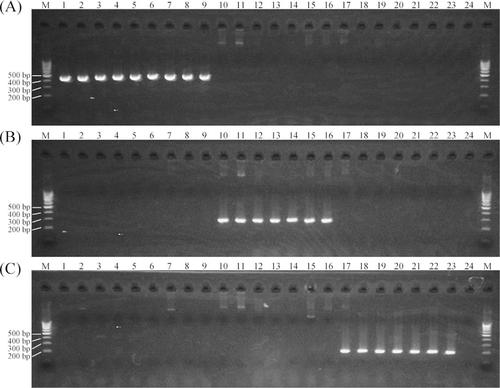
Aeromonas hydrophila, Flavobacterium columnare, and Yersinia ruckeri have been isolated from catfish species, which are known to be major hosts for E. ictaluri (Buller 2004). Aeromonas salmonicida, Lactococcus garvieae, Nocardia seriolae, Streptococcus iniae, Vibrio anguillarum, and V. cholerae serogroup non-O1 have been isolated from Japanese flounder, red sea bream, and Nile tilapia. Polymerase chain reaction products amplified from non-Edwardsiella pathogens in the genera Aeromonas, Flavobacterium, and Yersinia were examined using the three primer sets EDi, EDtT, and EDtA (Figure 4). Suitably sized diagnostic amplicons were not observed by PCR with the template from these non-Edwardsiella pathogens. The results indicate that PCRs using EDi, EDtT, and EDtA were specific for E. ictaluri, typical E. tarda, and atypical E. tarda, respectively. This suggests that the three PCR primer sets are useful for the diagnosis of E. ictaluri and E. tarda infections and for discrimination between the targeted Edwardsiella spp.
Agarose gel showing the polymerase chain reaction amplicons (bp = base pairs) produced using three primer sets, (A) EDi, (B) EDtT, and (C) EDtA, against non-Edwardsiella pathogens (lane 1 = Aeromonas hydrophila strain ATCC [American Type Culture Collection] 19570, 2 = A. salmonicida masoucida NCIMB [National Collection of Industrial, Marine, and Food Bacteria] 2020, 3 = Citrobacter freundii IAM [Institute of Applied Microbiology] 12471, 4 = Flavobacterium branchiophilum FPC520, 5 = F. columnare ATCC 43622, 6 = F. psychrophilum NCIMB 1947, 7 = Hafnia alvei JCM [Japan Collection of Microorganisms] 1666, 8 = Klebsiella pneumoniae IAM 142, 9 = Lactococcus garvieae FPC139, 10 = Nocardia seriolae OTTS024013, 11 = Pantoea agglomerans IAM 12659, 12 = Piscirickettsia salmonis ATCC VR1361, 13 = Pseudomonas anguilliseptica NCIMB 1949, 14 = Pseudomonas plecoglossicida ATCC 300383, 15 = Streptococcus iniae NUF812, 16 = Vibrio anguillarum NCIMB 6, 17 = V. cholerae WA1, 18 = V. ordalii ATCC 33509, 19 = Yersinia ruckeri ATCC 22908). Lane P1 represents E. ictaluri strain JCM 1690, P2 represents E. tarda typical strain E22, and P3 represents E. tarda atypical strain NB8030.
Polymerase chain reaction template from another key fish pathogen, Flavobacterium psychrophilum, has been previously prepared by heat extraction (Yoshiura et al. 2006). In the present study, the detection limits of the three PCR primer sets were evaluated using 1 μL of heat-extracted material from serial 10-fold dilutions of cells of E. ictaluri strain FPC1091, E. tarda typical strain E22, or E. tarda atypical strain NB8030. Minimal densities of initial template material for PCR using EDi, EDtT, and EDtA were 2.2 × 104, 1.3 × 105, and 1.3 × 105 CFU/mL, respectively. Edwardsiella ictaluri and E. tarda are capable of growth in broth or on agar of tryptic soy, brain–heart infusion, or SS medium. Wyatt et al. (1979) and Minagawa et al. (1983) reported that SS medium was useful for semiselective isolation of E. tarda from fish and water. We found that E. ictaluri FPC1091 grew from 102 to 106 CFU/mL in SS broth or tryptic soy broth after 24 h at 25°C. Edwardsiella tarda E22 and E. tarda NB8030 grew from 102 to 108 CFU/mL in tryptic soy broth or SS broth after incubation for 24 h at 25°C. Therefore, even if the initial concentration of E. ictaluri or E. tarda cells is below the detection limit of PCR, the cultivation of the bacteria by use of these media makes PCR detection possible.
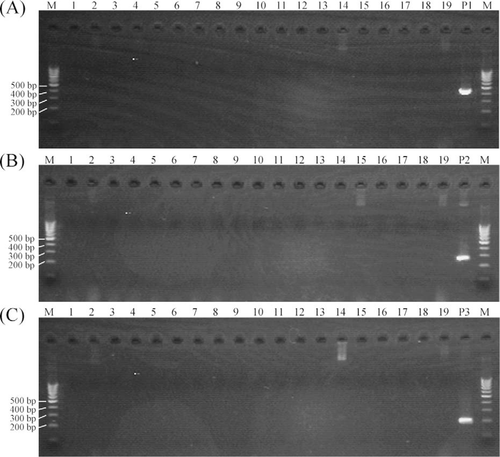
In recent years, E. ictaluri has damaged the aquaculture industries for striped catfish (Yuasa et al. 2003) and ayu (Sakai et al. 2008). Fish farming of Japanese flounder and red sea bream has been suffering seriously from infections with typical and atypical strains of E. tarda, respectively (Nakatsugawa 1983; Miyazaki and Kaige 1985; Kusuda and Salati 1993). Edwardsiella ictaluri and E. tarda have often been isolated from the kidney or liver of these diseased fish. In the present study, three PCRs using the primer sets EDi, EDtT, and EDtA could detect each target bacterial DNA in the affected kidney and liver of striped catfish, ayu, Japanese flounder, and red sea bream (Figure 5). Nonspecific amplification products, which interfered in the detection of the positive band, were not observed in any PCR. Therefore, these PCR primer sets would be suitable for direct detection of E. ictaluri or E. tarda in tissues.
Agarose gel showing the polymerase chain reaction amplicons produced using the primer sets EDi, EDtT, or EDtA against DNA extracted from liver or kidney of striped catfish (lanes 1–3 = infected with Edwardsiella ictaluri strain FPC1091; lane C1 = injected with phosphate-buffered saline [PBS]), ayu (lanes 4–6 = infected with E. ictaluri FPC1091; C2 = injected with PBS), Japanese flounder (lanes 7–9 = infected with E. tarda typical strain NE8003; C3 = injected with PBS), and red sea bream (lanes 10–12 = infected with E. tarda atypical strain FPC503; C4 = injected with PBS).
Edwardsiella ictaluri and E. tarda have been recently isolated from additional fish species; E. ictaluri was isolated from ayu in Japan in 2007 (Sakai et al. 2008), and E. tarda has also been recorded in the European eel Anguilla anguilla since 2003 (Alcaide et al. 2006). The number of recognized host species of E. ictaluri and E. tarda is likely to increase. Infection with Edwardsiella ictaluri was found in ayu in 3 of 47 Japan prefectures during 2007 (Sakai et al. 2008). The ayu is a commercially important fish in freshwater fisheries in local areas of Japan, and many rivers are stocked every year with juvenile ayu for game fishing. After the isolation of E. ictaluri, there are concerns about movement of the pathogen to uninfected areas and infection of other fish species. Avoidance of accidental transport of infected fish to non-epidemic areas is important for preventing of the spread of disease. Thus, a rapid technique for the species-specific detection of fish infected with E. ictaluri or E. tarda is urgently needed. The PCR methods described here provide tools for the rapid diagnosis of edwardsiellosis in fishes and are capable of discriminating key Edwardsiella spp.
Acknowledgments
We thank T. Nakai (University of Hiroshima) and E. B. Kholidin (Jambi Freshwater Aquaculture Development Center, Indonesia) for providing strains of E. ictaluri.