The Effects of Five Different Glycans on Innate Immune Responses by Phagocytes of Hybrid Tilapia and Japanese Eels Anguilla japonica
Abstract
The aim of this study was to evaluate the immune responses in hybrid tilapia (Nile tilapia Oreochromis niloticus × Mozambique tilapia O. mossambicus) and Japanese eels Anguilla japonica after treatment with five glycans: barley, krestin, MacroGard, scleroglucan, and zymosan. The effects of the glycans on the innate immune responses of the fish were investigated using the phagocytic index (PI), lysozyme activity, complement opsonization, and activation assay. The results of the lysozyme assay demonstrated that the lysozyme activities increased after treatment with glycans. Moreover, based on the PI, treatment with each of the five glycans resulted in increased phagocytic activities in anterior kidney and peripheral blood phagocytes in both tilapia and Japanese eels. The opsonic effect of complement on phagocytosis in tilapia and Japanese eels were investigated using baker's yeast, which served as the activator in the classical complement pathway (CCP) and in the alternative complement pathway (ACP). Tilapia and Japanese eel sera that were treated with glycans greatly enhanced phagocytosis. The classical pathway–hemolytic complement titer (CH50) of Japanese eels treated with glycans was slightly increased in vitro and in vivo. While glycan treatment enhanced the CCP of both species in vitro and in vivo, the alternative pathway–hemolytic complement titer (ACH50) was only increased in vitro and in vivo in glycan-treated tilapia. Thus, it follows that the ACP must have been activated in tilapia treated with glycans. However, in Japanese eels, the ACH50 of the ACP activation assay was undetected in vitro or in vivo due to possible unknown factors in the Japanese eel serum that caused lysis of the rabbit red blood cells. Our study investigated the effects of glycans used to enhance phagocytosis and activate both of the complement pathways involved in stimulating the innate immune responses of Japanese eels and tilapia.
Introduction
Glycans—specifically, β-1,3- and β-1,6-linked glycans (β-glycans)—are major cell wall components of yeast and mycelia fungi. They are known as antitumor glycans and as stimulators in the innate immune system of fishes and mammals (Parry et al. 1965, Di Luzio 1985). The mechanisms of yeast cell wall glycans against bacterial, fungal, and viral infection in vertebrates have been identified and studied (Seljelid et al. 1987; Yano and Mangindaan 1989; Selvaraj et al. 2005). Cellular responses of animals to β-glycans may be crucial for the immunomodulatory effects of some compounds (Sveinbjornsson and Seljelid 1994; Kumari and Sahoo 2006). The β-glycans have been shown to stimulate an innate defense in fish (Robertsen et al. 1990), and β-glycan-induced immunomodulation mechanisms have been the subject of several studies (Sakai et al. 2005). Robertsen et al. (1990) demonstrated that intraperitoneal injection of β-glycans from the cell walls of yeast Saccharomyces cerevisiae into Atlantic salmon Salmo salar results in increased resistance to several bacterial pathogens. Injection of β-glycans was followed by an increase in serum lysozyme and complement-mediated hemolytic activity, which contributed to the enhancement of innate defense (Engstad et al. 1992). It was demonstrated that anterior kidney phagocytes from glycan-treated rainbow trout Oncorhynchus mykiss have an elevated bactericidal capacity (Jørgensen et al. 1993). This increased potency could be correlated with an increased production of superoxide anion (O2−). Likewise, rainbow trout treated with yeast β-glycans demonstrated increased blood lysozyme activity together with increased bactericidal capacity for isolated anterior kidney phagocytes (Jørgensen and Robertsen 1995). Yeast glycan has also been shown to function as an adjuvant in an intraperitoneal-administered furunculosis vaccine in Atlantic salmon. Both antibody response to a bacterial protein antigen and the protective effect of the vaccine were enhanced by β-glycans (Rørstad et al. 1993).
It has been well documented that injections of foreign material into fish, as well as mammals, are accompanied by inflammatory responses (Fletcher and White 1976; Selielid et al. 1981). The ability of leukocytes to mobilize quickly at injury sites serves to minimize the spread of disease and initiate the immune response. The duration, number, and cellular composition of the responding leukocytes vary with the type of material injected (Matsuyama et al. 1988). Engstad and Robertsen (1993) showed that anterior kidney phagocytes from Atlantic salmon rapidly phagocytize glycan particles in vitro. The uptake was inhibited by incubating the phagocytes with β-1,3-glycans. The phagocyte response in fish is especially interesting because immunomodulators as well as vaccines are frequently administered by intraperitoneal injection (Jeney and Anderson 1993). Therefore, phagocytes appear to play a crucial role in the immunostimulatory effects of β-glycans in fish (Goldman 1988; Czop and Kay 1991; Konopski et al. 1991).
In our previous studies, five glycans—barley (Bar), krestin (Kre), MacroGard (MG), scleroglucan (SG), and zymosan (Zym)—were found to significantly raise the survival rates of bighead carp Hypophthalmichthys nobilis and milkfish Chanos chanos after infection with Aeromonas hydrophila (Wang and Wang 1996). Treated fish had a greater number of cells that were positively stained with nitroblue tetrazolium (NBT). An increase in bacterial killing was correlated with an increased production of nitric oxide in hybrid tilapia (Nile tilapia Oreochromis niloticus × Mozambique tilapia Oreochromis mossambicus) bighead carp, and grass carp Ctenopharyngodon idella (Wang and Wang 1996). Therefore, we investigated the effect of five different glycans on the innate immune responses of hybrid tilapia and Japanese eels Anguilla japonica. This investigation was performed by assaying end points such as phagocytic ability, lysozyme activity, and complement opsonization in tilapia and Japanese eels after treatment with glycans in vitro and in vivo.
Methods
Fish
Tilapia and Japanese eels weighing 20–30 and 100–150 g, respectively, were collected from fish farms in Taiwan. All fish were acclimated to laboratory conditions for at least 2 weeks. Both species were held at 25°C in UV-treated water and fed commercial pellets before the experiment. Tilapia and Japanese eels were assayed separately, and each species was divided into six groups. Each group included 10 tilapia or Japanese eels, and experiments were performed in triplicate. Fish were injected twice with Bar, Kre, Zym, SG, MG, or phosphate-buffered saline (PBS; served as a negative control) by intraperitoneal route at a dose of 10 mg/kg, followed by a 2-d interval prior to examination.
Glycan preparation
Bar, Kre, Zym (Sigma, St. Louis, Missouri); SG (System Bio, Newbury, UK); and MG (Biotec, Tromsø, Norway) were dissolved or suspended in sterile saline.
Collection of peripheral blood phagocytes
Fish were anesthetized with p-aminobenzoate (0.04 g/L), and blood was collected from a caudal vessel using a heparinized syringe. Blood samples (5 mL) were drawn from each fish by caudal venipuncture, collected in Vacutainer tubes containing sodium heparin, and kept at 4°C with gentle stirring. Subsequently, blood samples were diluted at 4°C with 10 mL of Hank's balanced salt solution. Fifteen milliliters of the diluted blood were maintained at 4°C with gentle stirring until use. Four milliliters of diluted blood suspension were loaded on a 3-mL Ficoll density gradient, which was then centrifuged (800 × gravity [g], 40 min, 4°C). Leukocytes were collected at the interface, washed, resuspended in RPMI-1640 medium, and then cultured at 25°C in 5% CO2 for 24 h. After culturing, the adherent cells were collected with 0.25% trypsin EDTA. They were washed three times with RPMI-1640 medium and then resuspended in RPMI-1640 medium. Enumeration of the cell viability was determined by the trypan blue exclusion method (Bridle et al. 2005), and cells were diluted to an adequate concentration.
Isolation of anterior kidney phagocytes
After anesthetic administration, anterior kidney phagocytes were isolated using a procedure adapted from Selielid et al. (1981). Briefly, cells suspended in RPMI-1640 medium (320 milliosmoles) containing 2% fetal calf serum, penicillin at a concentration of 100 units [U]/mL, streptomycin at 0.1 mg/mL, and heparin at 20 U/mL (Gibco, Refrewshire, Scotland) were layered on top of a 37%/51% discontinuous Percoll (Sigma) density gradient and then centrifuged at 4°C (400 × g) for 30 min. The cell-enriched band at the gradient interface was collected and suspended with RPMI-1640 (1 × 106 cells/mL). The cells were allowed to adhere for 2 h at 14°C before nonadherent cells were washed off. After cells were washed with PBS, their phagocytic activity was studied.
Serum isolation
Fish were injected twice by intraperitoneal method with 10 mg/mL of glycan, and there was a 2-d interval before examination. Fish injected by intraperitoneal method with PBS served as negative controls. Ten fish from each group were anesthetized, and blood samples were collected from the caudal vein using syringes. Blood samples were allowed to stand at room temperature for 15 min, and clots were then removed by centrifuging at 4°C (1,000 × g) for 5 min. Individual serum samples were then transferred to serum tubes and stored at −80°C until required.
Assay of phagocytic activity
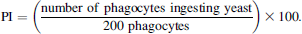
Lysozyme assay
Lysozyme activity was assayed according to the lysoplate technique described by Lie et al. (1986). For this purpose, 0.05 mg/mL Micrococcus luteus were cast in a 1% agarose gel (Bio-Rad, California) after adding 15 mL of 50-mM phosphate buffer (pH = 6.2). Fish serum (9 μL) was added to plate wells (3 mm in diameter). The plates were incubated for 17 h at 20°C. The diameters of the zones cleared by lysozyme activity were determined using a Measuring Viewer (Behring) and were found to be proportional to the log2 of the lysozyme activity in the range of 8–22 mm.
Opsonic effect of the fish complement component
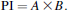
Assay of alternative complement pathway activity in vitro
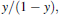
Assay of classical complement pathway activity in vitro
Classical complement pathway activity was assayed using a method described previously by Yano (1995). Sheep blood was suspended in 10-mM GVB (0.1% gelatin, 114-mM HCl, 2.76-mM sodium barbiturate, 142-mM NaCl, 0.1-mM MgCl2·6H2O, and 0.044-mM CaCl2·2H2O) at 1 × 109 cells/mL. Cells were then mixed with the heat-inactivated fish serum (1:1 in GVB) at 25°C for 30 min. After washing with GVB, the cell suspension (5 × 108 cells/mL) was stored at 4°C until required. Each of the five glycans was dissolved or suspended in GVB. To each of five individual test tubes, 1.0, 0.8, 0.6, 0.5, or 0.4 mL of diluted fish serum (1:80 in GVB) and glycan was added to give a final volume of 1.3 mL. A volume of 0.2 mL of sheep RBC suspension (5 × 108 cells/mL) was added to each of the five tubes. Each mixture was incubated at 25°C for 120 min. After centrifugation (1,000 × g for 5 min), y (percent hemolysis/100) was calculated from the OD at 541 nm (OD 541) using the centrifuged supernatant. Residual activity (classical pathway–hemolytic complement titer; CH50) was assayed according to the method described by Konopski et al. (1991).
Assay of alternative complement pathway and classical complement pathway activity in vivo
Each of the five glycans was dissolved or suspended in sterile saline and injected intraperitoneally into the fish at a dose of 10 mg/kg twice during a 2-d interval. Control fish received PBS injections. Blood was collected from fish by peduncle amputation. It was allowed to clot at 25°C for 30 min and cooled at 0°C for 1 h. Serum was then separated by centrifugation and stored at −80°C. Rabbit or sheep RBC suspension was added to each test tube of diluted fish serum, and each mixture was incubated. The ACP and CCP activities were assayed according to the in vitro methods described above.
Statistics
Results are presented as means ± SD. A one-way analysis of variance (ANOVA) test (Ainsworth et al. 1991) was applied to evaluate the means of lysozyme activity, complement opsonization, complement activation, monocyte receptor, and PI assays. In all tests, a P-value less than 0.05 was considered significant.
Results
Effect of Glycans on the Phagocytic Activity of Anterior Kidney Phagocytes
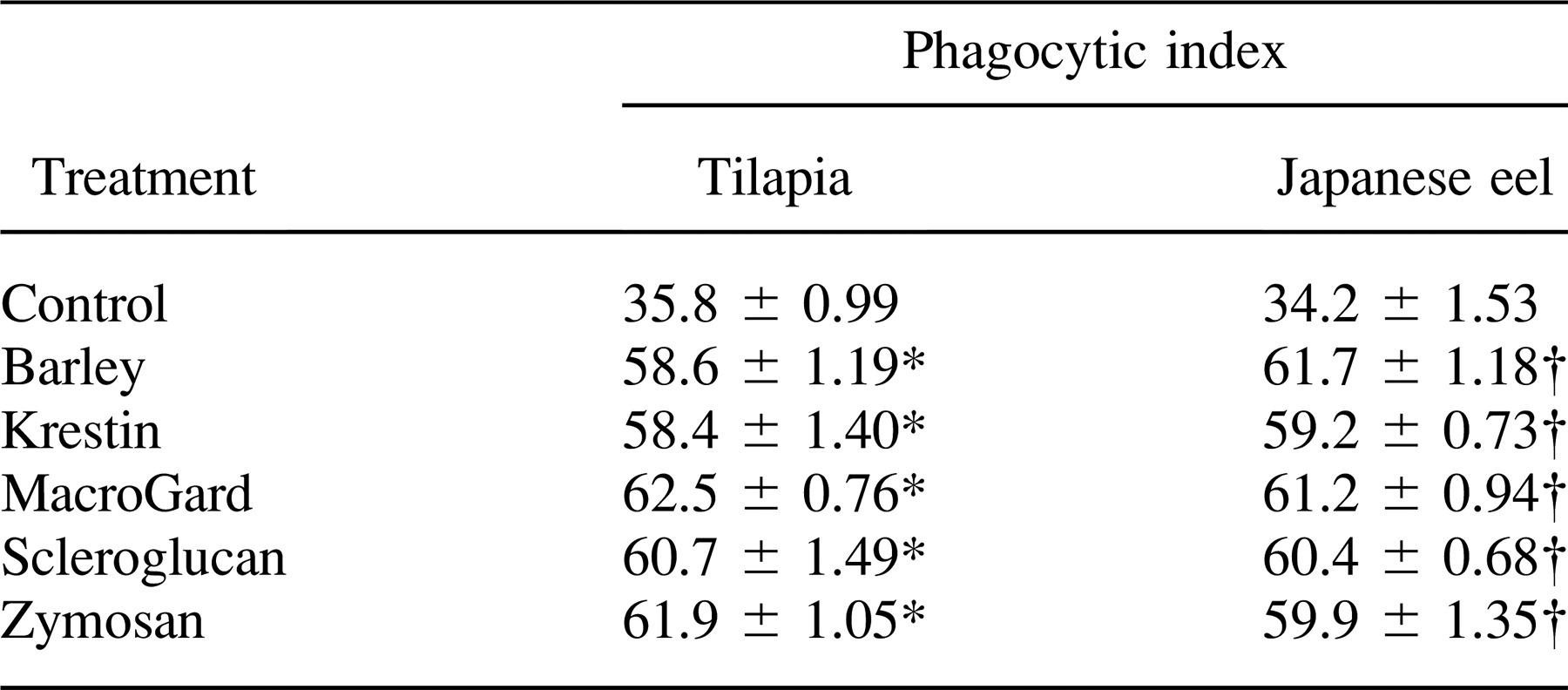
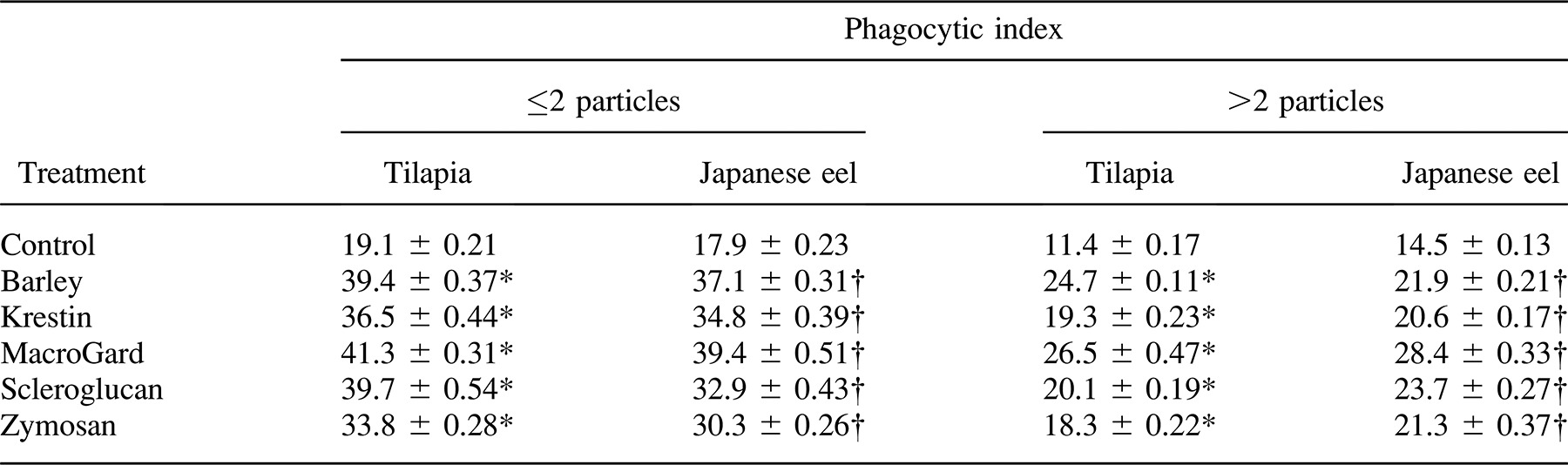
Tilapia and Japanese eels were both treated with five glycans. The phagocytic activities of anterior kidney phagocytes from treated and nontreated fish were assayed. Interestingly, our results showed that phagocytes containing phagocytosed yeast particles were observed at all times after glycan injection (Figure 1). Furthermore, we found that a much higher proportion of the phagocytes contained yeast in their cytoplasm as compared with controls (Figure 1). As shown in Tables 1 and 2, the phagocytic activities of anterior kidney and peripheral blood phagocytes from glycan-treated fish were significantly higher than those from nontreated fish. Our results indicated that there was a 1.6-fold increase in the phagocytic activity of anterior kidney phagocytes from tilapia injected with glycans and a 1.8-fold increase in Japanese eels. Furthermore, the phagocytosis assay of peripheral blood phagocytes showed that glycans significantly increased the percentage of phagocytosis in tilapia and Japanese eels (P < 0.05; Table 2).
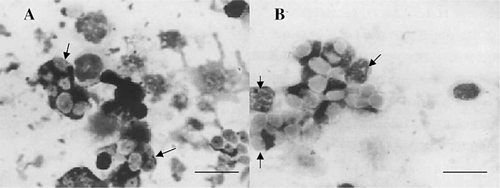
After treatment with MacroGard, anterior kidney phagocytes of (A) hybrid tilapia and (B) Japanese eels show phagocytized yeasts (arrows; scale bar = 5 μm)
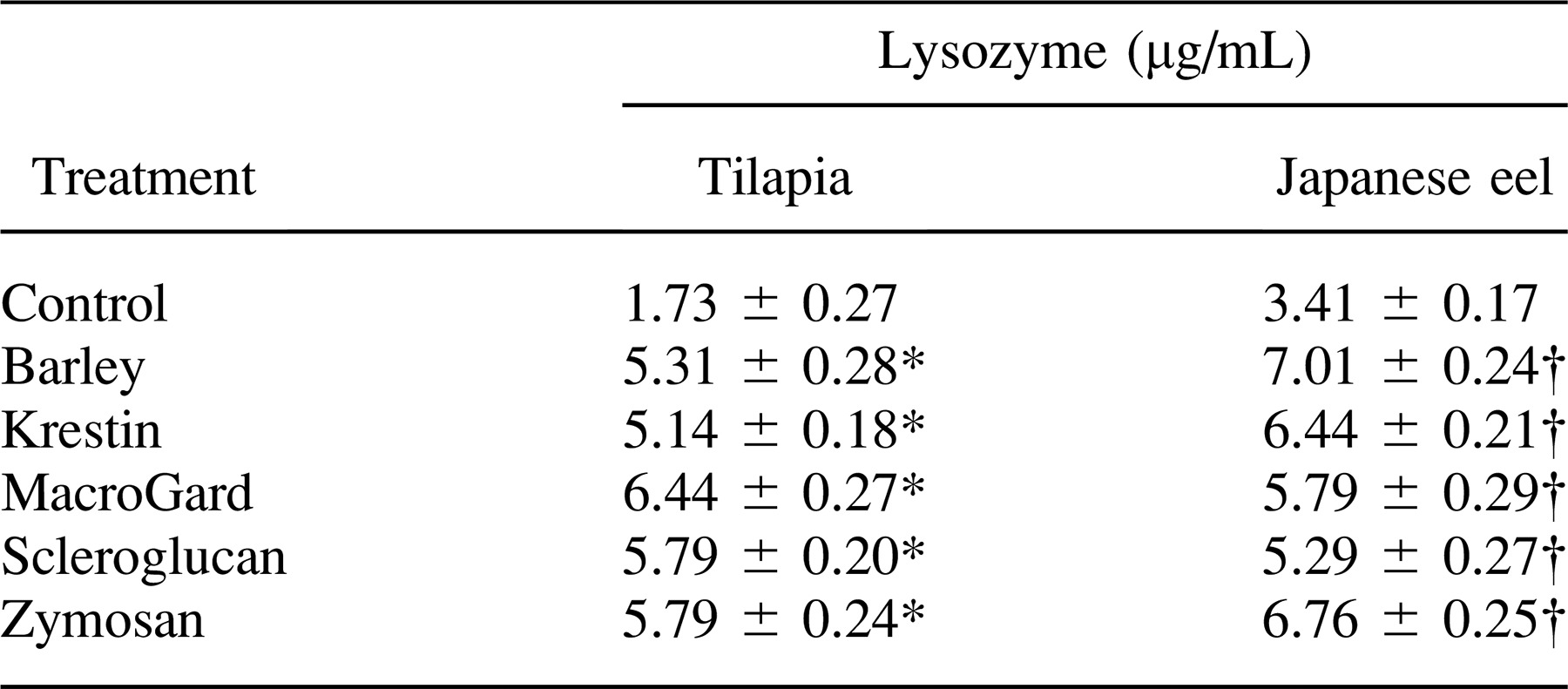
Effect of Injection of Glycans on Serum Lysozyme Activity
The effects of the five glycans injected intraperitoneally on the activity of serum lysozymes in tilapia and Japanese eels are illustrated in representative experiments presented in Table 3. There was a significant increase in serum lysozyme activity in glycan-injected tilapia and Japanese eels relative to that of PBS-injected fish (P < 0.05). The maximum lysozyme activity in serum from the glycan-treated fish was three to four times higher than the activity in serum from the control fish.
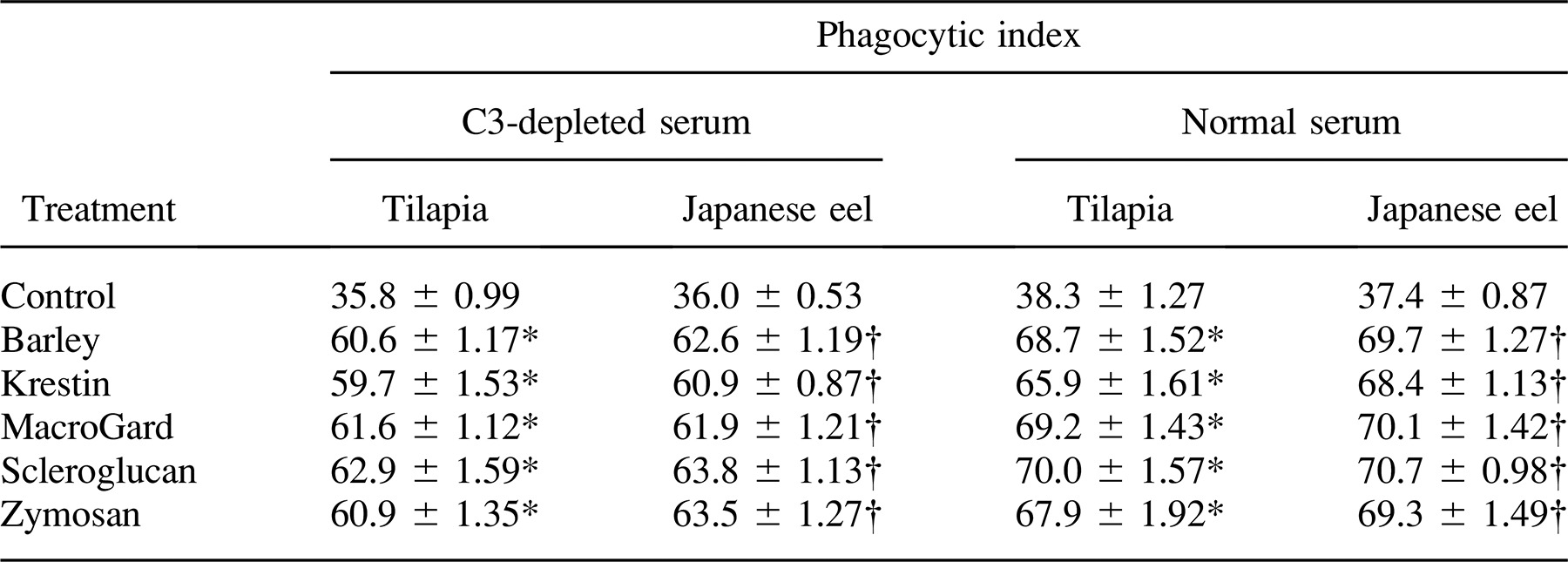
Effect of Complement on the Phagocytosis of Glycans
Each of the five glycans was incubated with tilapia and Japanese eel anterior kidney phagocytes, and the PI values were compared. A significant difference was observed between the controls and glycan-treated fish, indicating that glycans exerted an opsonic effect on the phagocytosis of yeast in tilapia and Japanese eel serum (Table 4). Glycan-induced phagocytosis was reduced by treatment with depleted tilapia and Japanese eel serum, although the values were still significantly higher than those of controls.
Activation of the Alternative Complement Pathway by Polysaccharides In Vitro and In Vivo
The hemolytic reaction was carried out, and the degree of hemolysis was calculated. Our data showed that there was significantly higher hemolytic activity in the glycan-treated tilapia serum than in the control tilapia serum (P < 0.05; Figure 2A). Tilapia serum with the addition of 0.5 mg/mL MG in vitro showed the highest hemolytic activity. Figure 2B shows the ACP (ACH50) of tilapia serum assayed in vivo. We found that each of the five glycans activated the ACP of tilapia to some extent. However, the ACH50 of the ACP assay for Japanese eels in vivo and in vitro was undetectable due to unknown factors in the Japanese eel serum that caused breakdown of the rabbit RBCs.
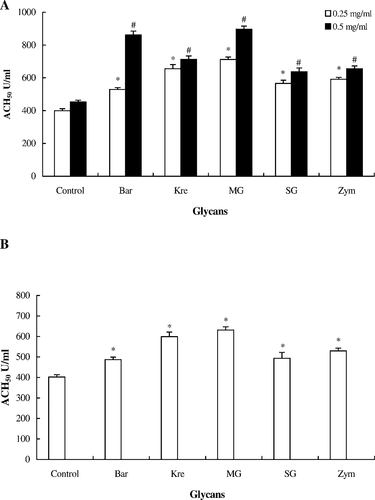
Activation of the alternative complement pathway (ACP) of hybrid tilapia serum treated with five glycans (Bar = barley; Kre = krestin; MG = MacroGard; SG = scleroglucan; Zym = zymosan) in vitro and in vivo. (A) In vitro: tilapia serum (100 μL) was incubated at 25°C for 90 min with 0.1 mL of rabbit red blood cells (5 × 108 cells/mL) and each glycan suspension (50 μL at a concentration of 0.25 or 0.50 mg/mL). The ACP activity (alternative pathway–hemolytic complement titer [ACH50]) in the supernatant was measured at an optical density (OD) of 414 nm. (B) In vivo: each of the five glycans was injected intraperitoneally into the tilapia at 10 mg/kg twice at a 2-d interval. Control fish received phosphate-buffered saline injections. The ACP activity in the serum was measured at an OD of 414 nm. The asterisk (*) and pound (#) symbols indicate significant differences between the experiment and control groups (one-way ANOVA: P < 0.05)
Activation of the Classical Complement Pathway by Polysaccharides In Vitro and In Vivo
The results showed that tilapia and Japanese eel serum had the highest hemolytic activity after glycan (0.25 and 0.50 mg/mL) treatment in vitro (Figure 3). According to the in vivo assay of CCP in tilapia and Japanese eel serum, our results revealed that the five glycans activated the CCP of tilapia and Japanese eels to some extent (Figure 4). The experimental groups of tilapia and Japanese eels were compared with controls by monitoring the hemolytic activities for complement activity. We found that there was a significant difference in the complement activity. These results indicate that the innate defense activity of Japanese eels was similar to that in tilapia.
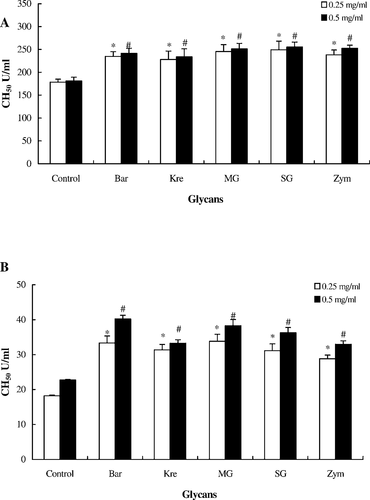
Activation of the classical complement pathway of fish serum treated with five glycans (0.25 or 0.50 mg/mL; abbreviations defined in Figure 2) in vitro: (A) hybrid tilapia and (B) Japanese eels. Sheep red blood cells (RBCs) were mixed with heat-inactivated serum at 25°C for 30 min. After washing with gelatin veronal buffer, serum (0.5 mL), glycan suspension, and 0.1 mL of sheep RBC suspension (5 × 108 cells/mL) was added and each mixture was incubated at 25°C for 120 min. After centrifugation, the classical pathway–hemolytic complement titer (CH50) was calculated based on an optical density of 541 nm
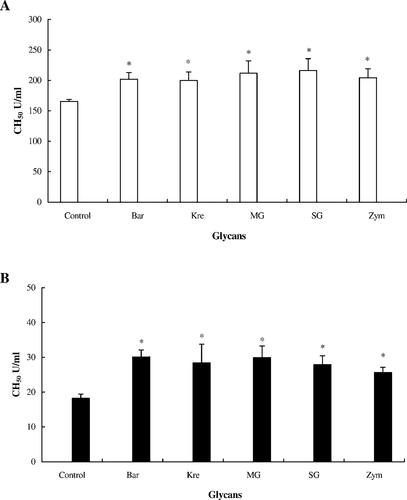
Activation of the classical complement pathway of fish serum treated with five glycans (0.5 mg/mL; abbreviations defined in Figure 2) in vivo: (A) tilapia and (B) Japanese eels. Each glycan was injected intraperitoneally into the fish at 10 mg/kg twice at an interval of 2 d. Control fish received phosphate-buffered saline injections. After collection of serum, 0.1 mL of rabbit red blood cells (5 × 108 cells/mL) was added to the diluted fish serum and was kept at 25°C for 120 min. After centrifugation, residual activity was assayed at an optical density of 541 nm
Discussion
Many studies have shown that β-1,3- and β-1,6-linked glycans appear to be important for the recognition of yeasts and fungi by vertebrate macrophages. These β-glycans also have the ability to stimulate the innate defense of vertebrates and to enhance the effect of vaccines (Yano et al. 1991; Engstad and Robertsen 1993). Mononuclear phagocytes are believed to be the main target cell for the immunomodulatory effects of β-glycans (Seljelid et al. 1987; Bridle et al. 2005). Previous studies have shown that intraperitoneal injection of β-glycans is followed by an increase in serum lysozyme and complement-mediated hemolytic activity, which may contribute to the enhancement of innate defense (Tolmasky and Krisman 1987; Engstad et al. 1992). In our studies, fish treated with glycans were able to enhance the function of lysozymes. The results from the lysozyme assay revealed the lysozyme activity indexes increase in tilapia and Japanese eels treated with each of the five glycans.
In mammals, β-glycans are known to be potent activators of ACP. Fungi, like other microorganisms, may be phagocytized through complementary receptors after being opsonized by the interaction of β-1,3 glycans structures and their complement (Czop and Austin 1985a, 1985b). Recent work in common carp Cyprinus carpio also indicates that yeast may be phagocytized by macrophages through specific receptors for β-1,3 glycans (Selvaraj et al. 2005). Czop and Austen (1985a) demonstrated that yeast β-glycans particles were phagocytized by human monocytes through a ligand-inhabitable receptor. A receptor isolated from human monocytes appears to be specific for yeast β-glycans (Czop and Kay 1991). Other researchers obtained results indicating that mouse macrophages have a specific receptor for β-glycans (Goldman 1988; Konopski et al. 1991). Willcocks et al. (2006) reported that dendritic cell-associated C-type lectin-1 (Dectin-1) was first identified in mice (muDectin-1) as a receptor for β-glycan, and they demonstrated that bovine (bo) Dectin-1 was also expressed in bovine immune cells, including bone marrow cells, monocytes, and natural killer cells. Dectin-1 is a common component of fungal cell walls (Ryan et al. 2002) and plays a key role in the innate immune defense by binding its ligand and then triggering phagocytosis. Human (hu) Dectin-1 exists as two main splice variants, A and B. HuDectin-1 is widely expressed in various cell types, including DC, monocytes, macrophages, neutrophils, and CD4+ T cells and B cells (Taylor et al. 2002; Willment et al. 2005). By studying phagocytosis, we demonstrated that similar recognition mechanisms for β-glycans exist in tilapia and Japanese eels. The phagocytic activities of anterior kidney phagocytes from glycan-treated fish were significantly higher than those in the control fish. Thus, the results suggest that yeast phagocytosis in fish phagocytes may be increased through a specific receptor similar to the human monocyte.
Based on the complement opsonization and complement activation assay, our findings demonstrate that the tilapia and Japanese eel complement enhances phagocytosis of foreign particles by anterior kidney phagocytes through the activation of the CCP and ACP. In addition, fixation of C3 on the targets is a critical prerequisite for phagocytosis. This complement activation through the ACP and its enhancement of phagocytosis is probably an important defense mechanism in fish in the early stages of bacterial infection. The presence of C3 receptors has been reported in rainbow trout (Nonaka et al. 1984a; Nonaka et al. 1984b), salmon (Johnson and Smith 1984; Sveinbjornsson and Seljelid 1994), and common carp macrophages (Matsuyama et al. 1992). The C3b receptors have even been proposed to occur in the phagocytes of sea urchins (Echinoidea; Bertheussen 1982) and earthworms Lumbricus terrestris (Laulan et al. 1988); these phagocytes vigorously ingest sheep erythrocytes that are coated with human C3b. This wide distribution of complement-like receptors in mammals, fish, and invertebrates emphasizes the importance of complement as a part of the host defense system against microorganisms. In the current study, we compared PI values of an assay in which yeast treated with C3-depleted Japanese eel or tilapia serum were incubated with anterior kidney phagocytes. The results showed that the phagocytosis of yeast is higher with normal C3-treated Japanese eel serum than with C3-depleted serum (Table 4). It is probable that the generation and degradation of C3b-like fragments occur on the target cells during the incubation of yeast with C3-depleted Japanese eel serum, but we have not yet identified these fragments. In lampreys, rainbow trout, and common carp, C3 has been isolated and the physicochemical characteristics were demonstrated to be similar to those of mammalian C3, but little information is available about the degradation of fish C3 on the target cell surface (Nonaka et al. 1984a, 1984b; Nakao et al. 1988). Further work is required to elucidate the types of C3-related receptors and their functions in fish.
Activation of the fish complement system is known to proceed through two distinct pathways (Nakao et al. 1988), the CCP and ACP, which are similar to those in mammals. The CCP is activated by an antigen–antibody complex and requires both Ca2+ and Mg2+. The ACP requires Mg2+ alone and can be activated without antigen–antibody complex using cobra venom factor, Zym, insulin, or lipopolysaccharides. The difference in the divalent cation requirement between the two pathways can be used for separate titration of ACP activity.
Matsuyama et al. (1988) reported that the serum of porgy Pagrus major, yellowtail jacks Seriola lalandi, and Japanese eels displayed high ACH50 values of 92, 142, and 134 U/mL, respectively. However, in our experiment, the ACH50 of the ACP activation assay of Japanese eels in vitro and in vivo were undetected due to a number of unknown factors in the Japanese eel serum that caused lysis of the rabbit RBCs. However, the CH50 and ACH50 of tilapia were higher than those of Japanese eels in the CCP after treatment with glycans in vitro and in vivo. We confirmed that the CCP does exist in tilapia and Japanese eels. This pathway is activated by glycan treatment in vitro and in vivo.
Acknowledgments
We would like to thank the Bio-Industrial System Company and the Biotic Mackzymal Company, who kindly provided samples for this study. The budget for this research was supported by Taiwan's National Science Commission (NSC 83-0409-B-005-089, NSC 84-0115-C-005-01-032B, and NSC 85-2313-B005-053).