Natural Disturbances and Fish: Local and Regional Influences on Winterkill of Fathead Minnows in Boreal Lakes
Abstract
We investigated the population dynamics of fathead minnow Pimephales promelas and the environmental factors of four small lakes in the boreal forest of Alberta, Canada, for 5 years to determine the influence of local and regional factors on the development of hypoxia and the occurrence of fish winterkill. Fathead minnow densities varied considerably among lakes and years, with dramatic (47–94%) year-to-year declines occurring when dissolved oxygen levels were extremely low in the intervening winter. Large declines (presumed winterkills) occurred after 25% of the “lake-winters,” affecting three of four study lakes and 2 of 5 years. A fifth population in the same region, monitored for 15 years, displayed both a comparable frequency and temporal synchrony of large density decreases, suggesting that winterkill is a pervasive natural disturbance in small lakes of the Boreal Plains. In contrast to patterns displayed by larger fish species, smaller individuals in the fathead minnow populations were more strongly affected than larger individuals. Oxygen levels in a given lake and winter were related to (1) the collective interactions of productivity and depth of the lake, (2) the local and regional hydrogeology, and (3) the current and antecedent climate. As a result, the relative effects of these local and regional factors strongly influence the natural dynamics of fathead minnow populations in these lakes. Given that humans can alter many of the important factors, the natural incidence of winterkill could be augmented if human activities are poorly managed.
Introduction
Natural disturbances play important roles in shaping biological communities (Pickett and White 1985). In north-temperate lakes, winter hypoxia is a key natural disturbance that can affect fish assemblage composition (Magnuson et al. 1989; Tonn 1990). Small-bodied fishes have lower oxygen requirements than larger species (Moyle and Cech 1996), and several small-bodied fishes have developed physiological and behavioral adaptations to cope with hypoxia (Gee et al. 1978; Klinger et al. 1982; Magnuson et al. 1985). Consequently, small-bodied species tend to dominate fish assemblages in lakes prone to winter hypoxia (Tonn and Magnuson 1982; Robinson and Tonn 1989; Tonn et al. 1995).
Variation in the severity of winter oxygen depletion within and among lakes has traditionally been linked to a small set of environmental factors (Greenbank 1945). Local lake characteristics (particularly water depth and productivity) are strongly related to winter oxygen depletion, with shallow, productive lakes being more prone to winter hypoxia than deeper or nutrient-poor lakes (Barica and Mathias 1979). In addition, regional climate patterns can influence the extent of winter oxygen depletion since the duration of ice cover and accumulation of snow can affect the input of oxygen to lakes (Greenbank 1945; Barica et al. 1983). More recently, variation in annual precipitation, in conjunction with a lake's position in the landscape, has also been shown to influence characteristics of lakes that should affect the occurrence of winter hypoxia (Webster et al. 1996, 2000; Devito et al. 2000). For instance, drought can reduce surface water and/or groundwater flow, thereby influencing lake depth and the input of oxygenated water (LaBaugh et al. 1998).
Although relations between environmental factors and the susceptibility of northern lakes to winter hypoxia have been identified, the effects of hypoxia on the population structure and dynamics of resident fishes are much less explored. Even small-bodied fishes that are tolerant of winter hypoxia can be affected if winter oxygen depletion is severe (Klinger et al. 1982). Therefore, differences in winter oxygen depletion among years and lakes may contribute to a natural variation in the abundance of small-bodied fishes. In spite of this, no studies have been conducted at appropriate spatial and temporal scales to examine how variation in local and regional environmental factors affects winter hypoxia within and among northern lakes and, in turn, the abundance of resident small-bodied fishes.
In the boreal region of western Canada, lakes are relatively shallow and productive and are often dominated by small-bodied fishes (Robinson and Tonn 1989; Paszkowski and Tonn 2000). This suggests that winter hypoxia is an important natural disturbance in these lakes, and that these systems offer an excellent opportunity to explore the effects of hypoxia on fish population dynamics. To examine such questions, we collected environmental and population data for fathead minnow Pimephales promelas inhabiting four lakes in boreal Alberta over a 5-year period. We predicted that fathead minnows inhabiting lakes whose local characteristics and regional setting make them vulnerable to winter hypoxia would be more frequently and severely disturbed and show higher variation in abundance and size structure than populations in less susceptible lakes.
Methods
Study lakes
The four study lakes lie within the Boreal Plains ecozone, in a roadless area about 200 km north of Edmonton, Alberta (Table 1). The lakes were located within 30 km of each other in the Terrestrial and Riparian Organisms, Lakes, and Streams (TROLS) project's South Calling Lake (SCL) study region (see Prepas et al. 2001).
Although the study lakes are all small and relatively shallow (Table 1), SCL20 and SCL100 have steeper basins and thermally stratify during the summer, and SCL200 and SCL800 are flat and mixed (Prepas et al. 2001). Despite the lakes' geographic proximity, their hydrogeologic settings differ due to spatial variability in the depth and composition of the underlying glacial till and the contour of the bedrock (Devito et al. 2000; Table 1). Both SCL20 and SCL800 have one ephemeral surface inflow and are in areas of groundwater recharge, while SCL200 has three surface inflows and is in an area of local groundwater discharge. SCL100 is a headwater lake located in an area of both local and regional groundwater discharge; it therefore receives water from beyond (as well as within) the topographical divide of its catchment (Devito et al. 2000). The outflows of all four study lakes are regulated by beaver dams, and the immigration or emigration of fish would be likely only if the dams were to collapse.
Fathead minnow dominated the fish assemblages of all four SCL study lakes. SCL20 contained an allopatric population of fathead minnow, while the other three populations were sympatric with brook stickleback Culaea inconstans and finescale dace Phoxinus neogaeus. In some years, white sucker Catostomus commersoni would invade SCL200 following the collapse of the beaver dam on the outflow; however, gill net surveys indicated that this population was neither persistent nor abundant (Tonn and Danylchuk, unpublished data). No piscivorous fish were present in any lake, and the only visible source of predation on minnows was piscivorous birds, such as resident common loons Gavia immer (all lakes) and irregularly visiting white pelicans Pelecanus erythrorhynchos.
Population surveys
We conducted mark–recapture surveys of the four fathead minnow populations annually from 1995 to 1999. Surveys were carried out in spring, before the onset of reproductive activity. We conducted surveys one lake at a time, alternating the sampling order among years.
We used unbaited Gee minnow traps (2-cm trap openings, 5-mm mesh) to collect fish. The number of traps set in a given lake (10–45 traps) was determined by the number of fish that could be processed in a day. Since fathead minnows in these lakes rarely inhabit water deeper than 2 m (W. M. Tonn, unpublished data), we set traps inshore within the 2-m isobath. The trap locations were determined randomly with the aid of a grid overlayed on a bathymetric map and a random number generator. The traps were set in the late afternoon and retrieved the following day; new trap locations were randomly assigned daily. Fathead minnows whose total length (TL) exceeded 38 mm (primarily age-1 or age-2 fish and older) were susceptible to the traps (Danylchuk and Tonn, unpublished data).
All captured fathead minnows were marked by fin-clipping. Because fathead minnows are sexually dimorphic (Flickinger 1969), we marked fish differentially based on gender and maturity (Danylchuk and Tonn 2001). At the end of each survey (7–9 d per lake), we used the cumulative data to derive abundance estimates for male, female, and juveniles of both sexes using the Schnabel method (Ricker 1975). We derived abundance estimates for each segment of the population separately since this would take into account the differential recapture rates among males, females, and juveniles and therefore provide more accurate estimates. The abundance estimates for males, females, and juveniles were then summed to provide a total abundance estimate for each population.
To facilitate among-lake comparisons, we converted abundance estimates to densities (number/m3) by estimating the water volume of the habitat sampled (i.e., the portion of the lakes <2 m deep) based on bathymetric maps. The densities of males, females, and juveniles were then summed for each lake to provide a total density estimate, with the variance for the summed density estimate being the sum of the variances for each component of the population (Zar 1996). To provide a measure of interannual variation in abundance, coefficients of variation (CVs, defined as 100 × SD/mean and corrected for small sample size; Sokal and Rohlf 1995) were calculated for each population for the 5 years of our study. To analyze year-to-year changes in fathead minnow abundance within each lake, we calculated the differences among annual estimates and determined whether the differences between consecutive years were significantly different from zero via the Z-test (Zar 1996).
We measured (TL to the nearest mm) a subsample of 100–200 fathead minnows each day. Each subsample was collected from several traps placed around the lake. When catches were low, all fathead minnows from a given trap were used to obtain the subsample for measurement; when catches were high (>50 fish per trap), we haphazardly measured approximately 25 fish from each selected trap. From these subsamples, we generated length-frequency distributions for each population in each year. Abundance estimates and length-frequency distributions were then used to determine the numbers of fathead minnows in each size-class. To determine if presumed winterkills were size selective, we compared the proportion of small (38–58 mm TL) fish before and after large (approximately 50%), statistically significant declines in abundance.
Physical and chemical variables
Winter dissolved oxygen profiles (0.25-m intervals) were taken several times per year at the deepest point in each lake as part of the TROLS core monitoring of the SCL study lakes using a YSI model 59 dissolved oxygen meter with stirrer. Because oxygen levels can be spatially variable (Wetzel 2001), we supplemented this monitoring with detailed spatial oxygen surveys in February 1997–1999. Sampling sites for these surveys (n = 2–17, depending on the lake size, weather, and ice conditions) were selected randomly. We also measured oxygen levels near surface inflows and sites of potential groundwater inflow to determine if oxygen refuges for fish existed. Mean dissolved oxygen concentrations were calculated for each site using the first 0.5 m below the ice-water interface. This stratum is the most oxygen-rich during winter stratification and is important for the overwinter survival of fish (Magnuson and Karlen 1970). Since ice and snow conditions can affect oxygen levels in lakes (Greenbank 1945), we also measured ice thickness and snow depth (nearest cm) at each site. To estimate the duration of ice cover, we visited the study lakes frequently in fall and spring.
To monitor changes in water levels, we installed staff gauges in the four study lakes in the spring of 1996. The water level was measured in each lake two to three times per month between ice-off and early September, and then monthly until ice-on. In 1996, we used changes in water levels between the installation of the staff gauges and the end of the open-water season as a relative indicator of annual change. For 1997 and 1998, we calculated annual change as the difference between the water levels at the ends of the previous and current open-water seasons. Annual precipitation data were obtained from Environment Canada climate stations within the region.
Euphotic zone water samples were collected about twice per month throughout the open-water seasons of 1995–1998 as part of the TROLS core monitoring of the SCL study lakes. We used data for total phosphorus (TP) and chlorophyll a (Chla; see Prepas et al. 2001 for details) as indices of primary production; because of restrictions on data availability, data from 1995 and 1996 are presented as 2-year averages.
Data analyses
Data were tested for normality and homogeneity of variance with Shapiro–Wilk W and Bartlett's test, respectively (Sokal and Rohlf 1995). Fathead minnow density (log (x + 1)) and abundance (log10), the proportion of small fish (arcsine square root), TP (log10), and Chla (log10) were transformed to meet the assumptions of parametric statistics (Z-test, t-test, analysis of variance [ANOVA]), and nonparametric methods (Mann–Whitney U-test, Kruskal–Wallis H-test) were used when these assumptions could not be satisfied. For all tests, the significance of the differences among variables was determined (marginally significant if 0.1 > P > 0.05 and significant if P < 0.05). All statistical analyses were performed using Statistica ’99 for the PC (StatSoft 1999).
Results
General Spatial and Temporal Patterns
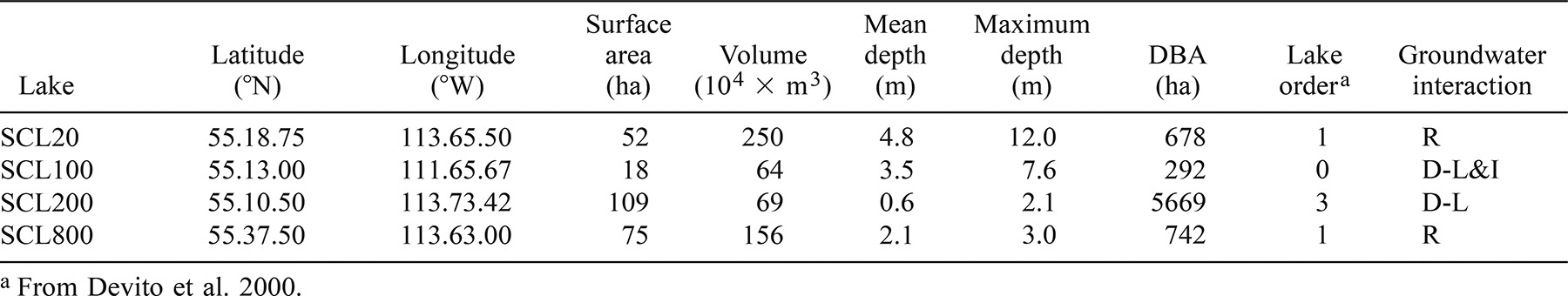
Abundance estimates for each fathead minnow population were relatively precise, with standard errors ranging from 4% to 20% within a given year. The density of fathead minnows (Figure 1) differed significantly among each of the study lakes (ANOVA [P < 0.001] and Tukey's highly significantly different [HSD] test). Densities over the 5-year period overlapped in SCL20 and SCL100, and also in SCL200 and SCL800. Within these two pairs, lakes were also similar in their degree of annual variability, with CVs being lower in the two higher density populations (SCL20 and SCL100) than in the lower density populations (SCL200 and SCL800; Figure 1).
Density of fathead minnows in the four boreal Alberta study lakes, from spring mark–recapture estimates in 1995–1999. Dashed lines represent the mean density for each lake over the course of the study; CV is the coefficient of variation, defined as 100 × SD/mean
SCL20 and SCL100
Winter dissolved oxygen concentrations were consistently higher in SCL100 than in the other study lakes and remained high even when winter conditions were severe elsewhere (Table 2). Correspondingly, the fathead minnow population in SCL100 was the most stable (Figure 1), and the size range and frequency distribution of fathead minnows in SCL100 as well were similar from year to year, with modes consistently falling between 58 and 62 mm TL (Figure 2).

Length-frequency distributions for fathead minnows in the study lakes (1995–1999)
The population in SCL20 was also relatively stable among years; however, density decreased 47% between 1995 and 1996 (Z-test, P < 0.01; Figure 1). Although the size range of fathead minnows did not change considerably following this decline (Figure 2), the proportion of small fish within the population decreased significantly (t-test, P < 0.05). The decrease in the density of minnows from 1995 to 1996 corresponded to exceptionally low levels of dissolved oxygen in the intervening winter (Table 2). These low winter oxygen levels coincided with the longest duration of ice cover, 222 d, of the four winters of the study period (Table 2).
SCL200 and SCL800
The fathead minnow populations in SCL200 and SCL800 displayed the greatest annual variations in density over the course of the study (Figure 1), including one and two year-to-year declines of greater than 60%, respectively. Concurrent with the decrease in density in SCL20, the population in SCL800 decreased by 62% between 1995 and 1996 (Z-test, P < 0.001; Figure 1). Even more dramatic was the decline between 1998 and 1999, when density decreased by 94% (Z-test, P < 0.001). Following both of these declines, the proportion of small fish in the population decreased substantially (1995 versus 1996: t-test, P < 0.001; 1998 versus 1999: Mann–Whitney U-test, P < 0.01), particularly in 1995–1996, when the length-frequency mode shifted from about 50 mm TL to 68–72 mm TL (Figure 2). Although winter oxygen levels were chronically low in SCL800, dissolved oxygen levels were slightly lower during the winter of 1998−1999 than in previous years (Table 2). Unlike the winter of 1995−1996, the duration of ice cover was not prolonged in 1998/1999; however, snow accumulation was high relative to other years (Table 2). Moreover, TP and Chla were higher during the open water season of 1998 than in previous years (ANOVA, P < 0.05; Tukey's HSD; Table 3), indicating an increased availability of organic material for decomposition. Furthermore, precipitation was low in 1998, and water levels were considerably lower prior to ice-on in 1998 than earlier (Table 4).

Synchronous with the decimation of the population in SCL800, a substantial decrease (>80%) in fathead density occurred in SCL200 between 1998 and 1999 (Z-test, P < 0.001; Figure 1). As with SCL800, the length-frequency distribution in SCL200 became skewed towards larger fish concurrent with the decreased density (Figure 2); this trend, however, was not significant (t-test, P > 0.1). Dissolved oxygen levels in SCL200 were below detection during the snowy winter of 1998/1999 and no spatial variation occurred within the lake (Table 2). In contrast, spatial surveys in previous winters had revealed considerable heterogeneity in oxygen levels, with concentrations near inlets measuring 4–6 mg/L. As in SCL800, the water level in SCL200 decreased substantially in 1998, when precipitation was low (Table 4). This decrease—in combination with an even larger reduction in 1997 that was due, in part, to the collapse of the beaver dam on the outlet—resulted in an overall drop in lake level of more than 50%. High Chla concentrations were also observed during 1998 (Table 3), as in SCL800, reflecting an increased production of organic matter.
Discussion
Our study suggests that winterkill is a pervasive and common disturbance for small-bodied fish populations inhabiting shallow Boreal Plains lakes. Large density declines followed winters of notably low dissolved oxygen levels in three of the four populations of fathead minnows and in two of five study years. Interestingly, the suite of lakes that experienced winter hypoxia differed between years, indicating that not all lakes in a region respond similarly to regional environmental factors that affect winter oxygen depletion. Fathead minnow populations in two of the study lakes, SCL200 and SCL800, were particularly prone to this disturbance, displaying year-to-year declines of greater than 80%, correspondingly large coefficients of variation in density, and, for SCL800, major shifts in size-frequency distributions. In contrast, the populations were more stable in SCL20 and SCL100.
Relative Influence of Local and Regional Factors
We suggest that the extent of winter oxygen depletion and the susceptibility of a lake to winterkill will vary depending on its inherent characteristics and the relative influence of a suite of environmental factors that can be arranged hierarchically from regional to local scales (Figure 3). At the regional level, an extended duration of ice cover can affect oxygen levels in lakes by reducing the atmospheric contributions of oxygen (Barica et al. 1983; Wetzel 2001). Synchronous population declines in SCL20 and SCL800 following the 1995–1996 winter (which had an ice-covered period about 1 month longer than normal) were therefore not surprising. Harsh winter conditions can also be marked by the heavy accumulation of snow and ice cover. Heavy snow and cloudy ice can affect oxygen levels in lakes by reducing the photosynthetic contributions of oxygen (Barica et al. 1983; Wetzel 2001). Severe and synchronous winterkills also occurred (in SCL200 and SCL800) following the 1998−1999 winter that provided the heaviest accumulation of snow. Interestingly, this was also a short winter, so predictions of the reduction or elimination of winterkill due to climate warming that are based solely on winter duration (Stefan et al. 2001) should be viewed with caution.
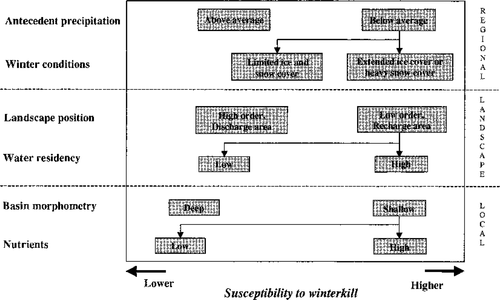
Conceptual model for the relative influence of local, landscape, and regional factors on the susceptibility of a lake in the Canadian Boreal Plains to experience winterkill of fish (from the work of K. Devito and I. Creed, personal communication; see Buttle et al. 2000)
The importance of regional climate is further illustrated by the synchronous occurrence of winterkill in other lakes in the region during the harsh winters of 1995–1996 and 1998–1999. In three other lakes monitored by the TROLS project, major winterkills of northern pike Esox lucius and white sucker were documented for 1995–1996 (W. M. Tonn, unpublished data). Both regional synchrony and a comparable overall frequency of winterkill was also documented for an allopatric population of fathead minnows in a small (3.2 ha, 4 m maximum depth) unnamed lake about 40 km south of the SCL study lakes (W.M.T., unpublished data). Using methods identical to those employed here, five declines of greater than 50% were documented during 15 years of study (Figure 4). Although winter conditions were not monitored at this site, two of the declines occurred following the winters of 1995–1996 and 1998–1999, synchronously with winterkills in the SCL lakes.

Long-term record of fathead minnow densities from a small lake south of the South Calling Lake study area. Densities are based on spring mark–recapture estimates from 1985 to 2000 (except 1991)
The input and storage of oxygenated water for the winter period, which will influence the probability of winterkill in a lake, is influenced by variation in antecedent precipitation (another largely regional factor) through its effects on local surface and groundwater flow (Webster et al. 1996, 2000; LaBaugh et al. 1998). In our study region, evaporation generally exceeds precipitation (Devito et al. 2000), making the contribution of water from the atmosphere especially important in regulating water flow (Winter and Woo 1990). When precipitation is unusually low, as it was in 1998, water levels decline and water residence times increase; coincidentally, nutrients and primary production increased in three of our study lakes, likely reflecting a reduced water flow and a greater internal loading of nutrients (Prepas et al. 2001). In combination with the heavy snow cover of the following winter, these changes in subregional conditions likely contributed to winterkill in two of the three lakes. The winter oxygen levels in SCL20 and SCL100 were less affected by the drought since these lakes are deeper and are therefore able to hold more oxygen at the start of winter.
Decreased stream inflows related to regional drought can also affect the survival of fish by eliminating oxygen refugia within a lake (Magnuson et al. 1989). Over the course of our study, spatial variation in winter oxygen levels was typically greatest in SCL200 (associated with its three small, inflowing streams), but this variation was eliminated in the winter following the drought, probably as a result of severely reduced inflows. A lack of winter oxygen refugia may have contributed to the severe winterkill in 1998/1999 as much as the low average oxygen concentrations.
At a subregional level, we suggest that the landscape position of SCL100 contributes to its limnological stability and reduced susceptibility to winterkill relative to other lakes in the SCL region. Since it is in an area of both local and regional groundwater discharge (Devito et al. 2000), SCL100 is less susceptible to changes in water inflow associated with annual fluctuations in climate (LaBaugh et al. 1998) and therefore less susceptible to the drought-related changes in water level, water residency time, nutrient concentrations, and primary production discussed above. Likewise, although SCL200 and SCL800 are both shallow and productive, SCL200‘s higher lake order and hydrogeologic position in an area of local groundwater discharge likely reduced the extent of winter oxygen depletion and buffered its fathead minnow population from the harsh winter conditions. Correspondingly, fathead minnows in SCL200 did not experience winterkill during the long winter of 1995–1996 whereas fathead minnows in SCL800 did.
Locally, the morphometry of a lake's basin is known to influence a lake's susceptibility to winter oxygen depletion (Barica and Mathias 1979), but other local factors can also play important roles. The collapse of the beaver dam on the outflow of SCL200, for example, contributed to the dramatic decline in water level in 1997–1998 that, in turn, contributed to the severity of winterkill in 1998–1999. Similarly, a near total winterkill followed a substantial drop in water level when the beaver dam at the outlet of the long-term study pond south of the SCL lakes collapsed in late summer 1986 (Figure 4). Clearly, the stochastic nature of beaver-controlled water bodies can influence the incidence of winterkill on a local scale.
Effects on Population Size Structure
The majority of studies on winterkill have focused on large-bodied fish species in north-temperate lakes (e.g., Cooper and Washburn 1946; Johnson and Moyle 1969). The loss of larger individuals from such populations may be due to their larger oxygen demands (Casselman and Harvey 1975). In contrast, we found that populations of fathead minnows that experienced winterkill demonstrated either no size-related trends (SCL200) or substantial decreases in the proportion of small individuals (SCL20 and SCL800). Although we did not sample individuals smaller than approximately 38 mm TL, Magnuson et al. (1985) found that young-of-the-year fathead minnows and northern redbelly dace Phoxinus eos emigrated from a hypoxia-prone lake in the fall, whereas adults remained within the lake. This suggests that susceptibility to and effects of winterkill may be different for small-bodied versus large-bodied species. Interestingly, St-Onge and Magnan (2000) found decreases in smaller size-classes of yellow perch Perca flavescens and white sucker following disturbances caused by forest fire and timber harvest.
Collectively, our results suggest that large declines in fathead minnow density and shifts in size structure were related to winter hypoxia. Although we cannot rule out other possible explanations for our long-term comparative study, alternative hypotheses seem much less likely. For example, because smaller fish have lower fat stores and higher rates of energy use than larger fish, smaller fish can be susceptible to overwinter energy depletion (reviewed by Shuter and Post 1990). The large decreases in fathead minnow density in our study lakes, however, occurred following both long and short winters. It is also possible that fluctuations in recruitment could have reduced densities and altered population size structure. The likelihood, however, that year-class failures would consistently and repeatedly anticipate critically low winter oxygen levels by 1–2 years (since minnows recruited to our traps at age 1 or 2) seems low. Moreover, it is unlikely that the magnitude of the population declines (47–94%) could have been accounted for by year-class failure, since these declines involved multiple age-classes (Danylchuk and Tonn, unpublished data).
Potential Implications of Human Disturbance
Our results indicate that the synergistic effects of local, landscape, and regional factors on winter oxygen depletion strongly influence the natural incidence of winterkill for small-bodied fish in Boreal Plains lakes. Some of these factors can also be influenced by human disturbance, suggesting that the natural risk of winterkill for fishes in northern lakes can be altered by human activities. For example, a reduction in the length of winter resulting from climate warming is predicted to reduce or even eliminate winterkill in shallow north temperate lakes (Stefan et al. 2001). This view, however, considers that the probability of winterkill is largely a function of winter duration, and ignores other local and regional influences. In some regions, including western Canada, climate change is also predicted to decrease precipitation and increase evaporation, which can reduce surface and groundwater flow, increase water residency times, reduce lake levels, and increase eutrophication in lakes (Schindler 2001). In spite of a short winter, the most severe winterkills during our study (1998–1999 in SCL200 and SCL800) followed a year of low rainfall that reduced surface flow and lake levels, and increased water residence times and nutrient concentrations. The 1998–1999 winter was also characterized by above-average snowfall, a climatic factor known to affect winter oxygen depletion rates (Greenbank 1945); interestingly, climate change models that predict lower summer precipitation for northwestern Canada also predict moister winters (e.g., Environment Canada 1999). Thus, the effects of climate change may actually increase winter oxygen depletion and winterkill in our region.
The incidence of winterkill could also be affected by human disturbance at the local or landscape level. In addition to enhancing evaporation through greater convection and wind velocity (Schindler et al. 1990), the harvest of timber near lakes could cause an increase in nutrient loading and primary production (Carignan et al. 2000), and, in turn, increase winter oxygen depletion. Land clearing and other activities associated with agriculture can also increase nutrient export to lakes, eutrophication (Cooke and Prepas 1998; Schindler 2001), and, therefore, the incidence of winterkill for resident fish. Although the effects of climate change and other, more direct human impacts on freshwater ecosystems have received considerable attention, those examinations are incomplete if they fail to consider the natural disturbance regime of these ecosystems, and thus the local- and landscape-level factors that affect the frequency and severity of winterkill.
Acknowledgments
We gratefully acknowledge the generous support for the TROLS project from NSERC; Alberta-Pacific Forest Industries; Weyerhaeuser Canada, Ltd.; Alberta Environmental Protection; Manning Diversified Forest Products; Syncrude Canada, Ltd.; Alberta Economic Development and Tourism; Ainsworth Lumber Co., Ltd.; Employment Canada; NRC; NWRI; R.L. & L. Environmental Services; CS Resources; and the University of Alberta. Additional support for our research was received from the Biodiversity Grants Program (Department of Biological Sciences, University of Alberta and Alberta Conservation Association), and grants from the Canadian Circumpolar Institute (A.J.D.); Alberta Sports, Recreation, Parks, and Wildlife Foundation (A.J.D); and NSERC (W.M.T.). Thanks to S. Teige, C. Decker, R. Dillon, J. Haag, L. Turner, K. Field, S. Reedyk, N. Scott, and W. Zyla who assisted in the field, and to N. Carmosini and M. Serediak for logistical support. The manuscript benefited from the constructive criticisms of L. Kallemeyn, B. Pinel-Alloul, K. Westcott, and C. Paszkowski. The preparation of this manuscript was completed while the first author (A.J.D.) was employed by the School for Field Studies.




