Population Viability of the Gulf of Mexico Sturgeon: Inferences from Capture–Recapture and Age-Structured Models
Present address: North Carolina Cooperative Fish and Wildlife Research Unit, Box 7617, David Clark Labs, North Carolina State University, Raleigh, North Carolina 27695, USA.
Present address: Natural Resource Ecology Laboratory, A246 Natural and Environmental Science Building, Fort Collins, Colorado 80523, USA.
Abstract
The Suwannee River, Florida, population of the Gulf of Mexico sturgeon Acipenser oxyrinchus desotoi, a subspecies of Atlantic sturgeon A. oxyrinchus oxyrinchus, was evaluated using a capture–recapture approach and an age-structured model to examine population trends from 1986 through 1995. The capture–recapture analysis revealed a positive rate of change (λ) in the adult population, indicating that it was slowly increasing from the mid-1980s through the mid-1990s. The age-structured model revealed that the population was highly sensitive to changes in egg-to-age-1 mortality, the percentage of females that spawn annually, and adult mortality. The model predicted that even slight increases in annual adult mortality (from 16% to 20%) would result in a decline in the Suwannee River Gulf sturgeon population. Population trends were consistent for both modeling procedures and were similar to those in published reports. Although this population is currently expanding, care should be taken to protect adult fish from any fishing or bycatch mortality. Given the particular attributes of Gulf sturgeon (such as late sexual maturation, the fact that few mature females spawn each year, and high early life mortality), managers should be patient and willing to monitor populations for extended periods of time (∼20 years) to detect changes in the adult population.
Introduction
The Suwannee River, Florida, contains the most abundant population of Gulf of Mexico sturgeon Acipenser oxyrinchus desotoi, a large, long-lived anadromous subspecies of Atlantic sturgeon A. oxyrinchus oxyrinchus historically found from Tampa Bay, Florida, to Louisiana (Wooley and Crateau 1985). Gulf sturgeon supported a commercial fishery on the west coast of Florida throughout the mid-20th century, with harvests between 1956 and 1973 ranging from approximately 453 kg to 26,184 kg annually (Huff 1975). Concern over decreased stock abundance caused the state of Florida to impose a moratorium on sturgeon harvesting in 1984 (Odenkirk 1991) and led to both federal and state protection. Effective management of this threatened species requires proper assessment of population growth and mortality trends to evaluate population viability (i.e., trends in abundance). However, despite the availability of substantial data on this population (e.g., Huff 1975; Carr et al. 1996; Chapman et al. 1997; Sulak and Clugston 1999), the long-term status and population trends of Gulf sturgeon in the Suwannee River remain unknown.
We used data from published and unpublished studies of the Suwannee River Gulf sturgeon population from 1986 to 1995 to evaluate population viability. Our objectives were (1) to evaluate the overall rate of population change (λ) for this population between those years and (2) to predict how changes in egg-to-age-1 mortality, the percentage of females that spawn annually, and annual adult mortality would affect population size and recruitment to age 1. This information is required to evaluate the recovery criteria for this species, evaluate current protection measures, identify future research needs, and provide a more complete understanding of the factors shaping the population dynamics of this unique species.
Model Descriptions and Empirical Data
For this study we incorporated data from published and unpublished studies of Gulf sturgeon into two models. First, we used an open-population, capture–recapture approach to assess population trends over a 10-year period. Second, we used an age-structured population model to predict the life history parameters (e.g., adult mortality and the percentage of adult females that spawn annually) that most strongly influenced adult abundance and recruitment to age-1. When data for Gulf sturgeon were unavailable, we substituted data from other sturgeon populations.
Assessing Trends in Gulf Sturgeon Population Abundance
The Gulf sturgeon population data used in this analysis were obtained from a long-term tagging study in the Suwannee River. Fish were collected primarily during March and April from 1986 to 1995 using fixed gill nets (see Chapman and Carr 1995 and Carr et al. 1996 for a complete description of sampling methods and location). Captured fish were weighed (± 0.1 kg), measured (± 0.1 cm, fork and total lengths), tagged externally with an individually-coded Monel tag, and released at the capture site. A total of 2,061 fish were included in this analysis. Of these, 1,580 (77%) were captured once, 409 (20%) were captured twice, 62 (3%) three times, and 10 (<1%) four times. The recapture histories of each fish were used in capture–recapture models.
We used open-population capture–recapture models (Program MARK; White and Burnham 1999) to generate estimates of the rate of population change (λ) and survival (S) of fish of ages 6 to 20 (Pradel 1996; Dreitz 2000). This approach allows for natural variability in life history variables (i.e., survival and recruitment) and provides predictions of the rate of population change, recruitment, and total mortality, all of which are difficult to obtain with threatened species whose number is small or with slow-growing, long-lived individuals such as sturgeon. This approach models population growth as follows:
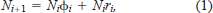
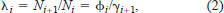
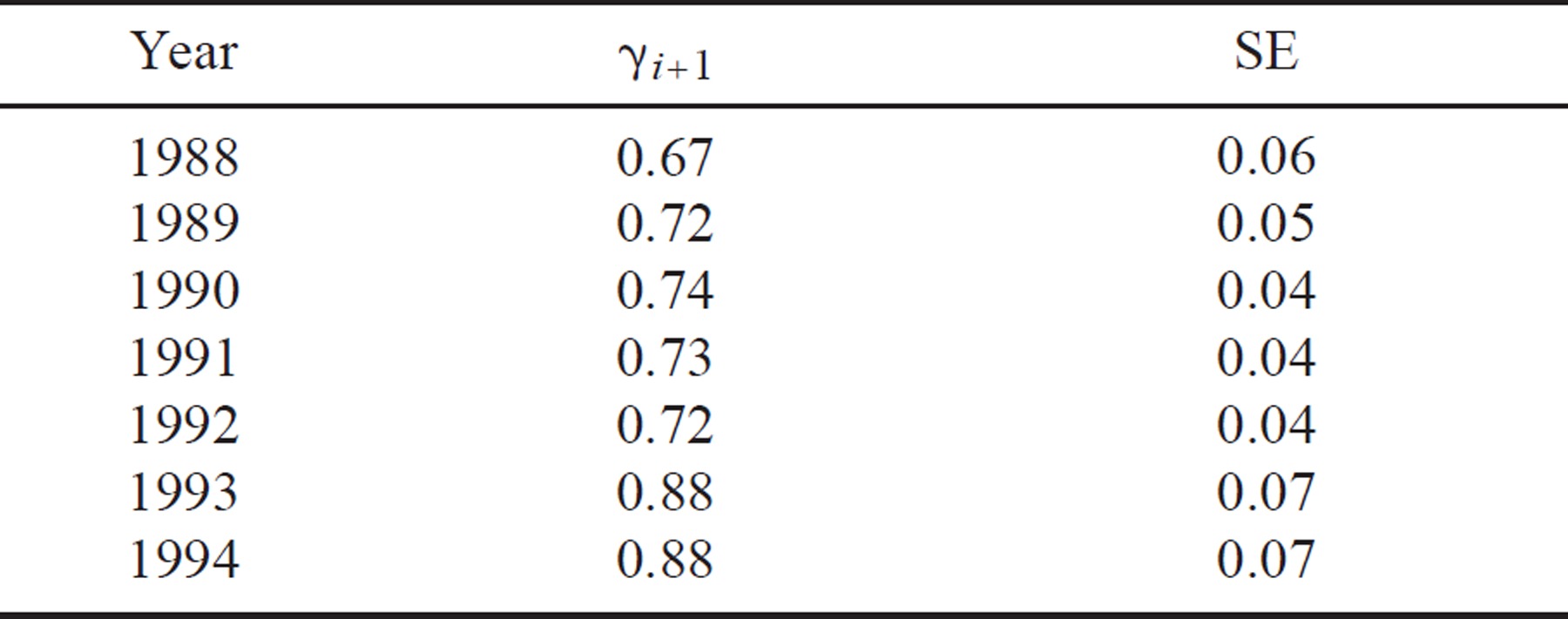
A λi value greater than 1 indicates that the population is increasing, a λi value less than 1 that the population is decreasing, and a λi value of 1 that the population is staying the same. Nichols et al. (2000) showed how γi+1 can provide information about the components of population growth. Thus, if γi+1 exceeds 0.50, survival has a greater influence than recruitment on population growth over the interval from i to i + 1. Likewise, if γi+1 is less than 0.50, recruitment has a greater influence than survival, and if γi+1 equals 0.50, both survival and recruitment are equally important to the observed λi.
The assumptions and overall approach of the temporal symmetry modeling (TSM) technique that we employed are generally the same as those for the Jolly–Seber model, in which the population is open to recruitment, immigration, emigration, and mortality (Jolly 1965; Seber 1965; Seber 1982, Hightower and Gilbert 1984; Pollock et al. 1990). This model allows for both losses and gains to the population between the individual sampling periods (years). The TSM also assumes that all individuals within the population have the same capture and survival probabilities, that marks are not lost, and that sampling time is short relative to the time interval between samples (Pradel 1996; Dreitz 2000; Nichols et al. 2000). The techniques used to predict the annual rate of population change (λi) are similar to the Jolly–Seber technique to predict survival (Cormack 1964; Jolly 1965; Seber 1965) but reverses the capture history in order to predict recruitment rate (Dreitz 2000).
We built models in MARK that considered only survival, recapture probability, and seniority probability and that estimated λ as a derived parameter. The models allowed these variables to remain constant or to vary through time. Model selection was made using QAICc, a modification of the Akaike information criterion (AICc; Burnham and Anderson 1998) that uses quasi-likelihood adjustments (i.e., a variance inflation factor) to correct for lack of independence or overdispersion in the data and improve the overall fit of the model (Dreitz 2000). A variance inflation factor (ĉ) was calculated using a bootstrap procedure on the full model (Dreitz 2000).
Assessing Critical Life History Parameters
The parameters critical to adult abundance and recruitment to age 1 were assessed using the age structure modeling software MOCPOP (Beamesderfer 1991; Table 1). The age-specific number of fish in the population in any year was calculated from the equation

The data used in the MOCPOP simulations are outlined in Table 1. Because of the threatened status of the Gulf sturgeon population, lack of research funding, and the handling restrictions placed on researchers by permitting agencies, little research on the age and reproduction of these fish has been conducted since the 1970s. We relied heavily on published life history parameters when data were available, particularly the extensive histology and aging work of Huff (1975) and the unpublished age analysis work by K. Sulak (U.S. Geological Survey, Gainesville, Florida). Fishing mortality was set at zero for all simulations because the fishery has been suspended since 1984. The sex ratios used were 50:50 (male:female), and female age at sexual maturity was set at 10 based on Huff (1975). Relationships between fork length and weight were generated from fish collected between 1986 and 1995. A von Bertalanffy growth curve was fitted to Suwannee River Gulf sturgeon collected between 1986 and 1998 using pectoral fin spines aged by K. Sulak (Figure 1). This period includes the range of years used in this study. Length−fecundity estimates from white sturgeon A. transmontanus were used (Beamesderfer et al. 1995) to meet model parameter requirements because few estimates existed for either Gulf sturgeon or the closely related Atlantic sturgeon A. oxyrinchus.
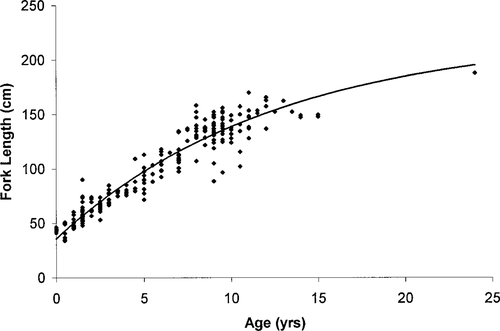
Observed fork lengths and ages of Suwannee River Gulf sturgeon (N = 237). The line illustrates the von Bertalanffy growth model fitted to this data and reported in Table 1
Although intensive studies have been conducted on spawning site selection within the Suwannee River (Marchant and Shutters 1996; Sulak and Clugston 1998; Sulak and Clugston 1999), little data exist on the number of female sturgeon that spawn each year or the mortality rates of the eggs produced. Sulak and Clugston (1998) estimated that 30–90 female fish spawn per year based on intensive egg sampling efforts conducted between 1993 and 1998. The spawning interval for Atlantic sturgeon is 3–5 years (Smith 1985), and Sulak and Clugston (1999) indicated that the same interval may be applicable to Gulf sturgeon. By extrapolating a 3–5 year spawning interval out to the population level and using population estimates (N = 7,650) from Sulak and Clugston (1999), we estimated that approximately 3–10% of the sexually mature females in the population spawn in any given year. These numbers are similar to the estimates made by F. A. Chapman (University of Florida, personal communication). To evaluate a range in the proportion of females that spawn each year, we simulated three levels (5, 15, and 25%).
A Beverton–Holt density-dependent relationship between female reproductive potential and egg survival was used to simulate egg production (Ricker 1975; Beamesderfer 1991):
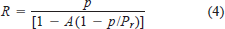
where R is actual egg deposition per female, p is potential egg deposition, A is the shape parameter of the curve, and Pr is the reproductive potential at equilibrium.
The value of Pr was calculated by running a 1-year simulation in MOCPOP with fixed recruitment and age 1 as the maximum age. Egg-to-age-1 mortality was calculated from the equation
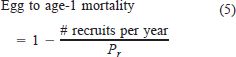
with 1,000 as the number of recruits each year. We selected that number because it was similar to the recruitment estimate from Sulak and Clugston (1999) and resulted in realistic egg mortality rates. We simulated three different ranges of egg-to-age-1 mortality (99.86–99.90, 99.91–99.95, and 99.96–100%) to assess how changes in first-year mortality would affect the population.
Mortality rates for juveniles (ages 1 to 3) and subadults−adult (ages 4 to 25) were obtained from Morrow et al. (1998), Sulak et al. (1999), and unpublished data provided by K. Sulak. Because Sulak and Clugston's (1999) estimate of subadult and adult mortality was based on the tagging rates of new individuals into the tagged fish population, as an independent estimate of total adult mortality we used the formula (after Gulland 1983)
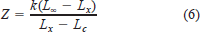
where Z is the instantaneous rate of total mortality, k is the shape parameter from the von Bertalanffy growth equation, L∞ is length at infinity from the von Bertalanffy growth equation, Lx is mean fork length at capture, and Lc is the minimum fork length at which Gulf sturgeon are vulnerable to capture.
Based on these estimates of total annual mortality (Table 1), we simulated three rates (10, 16, and 20%) to assess the effects of total adult mortality. A maximum age of 25 was used for all simulations (Sulak and Clugston 1999). The mortality rate for juveniles (ages 1 to 3) was obtained by taking the mean of the annual mortality rates reported for ages 3–9 in Morrow et al. 1998 (34%) and for ages 3–25 in Sulak and Clugston 1999 (16%), which is 25%.
We evaluated how changes in the percentage of females spawning each year, egg-to-age-1 mortality, and total subadult−adult mortality would affect the overall population size. For each simulation, all parameters were held constant at the values listed in Table 1 except for the parameter being evaluated. Parameters that were simulated over a range of values (percentage of females spawning, egg mortality, and annual mortality) were based on a random uniform distribution of the values within the specified range. A 50-year period of instability in population density and structure occurred when the simulations began. This instability, often called transient dynamics, resulted from the adjustment of the population to the applied rate of recruitment and mortality. After this period of instability, the populations formed a new, stable age distribution. Our results exclude the 50-year instability period.
Results
Assessing Trends in Gulf Sturgeon Population Abundance
Analysis of the capture–recapture data from 1986 through 1995 suggested that the population showed slight increases. An overall positive rate of population change was predicted for this period (λ = 1.05 ± 0.01). In estimating the rate of population change, we used a conservative model that allowed survival, recapture probability, and seniority probability to vary over time. Placing constraints on these parameters would potentially have limited our estimate of λ (Franklin, in press; J. D. Nichols, Patuxent Wildlife Research Center, U.S. Fish and Wildlife Service, Laurel, Maryland, personal communication). We used variance component analysis to average the results of our yearly λ estimates to predict a rate of population change during the study. Our model selection criteria selected two models as the best approximating models to estimate mortality. The two models had constant survival, allowed recapture probability to vary through time, and either set seniority probability to be constant or allowed it to vary. The apparent mortality estimate (1 − φ) generated from the TSM was constant at 17% (± 0.03%) for each year.
We selected two models to estimate seniority probability. The model holding seniority probability (γi+1) constant gave an estimate of 0.74 (± 0.03). The model with temporal variation gave estimates ranging from 0.67 to 0.88 (Table 2). Both the constant and variable estimates of seniority probability suggested that adult survival had a greater influence on λi than recruitment (i.e., γi+1 > 0.50; Table 2).
The Gulf sturgeon were fully vulnerable to the gear at approximately age 6 (∼1,000 mm TL) and few (N = 8) individuals older than age 20 (∼2,000 mm TL) were collected. Thus, all predictions derived from the capture–recapture dataset apply to subadult and adult individuals within this age and size range. We were not able to compensate for tag loss in this study. However, tag retention for a very similar tag was reported as 82–100% annually (Chapman et al. 1997).
Assessing Critical Life History Parameters
Our simulations predicted that Gulf sturgeon would be highly sensitive to changes in the percentage of females that spawn annually and to the egg-to-age-1 mortality rate. With a 5% adult female spawning pool, the model projected an equilibrium population size of 5,500 (Figure 2a) and annual recruitment of approximately 1,000 age-1 individuals. With an increase in the spawning pool to 15% of the adult females, the model projected an equilibrium population size of about 12,000 individuals and 2,000 recruits (Figure 2b). An increase in the spawning pool to 25% could increase the population to 16,000 individuals and 3,000 recruits (Figure 2c).
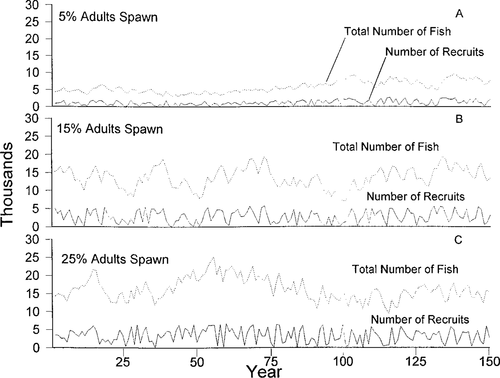
Total numbers of fish (dotted lines) and recruits (solid lines) predicted with different percentages of sexually mature adult females assumed to spawn. Panel A most closely approximates the observed population size range in the Suwannee River, Florida
We predicted mortality of 99.96% from egg to age-1 for Gulf sturgeon. Although this number may at first seem high, it is similar to estimates for striped bass Morone saxatilis (Rose and Cowan 1993; Bulak et al. 1997). Egg-to-age-1 mortality rates ranging from 99.96% to 100% resulted in a population of around 5,500 individuals (Figure 3c). At these mortality rates, the model predicts that around 1,000 recruits will be produced each year, with some year-class failure caused by the failure of any of the eggs to survive to age 1. With mortality 0.05 percentage points lower, population size increased 10-fold, with a 5-fold increase in the number of recruits (Figure 3b). Decreasing the mortality rate by another 0.05 percentage points resulted in a 20-fold increase in population with a 10-fold increase in recruits (Figure 3a).
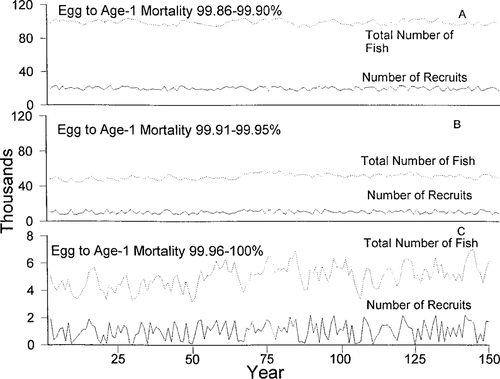
Total numbers of fish (dotted lines) and recruits (solid lines) predicted with different egg-to-age-1 mortality rates. Note that the scale of the y-axis is not the same for all panels. Panel C mostly closely approximates the observed population size range in the Suwannee River
Changes in adult mortality also had large effects on long-term population size. Total mortality of 16% provided around 5,000 adults in the population and around 1,000 recruits/year (Figure 4b). A decrease in annual mortality to 10% nearly doubled the projected number of adults and recruits at equilibrium (Figure 4a). At 20% total mortality, the model predicted that the Suwannee River population would decline towards extinction (Figure 4c). Thus, with a 4% increase in total adult mortality (from 16% to 20%), the population would fluctuate around 700 individuals with low production of recruits for approximately 35 years, then begin to decline over the following 50 years (Figure 4c).

Total numbers of fish (dotted lines) and recruits (solid lines) predicted with different annual adult mortality rates. Note that the scale of the y-axis is not the same for all panels. Panel B mostly closely approximates the observed population size range in the Suwannee River
Discussion
Population Trends
Independent estimates of population attributes such as survival and recruitment were generated by the age-structure and temporal symmetry models. Although there is variability associated with each of the parameters used in our models, the estimates of recruitment and total mortality generated by both models were very similar to each other and to published estimates generated by traditional fisheries assessment techniques for Gulf and other sturgeon species. Population size estimates generated by the MOCPOP model with the data in Table 1 were within the range of those reported by Chapman et al. (1997) and Sulak and Clugston (1999). Multiplying the number of individuals in the subadult and adult fish populations generated by the MOCPOP simulations (5,500 total individuals − 1,000 recruits = 4,500 subadults and adults) by 1 − γ (0.26, the proportion of subadult and adult fish that are new to the population according to the capture–recapture data) yielded a mean annual recruitment of 1,170 fish per year (both juveniles and adults). This estimate is close to the 1,210 recruits estimated by Sulak and Clugston (1999).
Chapman et al. (1997) also derived annual population estimates for Gulf sturgeon in the Suwannee River using the capture–recapture data from this study. Based on a regression of population size on year, they concluded that the population had remained stable. We approximated λ using data from Chapman et al. (1997) by regressing the natural logarithm of the population size estimate on the year and then exponentiating the slope of this regression. We estimated values of λ equal to 1.06 for both the weighted (by population size SE) and unweighted regressions. Similarly, the TSM model used in this study showed slight increases in population size over the same time period. Chapman et al. (1997) assumed survival was constant through time for each year that population size was estimated. The model we chose to estimate λ allowed survival to vary through time. Our model was more flexible and, coupled with our seniority probability estimates, this enabled us to determine which demographic component (survival or recruitment) had the most influence on this population (Nichols et al. 2000). Our estimates of the rate of population change (5% using TSM, 6% using Chapman's population size estimates) are small over the 10-year period and thus were not significant in the original regression models (Chapman et al. 1997). However, the small increases were significant in our variable-survival models, and we believe they are also biologically important.
Mortality
We estimated annual mortality to be about 16–17%, which was similar to the 16% reported by Sulak and Clugston (1999) and which resulted in an estimated population size similar to the range of the most recent estimates for the Suwannee River (Chapman et al. 1997; Sulak and Clugston 1999). Our mortality estimates were also similar to those reported for other sturgeon populations. Stevenson (1997) reported annual mortality rates of 16% for female and 4% for male Atlantic sturgeon in the Hudson River. Burch (1999) reported annual mortality rates of 17% for males and females in an exploited population of lake sturgeon A. fulvescens in Lake Winnebago, Wisconsin. Beamesderfer et al. (1995) reported mortality rates of 24% and 18% for two reservoir populations of white sturgeon in the Columbia River, Oregon−Washington. DeVore et al. (1995) reported annual mortality rates of 37% for age-classes (ages 12–17) that had been exploited and annual mortality rates of 10% for age-classes (ages 23–29) that had not been exploited in the unimpounded lower Columbia River.
Unlike our population in the Suwannee River, the sturgeon populations above were subjected to different levels of exploitation (commercial, recreational, or both), thus increasing their annual mortality rates. In theory, our estimated annual mortality rate is based solely on natural mortality since there should be no harvesting of sturgeon from the Suwannee River. Although the Gulf sturgeon fishery has been closed, some fishing mortality may still occur due to bycatch (Wooley and Crateau 1985). Juvenile Gulf sturgeon are known to congregate near shore (Huff 1975; Sulak and Clugston 1999; Fox et al. 2000) and may be incidentally harvested by commercial fishers. An amendment to the Florida constitution in 1994 that bans nearshore netting may reduce such mortality. Collins et al. (1996) reported that juvenile Atlantic and shortnose sturgeon A. brevirostrum were collected as bycatch in the American shad Alosa sapidissima and shrimp Penaeus spp. fisheries in South Carolina and Georgia. From the bycatch in shrimp trawls, Wooley and Crateau (1985) estimated 9.5% annual exploitation of Gulf sturgeon in Apalachicola Bay, Florida. We did not include bycatch mortality in our estimates because there is no large-scale commercial shrimp trawling in the nearshore areas surrounding the Suwannee River. Beamesderfer and Farr (1997) reported that annual exploitation of sturgeon greater than 5–10% almost always exceeds sustainable levels. However, the extent of the bycatch of Suwannee River Gulf sturgeon is unknown and should be evaluated. If it is substantial, efforts to reduce it could accelerate the recovery of Gulf sturgeon.
The total mortality of Gulf sturgeon in the Suwannee River has declined since the suspension of commercial fishing. From specimens returned from commercial fishermen, Huff (1975) reported annual mortality of 54% for Gulf sturgeon of ages 8–12 in the Suwannee River. Morrow et al. (1998) reported a 34% annual mortality rate for Gulf sturgeon in the Pearl River, Mississippi, and concluded that a reduction to 15–22% would be necessary for the sturgeon population to increase to a self-sustaining level. Given their late sexual maturity, long intervals between spawning, and (as our simulations suggested) the strong effects of adult mortality on the population, it is possible that the Suwannee River Gulf sturgeon population is only now beginning to benefit from the suspension of harvesting 17 years ago. A total of 21 years were required for the population to stabilize following a reduction in total annual mortality from the 54% reported by Huff (1975) to the 16% estimated here using MOCPOP simulations and constant recruitment (Figure 5). The effects of natural recruitment variability could increase the time required for recovery (Allen and Pine 2000) given the likelihood of several “weak” year-classes over this time interval. Our simulations suggest that at present this population could not sustain any exploitation. It is possible that other sturgeon species, which support recreational and/or commercial harvests, have different life history traits (i.e., a larger percentage of females that spawn annually) or larger areas of suitable habitat that ultimately allow for greater exploitation.
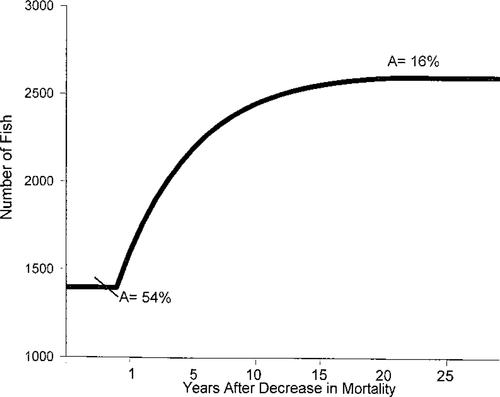
Number of years (x-axis) required for a given number of Gulf sturgeon (y-axis) to stabilize following a reduction in the annual mortality (A) rate from 54% to 16%
Our simulations suggested that even modest (e.g., 5%) increases in adult mortality could be detrimental to Gulf sturgeon in the Suwannee River, and we advise no exploitation of Gulf sturgeon in the river at this time. Seniority probability values were above 0.50, suggesting that adult survival was more important to population growth than recruitment rates. Thus, increases in adult mortality would cause more drastic population declines than changes in recruitment rate. Additionally, the MOCPOP simulations were highly influenced by the percentage of females that spawn annually and adult mortality. We believe that extreme care should be taken to protect adults in this population. Annual recruitment appears to result from relatively few females (30–90; Sulak and Clugston 1999), and removal of mature females for spawning and recovery efforts could reduce recruitment of wild fish. The effects of recovery efforts such as hatchery stocking were not revealed in this study and should be evaluated in future simulation studies. However, the population exhibited modest increases from 1986 to 1995, which may result in recovery if managers continue long-term protection of adults.
Assessing Model Predictions
Our models demonstrated that the percentage of females that spawn annually strongly influenced population size. The positive increase (∼5%) in population size that we detected was occurring in the sexually mature segment (or nearly so: age 6 and older) of the population, which may increase the number of females available to spawn and ultimately increase recruitment. Our model provided realistic population and recruitment estimates when 5% of the mature females spawned annually (Figure 2). The true percentage of spawning-age females that spawn in the Suwannee River each year is unknown but has been suggested to be around 3–10% (Sulak and Clugston 1999; F. A. Chapman, University of Florida, personal communication). However, studies are needed to assess the percentage of females that spawn each year.
Quantifying egg-to-age-1 mortality is difficult. As with most fishes that exhibit type III survival curves (Houde 1987), our model found that slight changes in egg-to-age-1 mortality would strongly influence the recruitment and subsequent population size of Suwannee River Gulf sturgeon. Egg-to-age-1 mortality in the range of 99.96–100% yielded realistic estimates of recruitment and population size. Stochastic variation simulated the potential variation in recruitment due to environmental factors. However, field studies are needed to address egg-to-age-1 mortality rates, particularly during the egg and larval stages when mortality would most strongly affect recruitment to adulthood (Houde 1987).
Beamesderfer and Farr (1997) demonstrated how evolutionary adaptations such as delayed maturation (which allows for rapid juvenile growth), high fecundity, and large adult body size improve spawning success and survival in years with suitable conditions but also make such fish susceptible to overfishing. Many life history traits are related to diverse, life-stage-specific habitats and require managers to think of habitat in a systemwide manner and not just focus on site-specific conditions such as flow and substrate (Beamesderfer and Farr 1997). Gulf sturgeon utilize diverse habitats, ranging from riverine areas to estuaries and nearshore communities (Fox et al. 2000) and possibly to deep oceanic benthic habitats (Sulak and Clugston 1999). Because of this, Beamesderfer and Farr (1997) advocate a combination of management strategies that integrate habitat protection and recovery with harvest restrictions and supplemental stocking.
This study utilized models to project trends that may be occurring in Suwannee River Gulf sturgeon. The predictions made through these modeling exercises are similar to field estimates (with associated variances) and are useful for addressing trends in management decisions (Johnson 1995). However, these models allowed us to identify trends for the Suwannee River Gulf sturgeon population that could aid management decisions. The parameters used in this model were gathered from a wide variety of studies in the Suwannee River conducted over a 25-year period as well as from studies of other sturgeon species. Changes have occurred over this period to both the habitat and the population of Suwannee River Gulf sturgeon that could affect the estimates used in this model, ultimately affecting the predicted results. Parameters for other sturgeon species may not apply to Gulf sturgeon (Stevenson and Secor 2000). Nevertheless, we compiled empirical data for this population and used two approaches for analysis, both yielding similar results. Therefore, we believe the model predictions are realistic.
Conclusions
While imperiled, the Suwannee River Gulf sturgeon population currently shows signs of recovery. With continued protection, this population should slowly increase. Researchers should refine some of the life history parameters, such as the length−fecundity relationships and female spawning interval, as well as develop indices to monitor year-class strength. It is possible that sturgeon, like many fishes, demonstrate variable recruitment, where a few large year-classes make up a high percentage of the adult standing stock (Allen and Miranda 1998). However, unlike short-lived fishes, the long life and late sexual maturation of sturgeon may distribute the strong year-classes over a large number of years. Historical catch records show that the Gulf sturgeon population in the Suwannee River has the potential to become relatively large. With further research, habitat protection, close monitoring of recruitment, and an intensive management plan, we believe that these fish will realize this potential.
Acknowledgments
We thank the many sturgeon research team members at the University of Florida, CCC, and the U.S. Geological Survey (USGS) for their hard work over the past 15 years and their willingness to provide data and insight on many aspects of this work. We also thank K. Sulak of USGS for providing length-at-age data for Suwannee River Gulf sturgeon. Jim Hines, J. Morrow, P. Kirk, D. Colle, F. Chapman, S. Carr, K. Pollock, D. Fox, and A. Huff all greatly improved this manuscript with their technical insights. This work was funded by the Florida Fish and Wildlife Conservation Commission and represents publication R-08260 of the Florida Agricultural Experiment Station.





