The Influence of Acidic Runoff Episodes on Slimy Sculpin Reproduction in Stone Run
Runoff events on April 20 and May 8, 1998, resulted in damage to the Benner Run cage and the escape of 5 fish.
Abstract
Much research has been devoted to the effects of acidic runoff episodes on populations of brook trout Salvelinus fontinalis. Less is known about slimy sculpin Cottus cognatus and why their numbers have declined in acidified streams. Adult tolerance of low pH and aluminum (Al) toxicity is similar in these two species. Slimy sculpin spawn in the spring, when high stream flows elevate concentrations of toxic Al and decrease stream pH in acid-sensitive watersheds. We hypothesized that acidic episodes in spring were a source of stress for slimy sculpin and hindered their reproduction. We tested this hypothesis by examining the mortality, behavior, whole-body sodium concentrations, and spawning among slimy sculpin exposed to ambient conditions during the spring spawning period in two Pennsylvania streams, Stone Run (an episodically acidified stream that formerly contained slimy sculpin) and Benner Run (a stream with slimy sculpin that does not experience severe acidic episodes). Our hypothesis was supported by the higher mortality, hypoactivity, lower body sodium concentrations, and lack of spawning among slimy sculpin in Stone Run relative to those in Benner Run. Reproductive disturbance caused by stressful concentrations of Al and hydrogen ions may have led to the recruitment failure and collapse of the slimy sculpin population in Stone Run.
Introduction
The northern Appalachian Plateaus Province in Pennsylvania receives high amounts of acidic deposition in the form of SO4–2 and NO3–2 ions (DeWalle and Swistock 1994). Most headwater streams in this region are low in acid-neutralizing capacity and pH and are vulnerable to acidification (Carline et al. 1992). Acidic rainfall and meltwater passes through the poorly buffered soils of the Appalachians, leaching Al into streams (Swistock et al. 1989). Periods of low pH and elevated Al concentration, called acidic episodes, result during increases in stream flow (Wigington et al. 1990). Exposure to low pH and elevated concentrations of Al can result in rapid loss of body sodium in fish; failure to maintain sodium balance can lead to death (Packer and Dunson 1970, 1972; Gagen and Sharpe 1987a, 1987b; Baker et al. 1990). In a survey of 50 Allegheny Plateau streams, Heard et al. (1997) documented a widespread loss of fish species in streams that had experienced acidic episodes.
The fish community of Pennsylvania's headwater streams typically includes brook trout Salvelinus fontinalis and sculpins (Hallam 1959; Cooper 1983). The slimy sculpin Cottus cognatus is the most widely distributed member of the family Cottidae in northern North America (Scott and Crossman 1973). In Pennsylvania, the slimy sculpin is found primarily in the Susquehanna River drainage, and the mottled sculpin Cottus bairdi is found primarily in the Ohio River drainage. Adult slimy and mottled sculpins are as tolerant of acidic conditions as brook trout (Gagen et al. 1993). However, sculpins are absent from a number of streams that experience acidic episodes yet that contain brook trout (Sharpe et al. 1987; Heard 1995; Baker et al. 1996). The slimy sculpin has been identified as a species that is sensitive to acidification because recruitment failure and population decline occur in waters with pH less than 5.4 (Baker and Christensen 1991). Based on the widespread distribution and association of the slimy sculpin with important game and commercial fishes, it has been identified as a good indicator species for changes in lacustrine fish communities due to acidification (Matuszek et al. 1990). As common associates of brook trout, slimy sculpin may also serve as a good indicator of the acidification of headwater streams in Pennsylvania.
Stone Run, a mountain stream in the northern Appalachian Plateaus Province, was surveyed in August 1961 during a comprehensive stream survey of fish distribution in Pennsylvania (Cooper 1983). Cooper found slimy sculpin, brook trout, brown trout Salmo trutta, and blacknose dace Rhinichthys atratulus in Stone Run. However, recent surveys (1995–1997) have found only brook trout in Stone Run (Heard et al. 1997; Dolte 1998). Releases of slimy sculpin into Stone Run (Gagen et al. 1993) have not resulted in their recolonization (Dolte 1998). Cooper recorded base-flow pH (6.40) and methyl orange alkalinity (5 mg CaCO3/L) for Stone Run (Heard 1995). As reported by Heard (1995), base-flow pH was 5.90 in 1994 and alkalinity was 0–1 mg CaCO3/L. In addition, Stone Run suffers from acidic episodes that coincide with the spawning season of the slimy sculpin (Wigington et al. 1996).
Previous researchers have hypothesized that the disappearance of slimy sculpin from episodically acidified streams may be the result of the greater sensitivity of early life stages to acidity, interspecific differences in avoidance behavior during acidic episodes, or reduced mobility that limits recolonization following acidic episodes (Gagen et al. 1993; Baker et al. 1996). The objective of this study was to demonstrate that slimy sculpin exposed to sublethal acid stress do not spawn successfully. To address this question, we compared the mortality, behavior, whole-body sodium content, and spawning among slimy sculpin introduced into Stone Run with those of conspecifics introduced into Benner Run, a reference stream with a resident slimy sculpin population that does not experience high Al concentrations. Field bioassays conducted by Gagen et al. (1993) and Baker et al. (1996) showed that spring acidic episodes in Stone Run were more toxic to slimy sculpin than acidic episodes in Benner Run. We hypothesized that slimy sculpin introduced into Stone Run would become hypoactive or moribund and would not spawn but that those introduced into Benner Run would spawn successfully. Plasma concentrations of sodium and chloride have been used to assess the stress of acidic field conditions on groups of fish (Muniz and Leivestad 1980a, 1980b; Leivestad et al. 1980). Gagen (1986) recommended analysis of whole-body sodium content as an alternative way of assessing Al toxicity for two reasons: the concentration of plasma sodium is confounded by the size of the fish, and blood samples are hard to obtain from small fish. We hypothesized that slimy sculpin exposed to acid stress in Stone Run would have lower whole-body sodium content than those exposed in Benner Run.
Study Areas
Stone Run and Benner Run are second-order streams draining minimally disturbed forested watersheds in the Northern Appalachian Plateaus Province of Pennsylvania. Stone Run is located in northern Clearfield County, approximately 40 km northwest of Benner Run, which is located in northern Centre County. Both streams drain watersheds of similar size and gradient. A more detailed description of the physical and biological characteristics of these watersheds can be found elsewhere (DeWalle et al. 1993; Gagen et al. 1993). We selected Six Mile Run, a second-order stream located 11 km southwest of Benner Run, as the source for the slimy sculpin used in the study because its base-flow chemistry, which was measured in 1994, was similar to that of our study streams and it contained an abundant slimy sculpin population (Heard 1995).
Stone Run and Benner Run were part of the Episodic Response Project sponsored by the U.S. Environmental Protection Agency that was conducted from fall 1988 through spring 1990. During the project, a permanent flow and water quality monitoring site was established on each stream. Rating curves were developed for the streams that related stream stage to flow. These curves were updated annually based on periodic stream gauging. A continuous record of stage was obtained during our study with a Belfort Instruments FW-1 water level recorder. These data, in digital format, were stored in a Campbell Scientific CR-10 data logger. We estimated stream flow using stage data and the rating curves. Precipitation amounts were collected and recorded monthly with a Belfort Instruments nonrecording precipitation gauge located on each watershed.
In spring 1998, study reaches were selected on Stone and Benner runs. The Stone Run reach was 11 m long × 6 m wide and the Benner Run reach 9 m long × 4 m wide. Both reaches included a run and a pool and were bounded by riffles. In spring 1999, a new study reach was selected on Benner Run that measured 62 m × approximately 6 m. The Stone Run study reach was expanded to 62 m in 1999 to match that of Benner Run. We selected these longer reaches in an attempt to increase both the number of slimy sculpin recovered and the number of nests found during our surveys. Study reaches on both streams contained a variety of nest-size rocks and were open to immigration and emigration of fish. We assumed that the habitat was suitable for slimy sculpin based on the current or historical occupation of these areas by this species.
Methods
Water quality
We visited Stone and Benner runs to gather data during two consecutive spring seasons. In 1998, we visited both streams every 3 d between March 1 and May 14. In 1999, we visited the streams every Monday, Wednesday, and Friday between April 1 and May 18. Both streams were visited on the same day. We collected water samples by hand in clean polyethylene bottles at the study reach every time we visited the streams. These samples were stored on ice during transport to the Water Laboratory of the Environmental Resources Research Institute at the Pennsylvania State University for analysis. The samples were analyzed within 24 h for pH using a Fisher Scientific Accumet model 20 pH/conductivity meter and then acid-fixed for Al determination. Driscoll et al. (1980) determined that the reactive, inorganic monomeric form of Al (AlIM) is the form most toxic to fish. Pagano (1990) reported that more than 90% of the total dissolved aluminum (AlTD) in Stone Run was in this form. During storm events, Benner Run had a substantial proportion of organic monomeric Al. Therefore, the AlIM form was a smaller fraction of AlTD in this stream (Pagano 1990). We chose to analyze samples for AlTD as an estimate of AlIM. Before analysis, aliquots for Al determination were filtered through a Millipore filter paper with a 0.1-μm pore size. Aluminum analysis was conducted using a Perkin-Elmer atomic absorption spectrophotometer with a 5100 ZL furnace module. The sampling protocol included field blanks and replicate samples to ensure laboratory quality assurance. Data were examined to meet U.S. Environmental Protection Agency quality assurance objectives for Al and pH determination (Kaeser 1999).
Mortality bioassay
Slimy sculpin of at least 50 mm in total length were collected by electrofishing (110–130 V AC; Coffelt Electronics electro-shocker model BP-1C) from Six Mile Run on February 26 and April 17, 1998, and March 31, 1999. Fish were transported to the Bellefonte fish hatchery in Bellefonte, Pennsylvania, and held 24–48 h in an aerated, 4,480-L fiberglass fish carrier. Fish were anesthetized in small groups with 55 mg MS-222/L and then measured, sexed, and given a distinguishing pelvic fin clip (the right pelvic fin on males and the left pelvic fin on females).
We conducted two in situ bioassays of the slimy sculpin in spring 1998. The first occurred in March before the spawning period and the second in late April and May during spawning and nest defense. Two bioassay cages measuring 30 × 38 × 68 cm were submerged in each study reach. Twenty fish were placed in each cage and exposed to ambient stream water quality for 20 d. We checked for mortality every 72 h. Fish were not fed during the exposure trials. All of the cages used in 1998 had wooden frames with fiberglass mesh sides. The following year we replaced the fiberglass mesh windows of all cages with aluminum window screening because debris had punctured the fiberglass windows, allowing fish to escape. To determine whether the use of aluminum screening affected the ambient levels of Al in the stream water, we took grab samples immediately upstream and downstream of an observation cage on each stream during 12 visits in April. These samples were handled in the same manner as all other grab samples and were analyzed for AlTD. The mean AlTD of the upstream group was not statistically different from that of the downstream group (two-sample t-test; n = 24, P = 0.66).
Whole-body sodium bioassay
To test for differences in body sodium content, we removed 4 or 5 fish from each bioassay cage in Stone and Benner runs on day 20 of the two exposure trials in 1998. In spring 1999, we employed a different procedure. As in 1998, we conducted two exposure trials using the bioassay cages, putting 20 slimy sculpin into each cage on April 1 and April 22. But instead of following the protocol for a 20-d mortality bioassay, we removed 5 fish from each cage at 5-d intervals (5, 10, 15, and 20 d) until no fish remained. We followed this protocol in 1999 to determine whether body sodium loss occurred rapidly during exposure. All fish removed were anesthetized and immediately killed, then placed in individual plastic bags and held on ice during transport to the laboratory. All fish were frozen within 2 h after being removed from the cages. Within 2 months these fish were digested in reagent grade nitric acid after determination of wet and dry (12 h at 100°C) mass (±0.01 g). The whole-body sodium concentration was determined from an analysis of the digested fish solutions diluted with deionized water using atomic absorption spectrophotometry, following the procedure of Gonzalez and Dunson (1987).
Slimy sculpin behavior
To observe fish behavior, we placed three male−female pairs of slimy sculpin in observation cages measuring 43 × 60 × 90 cm. In 1998, we placed one observation cage in each stream on March 19. The following year we placed two observation cages in each stream, submerging three pairs of slimy sculpin in observation cage 1 on April 1 and three pairs in observation cage 2 on April 14. Four nesting tiles were provided in each cage. These tiles consisted of a square (16 × 16 cm) piece of slate elevated on one side by two 3.75-cm wooden dowel legs glued to the tile. Downhower and Brown (1977) demonstrated that mottled sculpin nested under similar tiles in a field study. We visited the cages every 3 d in 1998 and every Monday, Wednesday, and Friday in 1999 and recorded the number of movements made by fish during a 5-min observation period. A movement was defined as a change in the location of an individual fish of at least one body length that was preceded and followed by a rest period of at least 3 s. Water temperature was recorded at the time of observation. During five visits in 1998, equal numbers (50–150) of sowbugs Asellus sp. were placed into the observation cages. We fed the fish in the observation cages seven times during spring 1999 with an assortment of scuds Gammarus sp. and the larvae of mayflies Ephemerella sp. collected from Logan Branch in Centre County, Pennsylvania. Equal numbers of invertebrates (40–100) were added to each cage. Immediately following the addition of food, we recorded the number of items consumed within a 5-min period. Tiles were periodically inspected for fish and egg masses. Dead and missing fish were replaced whenever possible with fish subsequently collected from Six Mile Run.
Slimy sculpin reproduction
To determine whether slimy sculpin would spawn in the study streams, we released equal numbers of fish into the two study reaches in 1998, 56 per stream on February 27–28 and 60 per stream on April 18; on April 1, 1999, we released 238 fish per stream into the study reaches. Equal numbers of males and females were released and all females were gravid. We periodically collected and inspected female sculpin at Six Mile Run during the spring season. The onset of spawning was marked by the earliest collection of spent adult females (i.e., without eggs). It was observed in Six Mile Run on April 22 during both 1998 and 1999. Following this determination, we waited approximately 3 weeks before conducting a search for nests. On May 14, 1998, and May 15, 1999, we conducted searches for slimy sculpin and nests in the Stone and Benner run study reaches. To conduct a search, we first installed a blocking seine at the downstream end of the reach. The search then entailed a thorough underwater examination of the substrate using snorkeling gear. We inspected the undersides of all large rocks and logs for sculpin and egg masses. Males found guarding nests were captured using a small aquarium net. Sculpin remaining in the study reaches were collected by electrofishing.
Data reporting and statistics
The mean values for AlTD concentration and pH during the study periods were calculated from grab sample data. A two-sample, one-sided t-test was used to assess statistical differences between the mean AlTD and pH concentrations in Stone and Benner runs, the mean AlTD of upstream and downstream grab samples taken at the aluminum-screened observation cages in 1999, and the mean discharge for each stream during the study periods. We examined whole-body sodium data using a fixed-effects analysis of variance (ANOVA) model. This analysis was limited to data from fish exposed for 20 d. Therefore, differing exposure duration as in 1999 was not analyzed as a factor. Stream (Stone versus Benner) and year (1998 versus 1999) were the only statistically significant factors in the model (P < 0.001). Neither the factor exposure trial nor any of the factor interactions were statistically significant (P > 0.10). Mean whole-body sodium values are reported for groups of slimy sculpin exposed for 20 d. Whole-body sodium concentrations were expressed in terms of dry mass because net water loss may accompany net sodium loss and bias estimates based on wet mass (Gagen 1986). The reporting of movement and feeding data is restricted to observations made for equal numbers of fish in both Stone and Benner run cages unless otherwise noted. We used a Mann–Whitney nonparametric test to assess statistical differences between Stone Run and Benner Run slimy sculpin movement in spring 1998. Movement data from 1999 could not be tested using the Mann–Whitney test because no movement was observed in Stone Run; instead, we used a sign test to test the null hypothesis that the median movement of Benner Run fish in 1999 was equal to zero. Regression analysis was used to determine whether variations in the movement and feeding data could be explained by stream temperature.
Results
Water Quality
Water from Stone Run was significantly (P ≤ 0.01) more acidic and contained more Al than that from Benner Run in spring 1998 (Table 1). The AlTD concentration exceeded 0.2 mg/L in 19 of the 20 Stone Run grab samples taken over the study period. Between early February and June 1998, the Stone Run watershed received 41.7 cm of precipitation and the Benner Run watershed 55.3 cm. During the study, the mean (±SD) discharge in Stone Run was 0.033 ± 0.017 m3·s−1·km−2 and that in Benner Run was 0.057 ± 0.027 m3·s–1·km–2.
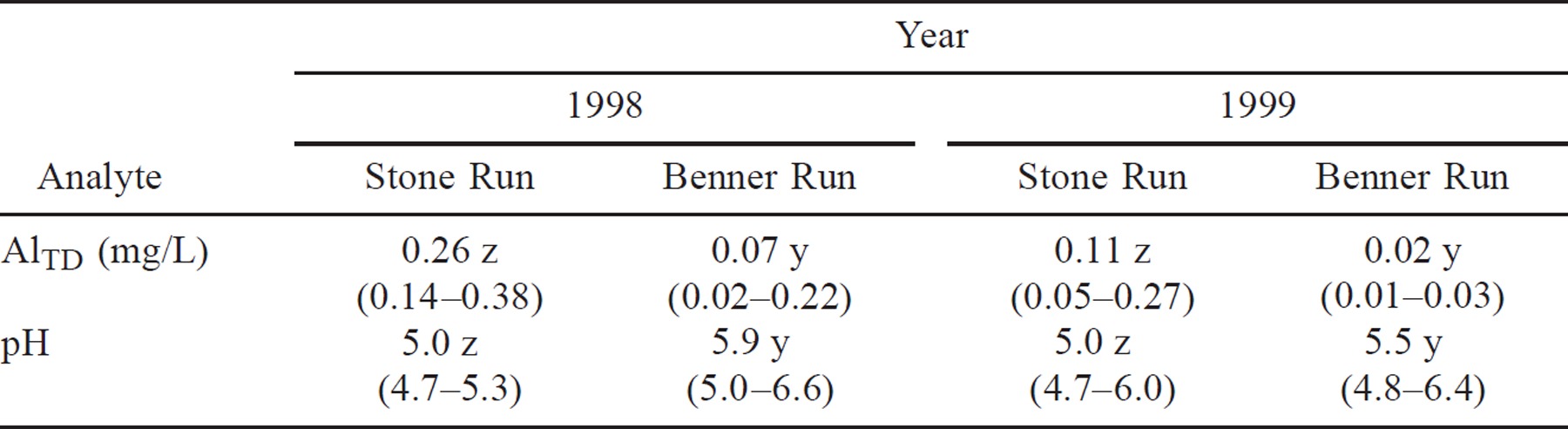
In spring 1999, Stone Run was again significantly (P ≤ 0.01) more acidic and had a higher mean AlTD concentration than Benner Run (Table 1). Precipitation was reduced relative to that of spring 1998: between late February and June 1999, the Stone Run and Benner Run watersheds received 24.4 and 26.7 cm of precipitation, respectively. The mean discharge was significantly (P < 0.001) lower in both Stone Run (0.024 ± 0.017 m3·s–1·km–2) and Benner Run (0.028 ± 0.014 m3·s–1·km–2) than during the spring 1998 study. After April 23, 1999, the Al concentration in Stone Run was below 0.1 mg/L in 7 of the 8 grab samples collected. In addition, pH increased in both streams over the study period.
Slimy Sculpin Responses
Mortality of slimy sculpin was only observed in the Stone Run cages. The highest mortality (25%) occurred during spring 1998 in the second bioassay exposure trial (Table 2). Although we did not conduct 20-d mortality bioassays in 1999, some mortality occurred among the fish being held in Stone Run for sodium analysis (≤12.5%). In both years, all fish held captive in Benner Run survived.
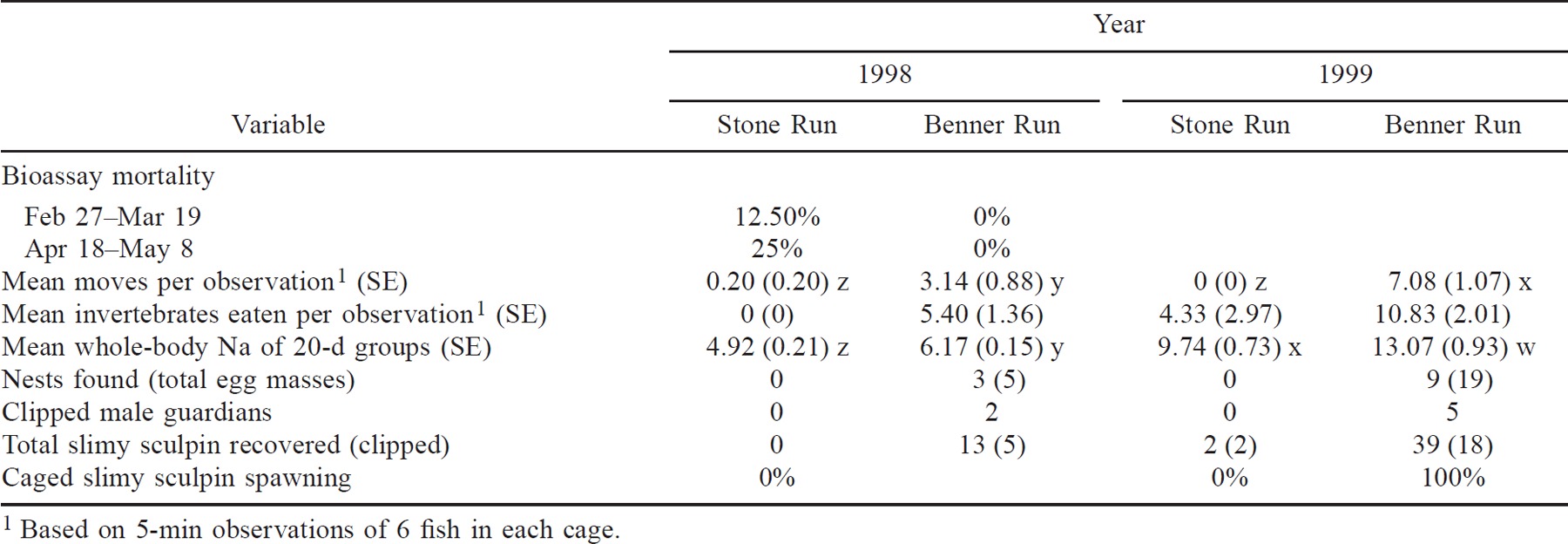
The slimy sculpin exposed for 20 d in Stone Run during spring 1998 and 1999 had significantly lower whole-body sodium than those exposed in Benner Run (two-way ANOVA: P < 0.01; Table 2). Whole-body sodium was lower among fish in both streams in 1998 than in 1999 (Table 2). Rapid sodium loss was not evident in sculpin removed at 5-, 10-, and 15-d intervals in 1999.
Our observations revealed behavioral differences between the slimy sculpin held captive in Stone Run and those held in Benner Run during each study year. The fish in Stone Run rarely moved during the 5-min observations (Table 2). It was also rare to find them outside of the tile shelters upon lifting the cage lids. However, the Stone Run fish responded to tile movement during our inspections by moving to another tile. Despite improved water quality during spring 1999, the movement among Stone Run fish did not increase. In contrast, the slimy sculpin in the Benner Run observation cages moved during the observation periods in both 1998 and 1999 (Table 2). Movement data from both years were plotted against the AlTD concentrations of the grab samples taken at the approximate times of observation (Figure 1). In general, movement was observed when the AlTD concentration was less than 0.05 mg/L.
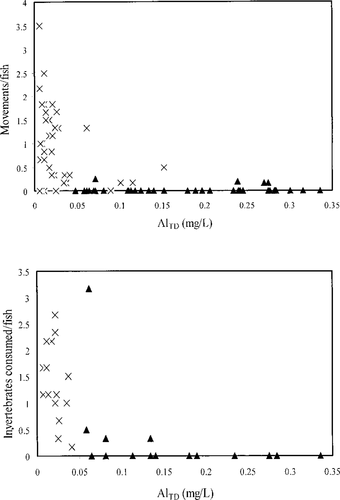
Relationships between total dissolved aluminum (AlTD) and movement of slimy sculpin (upper panel) and number of invertebrates consumed (lower panel) per 5-min observation. Crosses are observations from Benner Run, triangles observations from Stone Run
The slimy sculpin in Stone Run rarely responded to the addition of food (Table 2) and generally did not prey on invertebrates in their cages. Many invertebrates remained in the cages between visits, and their numbers increased as the study progressed. One noteworthy exception to this occurred on April 30, 1999, when fish suddenly responded to the addition of invertebrates. Additional feeding was observed on May 5 and May 10. On May 10 the fish consumed more food (19 invertebrates) during the 5-min observation period than had previously been recorded for the entire spring 1999 study.
When invertebrates were added to the Benner Run cages, the fish consistently responded by darting out from the tiles to consume them (Table 2). During both study years, invertebrates were always missing from the cages by the time of the next visit. As with the movement observations, feeding was observed when AlTD concentrations were relatively low (<0.075 mg/L; Figure 1). Movement and feeding data from Benner Run were not significantly (P ≥ 0.23) correlated with stream temperature.
We obtained evidence that some stocked slimy sculpin spawned in both 1998 and 1999. In 1998, we discovered 3 nests containing a total of 5 egg masses in the Benner Run study reach during our nest survey (Table 2). By contrast, we did not find any nests, eggs, or fish in the Stone Run reach. Spawning did not occur in the observation cage in either stream. However, the cage on Benner Run was damaged twice during high flows, and five fish escaped. During the 1999 nest survey, we found 9 nests containing a total of 19 egg masses in the Benner Run study reach (Table 2); no nests were found in the Stone Run study reach, but two clipped male slimy sculpin were captured. Between May 5 and May 18, all six females in the Benner Run observation cages spawned under the nesting tiles. We observed males defending the space around the tiles that contained eggs as well as fanning the eggs with their pectoral fins. By May 18, only six fish remained in the Stone Run observation cages and no spawning had occurred.
Discussion
Simonin et al. (1993) defined acidic episodes as continuous time periods with stream AlTD concentrations greater than 0.100 mg/L. By this definition, episodic conditions existed in Stone Run for the entire spring 1998 slimy sculpin spawning period. In spring 1999, episodic conditions occurred only during the first half of the spawning period. A few acidic episodes occurred during the spring 1998 spawning period in Benner Run (Kaeser 1999). Acidic episodes did not occur in Benner Run during the spring 1999 spawning period. Others (Swistock et al. 1989; DeWalle et al. 1995) have reported a strong positive correlation between Al concentration and stream flow in headwater streams of the Northern Appalachian Plateau. Less precipitation during the 1999 spawning period resulted in lower stream flows and improved water quality in both Stone and Benner runs.
Gagen et al. (1993) and Baker et al. (1996) used in situ bioassays as a tool for making comparisons of relative toxicity between Stone and Benner runs. Despite the high Al and low pH conditions that existed during spring 1998, mortality of slimy sculpin during in situ bioassays in Stone Run was relatively low. Gagen et al. (1993) reported 30–85% mortality during 20-d exposures in Stone Run with mean AlTD concentrations between 0.15 and 0.25 mg/L and mean pH between 5.0 and 5.2. Van Sickle et al. (1996) predicted a 50% bioassay mortality for slimy sculpin in Pennsylvania streams when the AlIM concentration exceeds 0.200 mg/L for 10 d of a 20-d exposure period. Thus, the mortality that we observed during our bioassays was low relative to that found by earlier studies. However, the observed mortality provided evidence that conditions in Stone Run were stressful and toxic during the spawning period in both years. The survival of all slimy sculpin in Benner Run in both years is evidence that water quality was not lethal during the spawning period.
The lower body sodium of slimy sculpin exposed in Stone Run during both study years was consistent with our hypotheses regarding the effects of water quality between the two streams. In addition, the lower body sodium of fish exposed during spring 1998 was consistent with the between-year differences in water quality at both sites. The whole-body sodium data from fish exposed for 5, 10, or 15 d in 1999 did not follow a pattern of increased sodium loss because water quality was improving. In a study of the whole-body sodium loss of three trout species exposed to acidic conditions, Gagen and Sharpe (1987b) demonstrated that losses as high as 40% can occur. Wood and McDonald (1982) associated the death of rainbow trout Oncorhynchus mykiss exposed to low pH and high Al with a 60–70% loss of whole-body sodium. Although we did not measure the absolute loss of body sodium, we believe that the differences in body sodium between the Stone and Benner run exposure groups demonstrate that slimy sculpin also experience substantial sodium loss when exposed to acidic conditions. The lower whole-body sodium of fish exposed in Stone Run during both years indicates that these fish were under more stress than those exposed in Benner Run throughout the spawning season. Whole-body sodium analysis proved to be a useful indicator of the relative differences in stress associated with sodium loss between streams within a given year. However, the slimy sculpin in Stone Run did not spawn in 1999 despite having higher body sodium levels than those in Benner Run in fish 1998, which did spawn. Therefore, the procedure does not appear to be a sensitive indicator of whether or not spawning will occur.
The habits of confined slimy sculpin facilitated the collection of observational data. Sculpin are sedentary fish (Brown and Downhower 1982) and spend much of their time resting on the stream bottom and under cover (Bailey 1952). In addition, the slimy sculpin is a nocturnal ambush predator and is more active at night (Mousseau and Collins 1987). Therefore, we did not expect much movement or feeding during our visits to the cages. Activity in our observation cages was usually limited to a series of short movements accomplished by fanning the pectoral fins. When the cage lids were lifted to prepare for observation, Benner Run fish were often observed darting under the tiles. At times, a plume of disturbed sediment on the cage bottom was the only evidence of their movement. These fish resumed activity during observation, to the extent that they occasionally left the tiles to move around or feed.
Our observations revealed striking differences in activity level between the slimy sculpin confined in Stone Run and those in Benner Run. Swimming activity is a sensitive indicator of sublethal exposure to acidic conditions for many fishes (DeLonay et al. 1993). Hypoactivity is commonly reported in studies of acidification and fish behavior. The sculpin held in the Stone Run observation cages were consistently hypoactive throughout the spawning season; they remained inactive despite improving water quality in late spring 1999. DeLonay et al. (1993) reported impaired activity and feeding inhibition among golden trout Oncorhynchus aguabonita alevins exposed to relatively low AlTD (0.05 mg/L) and a pH of 5.0 that persisted for more than 2 weeks following a 7-d exposure. Likewise, we observed that slimy sculpin activity at Stone Run remained impaired for weeks following improvement in water quality. We believe that this persistent hypoactivity during the spawning season was the result of low pH and high Al stress and that the willingness of Stone Run slimy sculpin to respond to food in May 1999 is evidence that they were recovering from the stress of acidic exposure. The difference in activity frequency at low Al concentrations and that at high Al concentrations is indicative of a threshold-type behavioral response to toxic exposure (Figure 1).
We recovered few stocked slimy sculpin in Stone and Benner runs in 1998 and 1999. We cannot rule out the possibility that they moved downstream from our study reaches during acidic episodes. Gagen et al. (1994) reported net downstream movement of brook trout during acidic episodes in Stone Run. Although electrofishing seems to be an effective means of capturing slimy sculpin, capture efficiency is probably low because these fish are well camouflaged against the substrate, lack swim bladders, do not float when stunned, and are often under rocks when immobilized. Morgan and Ringler (1992) noted difficulty in depleting slimy sculpin from study reaches by means of electrofishing due to these factors. Bailey (1952), Brown and Downhower (1982), and Hill and Grossman (1987) reported low (<40%) recapture rates of mottled sculpin during the nonspawning season. We speculate that the difficulties associated with capturing these fish are enhanced during the spawning season (when males are guarding nests) and that the low recovery in our study reaches is the result of movement out of the reaches as well as of low slimy sculpin recapture rates in general.
The results of three independent measures of stress (mortality, whole-body sodium content, and behavior) showed that the slimy sculpin in Stone Run exhibited stress and hypoactivity throughout the spawning season. We believe that these factors prevented successful reproduction during the 1998 and 1999 spring seasons. Male slimy sculpin that were introduced into Benner Run spawned and occupied nests in the study reaches during both years. Similar activity was not recorded at Stone Run. The fish in all of the observation cages in Benner Run spawned in spring 1999, while those in Stone Run did not. Based on these results, we conclude that the electrofishing, handling, and transport stress associated with capture and stocking did not affect the ability of the slimy sculpin to spawn but that low pH and high Al concentrations in Stone Run resulted in the lack of observed spawning in this stream.
Recruitment failure is a commonly reported cause of fish population declines associated with acidification (Baker and Christensen 1991). The slimy sculpin has a life span of 4 to 5 years, and individuals reach sexual maturity by the end of 2 years (Koster 1936). Because there are few year-classes capable of reproduction, populations are susceptible to dramatic loss from recruitment failure. Rahel and Magnuson (1983), Matuszek et al. (1990), and Mills et al. (1987, 2000) reported extirpation of mottled and slimy sculpin populations from lakes when pH decreased from 6.4 to 5.6. We observed spawning in Benner Run, where mean pH ranged from 5.5 to 5.9 during our study years. No spawning occurred in Stone Run, where mean pH was 5.0 or less during our study years. Based on our data, we propose an AlTD threshold of 0.05–0.10 mg/L and a pH threshold of 5.5 or more for the successful spawning of slimy sculpin confined in situ.
Our data seem to indicate that acidic episodes produced moderate mortality and severe hypoactivity in adult slimy sculpin during the spawning period in Stone Run. Spawning did not occur among hypoactive fish that survived exposure to acid runoff. Hypoactive behavior among Stone Run fish appeared to be the most sensitive indicator of the probable failure of spawning. Successive years of acidic runoff episodes and concomitant spawning failure could lead to the demise of slimy sculpin populations in streams. Other spring-spawning fishes may be similarly affected by spring acidic runoff episodes.
Acknowledgments
The research described in this paper was funded by the U.S. Environmental Protection Agency's Long-Term Monitoring: Northern Appalachian Plateau Project (agreement CR-8818735-01-0). The paper has not been subjected to the agency's peer and administrative review. Additional support was provided by the School of Forest Resources and the Environmental Resources Research Institute, the Pennsylvania State University. We are grateful to the three anonymous reviewers whose suggestions improved manuscript organization and clarity.




