Biomarkers Indicate Health Problems in Brown Bullheads from the Industrialized Schuylkill River, Philadelphia
Abstract
We used biomarkers to assess the health of two populations of brown bullheads Ameiurus nebulosus one from an urban, industrialized section of the Schuylkill River (SR), Philadelphia, Pennsylvania, and the other from Hopkins Pond (HP), in suburban New Jersey, which was not affected by industry. We evaluated health using histopathology, condition factor (K), hepatosomal index (HSI), prevalence of parasites, health assessment index (HAI), and population age structure. Sediment analysis revealed that both sites contained similar levels of polynuclear aromatic hydrocarbons (PAHs). Brown bullheads from the SR were longer and heavier than brown bullheads from HP and exhibited more external lesions. There were no hepatic lesions in brown bullheads from either site. Condition factor was higher in SR fish, but due to length differences between the two populations, was not a reliable indicator of general health. Hepatosomal index was similar between both populations, although the presence of numerous cestodes in the visceral organs, including the liver, of HP brown bullheads confounded the interpretation of the HSI. The incidence of parasitism was significantly greater in HP fish than in SR fish. The HAI was significantly greater in SR brown bullheads, indicating more organ abnormalities in SR fish than in HP fish, including more abnormalities of the fins, barbels, and mouth. Population age structure was older for HP brown bullheads than for SR fish, indicating a shorter lifetime for SR brown bullheads. Although PAH levels were similar between the two sites, SR brown bullheads were less healthy than HP brown bullheads, as indicated by several biomarkers. Histopathology, health assessment index, and population age structure were the most informative biomarkers.
Rivers that flow through large urban or industrial centers are frequently heavily polluted by industrial and municipal wastes, and aquatic organisms exposed to these wastes may suffer detrimental consequences. Even those species that can tolerate changes in habitat quality are often affected by chronic exposure to toxicants and as a result show sublethal health effects.
McCarthy and Shugart (1990) suggested that biomarkers can be sensitive indicators in demonstrating that toxicants have entered organisms, have been distributed within tissues, and have elicited a toxicological effect in target organs. Biomarkers can include histopathological changes in tissues (Hinton and Laurén 1990), such as induction of tissue lesions (Meyers and Hendricks 1982) and carcinogenic and neoplastic effects (Black 1983; Baumann et al. 1987; Baumann et al. 1991), induction of heat shock proteins (Sanders 1993), decreased reproductive capability or abnormal development (Weis and Weis 1987, 1989), mortality during sensitive developmental stages (Spotila and Paladino 1979), and anatomical deformities (Meyers and Hendricks 1982).
The susceptibility of bottom-feeding fish to neoplasms has made this guild a useful indicator of carcinogenic toxicants in sediments (Dawe et al. 1964). In particular, the brown bullhead Ameiurus nebulosus has been widely used as a model to study the effects of toxicants on fish (Black 1983) and as a bioindicator of sediment contamination. Brown et al. (1973) reported a higher tumor frequency in brown bullheads from the Fox River in Illinois than in those from unpolluted Canadian waters. Black et al. (1980) and Black (1983) reported elevated tumor frequencies in brown bullheads from the Buffalo River and demonstrated a causal relationship between high levels of polynuclear aromatic hydrocarbons (PAHs) in sediments from the Buffalo River and neoplasms in brown bullheads by initiating neoplasms after applying PAH-containing sediment extract to the epidermis of brown bullheads. Baumann et al. (1987) found high tumor frequencies in brown bullheads from the Black River in Ohio that were correlated with high levels of PAHs in sediments from the Black River and with levels of PAHs in fish tissues.
Such sublethal effects have been evident since the beginning of the 20th century. McFarland (1901) was among the first to describe an epithelioma of the mouth and skin in a catfish species (white catfish Ameiurus catus) in the Philadelphia area. Lucké and Schlumberger (1941) reported 166 catfish with tumors on the lips or dental plates, mostly from the Schuylkill and Delaware rivers in the Philadelphia area. Since that time, considerable effort has been expended to reduce the input of pollutants into these rivers and to remove contaminated sediments. Yet it is not known what effect this effort has had on improving the health of local fish populations (Gastrich 1992).
Urban ecosystems are a dominant feature of the eastern United States, and most fisherman live in cities and their suburbs. Many of those fisherman catch and eat fish from local rivers, lakes, creeks, and ponds. A logical assumption would be that fish in an industrialized site would live in a more contaminated environment and be in poorer health than fish in a nonindustrialized site. The objective of this study was to assess the health of two populations of brown bullheads from the largely urbanized Philadelphia region. One population was from the urban, industrialized section of the Schuylkill River in Philadelphia, and the other population was from suburban Hopkins Pond in nearby Haddonfield, Camden County, New Jersey. We evaluated the health of individual fish using several biomarkers, including lesion prevalence, condition factor, hepatosomal index, health assessment index, and parasite load.
Study Sites
The Schuylkill River (SR), in southeastern Pennsylvania, receives urban outflow through much of its course. The lower reaches of the SR are characterized by heavy industrial usage, especially by petrochemical plants. This portion is channelized and tidally influenced by the Delaware River. The Delaware River is also characterized by heavy industrial usage in this area, including petrochemical plants and the Philadelphia Naval Shipyard. We sampled brown bullheads from the lower SR, between the Fairmount Dam in Philadelphia and the mouth of the Schuylkill River.
Hopkins Pond (HP) is a 2.4-ha eutrophic pond with a mean depth of 2 m. The pond is in a woodland surrounded by a residential area and is used for recreation activities, primarily fishing. Boats with gasoline-powered engines are not permitted. The immediate area around the pond is a park and is dominated by an eastern hardwood forest assemblage (including yellow poplar Liriodendron tulipifera, beech Fagus grandifolia, and oak Quercus spp.). The rest of the watershed is residential and is not subject to industrial outflow, although the pond receives some runoff from nearby roads, lawns, and driveways.
Methods
Collecting methods
We collected 42 brown bullheads by gill net, trap net, and hook and line between August 1991 and August 1993 (SR, N = 23; HP, N = 19). Fish were placed on ice from the time of capture until they were brought to the laboratory. We measured total length (±0.5 mm) and mass(±0.5 g) and recorded external pathological abnormalities. We examined all fish internally for gross lesions. We collected and preserved parasites, following procedures outlined by Cable (1977), and sexed fish by gonadal examination. We removed the liver, measured its mass (±0.5 g), and placed a sample in 10% formalin for histopathological examination. We excised gross external abnormalities and preserved them in 10% formalin for histopathological examination.
We removed the pectoral spine and placed it in an envelope for drying. The spine was embedded in a cork and polished with 3/0-grade fine sandpaper, and age rings were read under a dissecting microscope. Fish whose spines were not collected were assigned ages by age–length regression analysis. Age was estimated from the pectoral spine for 14 fish from SR and 11 fish from HP. Age was estimated by age–length regression for 9 fish from SR (age = 0.188 × length − 2.596, r2 = 0.356) and 8 fish from HP (age = 0.317 × length − 2.874, r2 = 0.493).
We sectioned tissues to a 5-μm thickness and stained sections with hematoxylin and eosin. Three sections were randomly taken per liver.
Indices of health
The condition factor (K) for each individual was determined by the following formula (Carlander 1969):
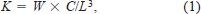
where W = mass (g), L = length (mm), and C = 102 (factor to bring the value of K near unity). The hepatosomal index (HSI) was the ratio of liver mass to total mass.
The health assessment index (HAI) is a method of quantifying anatomical and physiological stresses on organisms that allows for statistical comparisons between different populations (Adams et al. 1993). Goede and Barton (1990) introduced a necropsy-based system of health assessment designed as a method of providing a database for detecting trends in the health and condition of fish populations. The system was not meant to serve as a diagnostic tool but instead to serve as an overall measure of health. This system had the advantage of being a fast, easy, and inexpensive method that could provide rapid, analyzable results in a field situation. The necropsy method relied on several important assumptions (Goede and Barton 1990): (1) tissue and organ function change to maintain homeostasis in fish under stress; (2) when faced with a continuing stress, the change in tissue or organ function results in gross changes in structure; (3) there is a high probability that the fish is normal if all organs and tissues appear normal; and (4) the deviation from normal or a control in the appearance of tissues or organs is in response to an environmental stressor. Adams et al. (1993) modified the field necropsy method outlined by Goede and Barton (1990) to provide a method of quantifying the results to allow statistical comparisons between data sets and to include variables that categorize the extent of damage to the tissue or organ. The health assessment index can thus be compared statistically within populations over time or between populations by parametric or nonparametric means. The HAI we used was a variant of the method developed by Adams et al. (1993). Variables used in this study (Table 1) included a broad range of organs and tissues that would elicit a response in the face of an environmental stressor.
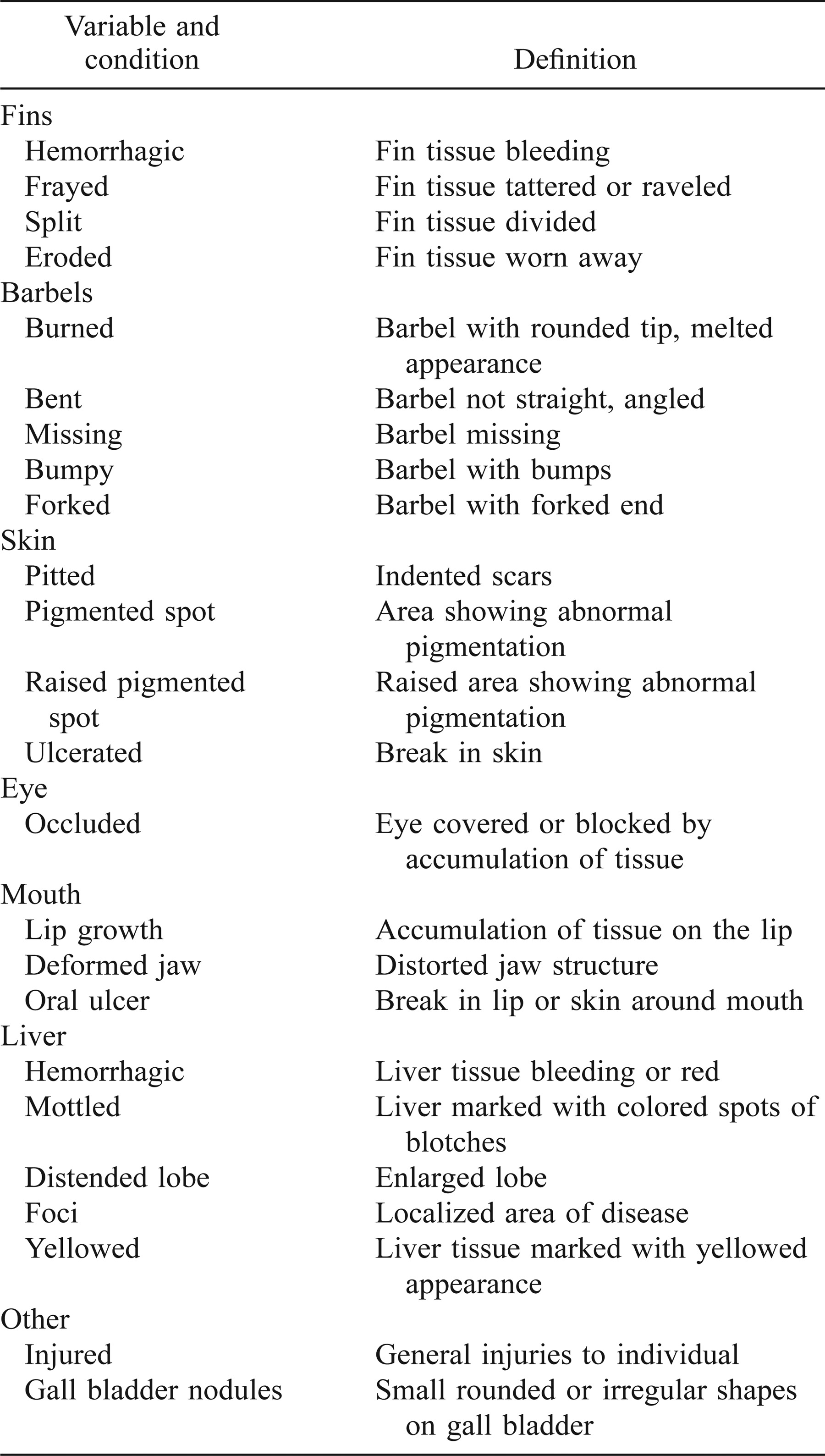
After we conducted a necropsy on an individual, we assigned a numerical value to the variables. If the condition was present, a value of 10 was assigned to that variable. If the condition was not present, a value of 0 was assigned to the variable. The assigned values were summed for each individual to give a HAI for each individual. The HAI for the sample population was calculated by summing the individual HAIs and dividing by the number of fish in the sample population. Hence a low HAI value indicates good health, and a high HAI value indicates poor health.
Sediment analysis
We collected three sediment samples from each site in 1993 with an Eckman bottom dredge and placed them into clean, acid-washed sample bottles with Teflon-lined caps. Samples were placed on ice until they were returned to the laboratory, at which time they were placed in a freezer at −20°C until analysis according to the criteria set forth in EPA Method 8310 for polynuclear aromatic hydrocarbon target compounds (USEPA 1986). Results are reported as micrograms per kilogram dry mass.
Statistical analysis
Length and mass values were compared with one-way analysis of variance (ANOVA). Gender ratios were compared with a chi square test. Index values were compared with Mann–Whitney U-tests. Anatomical abnormalities were compared with a 2 × 2 G-test of independence using the Williams correction factor. Linear regression was used to compare the relationship between population characteristics and biomarker values. Levels of significance were determined at alpha = 0.05.
Results
Chemical Contaminants
Sediment concentrations of PAHs were similar in the Schuylkill River and Hopkins Pond, although levels of several carcinogenic PAHs were higher in HP (Table 2). Of the 16 polyaromatic hydrocarbons analyzed, 10 were detected in HP, and 13 were detected in the SR (Table 2). Six of those compounds (benzo[a]anthracene, benzofluoranthrene, benzo[k]fluoranthrene, benzo[a]pyrene, dibenzo[a,h]anthracene, and indeno[1,2,3-cd]pyrene) were listed as carcinogenic substances by the International Agency for Research on Cancer (IARC 1983). Compounds indicated as undetected could have been present below the minimum detectable level for that analysis. Differences in the minimum detection limit of each sample were due to differences in moisture content of each sample.
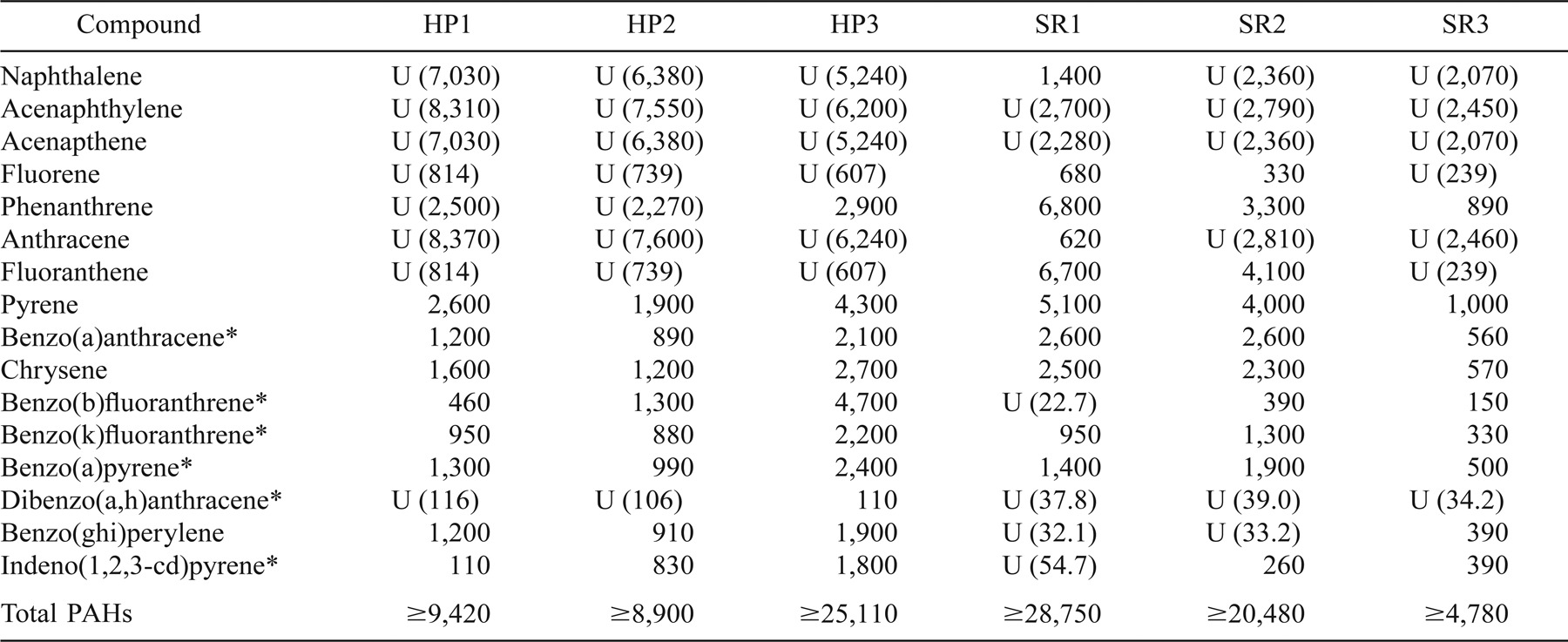
Some compounds showed considerable variation (one order of magnitude) in the amount detected within a site. Total mean PAH levels in sediments were higher for the SR (21.8 mg/kg) than for HP (16.8 mg/kg). However, some compounds were present at greater levels in HP than in the SR, and HP contained greater amounts of carcinogenic PAHs in the sediment than did the SR.
Population Characteristics
Mean age for SR brown bullheads was 2.8 years (SD = 1.24, range = 1–6, N = 23), and mean age for HP brown bullheads was 3.6 years (SD = 1.71, range = 1–7, N = 19). Brown bullheads from HP had an older age structure than fish from SR (Figure 1). Schuylkill River brown bullheads were longer (P < 0.0001) and heavier (P < 0.0001) than Hopkins Pond fish (Table 3). The number of females outnumbered the number of males at both sites. The SR had a 13:8 female: male ratio, while HP had a 15:3 ratio. These gender ratios were not significantly different from each other (chi square = 2.198, P = 0.138). The sex ratio for SR was not significantly different than a 50:50 sex ratio (chi square = 1.19, P = 0.275), but the sex ratio for HP was significantly different than a 50:50 sex ratio (chi square = 8, P = 0.005).
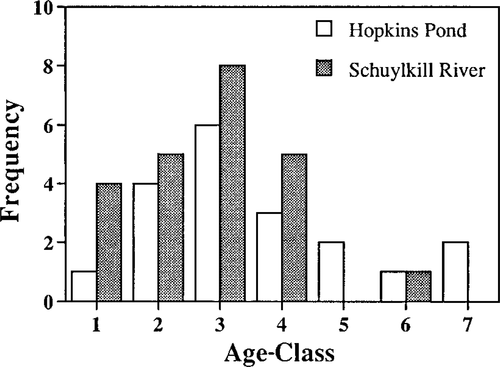
Population age structure for brown bullheads from Hopkins Pond, Camden County, New Jersey, and the Schuylkill River, Philadelphia, Pennsylvania
Parasitic Infection
All brown bullheads taken from HP were infected with Proteocephalus species (Class Cestoda, Order Proteocephalidea, Family Proteocephalidae). These cestodes occurred throughout the viscera, including in the peritoneal wall, in the pericardial cavity, and were embedded in many of the visceral organs, including the liver and gonads. In many individuals, infection was quite severe. One individual had 137 cestodes in the peritoneum, 158 cestodes in one-half the liver, and 19 in the kidneys. Another individual had 28 cestodes in the ovaries.
One brown bullhead from the SR showed obvious parasitic infection, a digenetic trematode in the bile duct (Family Allocreadiidae).
Histopathology
There were no neoplasms in livers from brown bullheads from either the SR or HP. However, 100% of the livers from HP brown bullheads were infected with parasites (see above) and displayed granulomas around areas of parasitic invasion. No livers from SR brown bullheads had parasitic infections.
The frequency of epidermal lesions was higher in SR brown bullheads than in HP fish (21.7% versus 0%, respectively). Lesions were located either on the lip (2 papillomas) or skin (one papilloma, one malignant neoplasm, and one hyperplasia). Lesions were generally found in older fish, although one young fish (age 1) exhibited a skin hyperplasia (Figure 2).
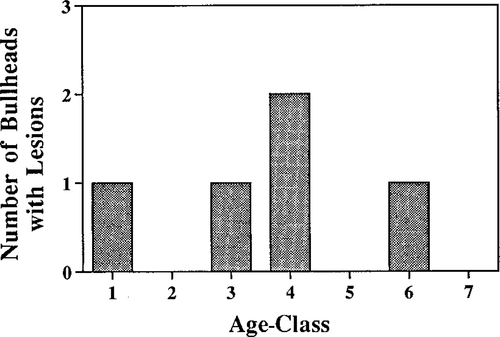
Epidermal lesion frequency by age-class in brown bullheads from the Schuylkill River, Philadelphia, Pennsylvania
Condition Factor
Coefficient of condition, K, was significantly higher for SR brown bullheads than for HP brown bullheads (P < 0.05; Table 3). Coefficient of condition was not significantly different between sexes overall. There were no significant differences in K between sexes within sites or in K within sexes between sites. There was no statistically significant relationship between age and K at SR (R2 = 0.003, P = 0.8037), but there was a statistically significant positive relationship between age and K at HP (R2 = 0.285, P = 0.0185).
Hepatosomal Index
The hepatosomal index was similar in SR brown bullheads and HP brown bullheads (Table 3). The HSI was not significantly different between sexes overall, between sex at either site, nor between sites within each sex. There was no statistically significant relationship between HSI and age at SR (R2 = 0.007, P = 0.6974), but there was a statistically significant positive relationship between age and HSI at HP (R2 = 0.229, P = 0.0380).
Health Assessment Index and Anatomical Deformities
Mean health assessment index (HAI) was significantly higher for SR brown bullheads (range = 0–70) than for HP brown bullheads (range = 0–20, P = 0.0099; Table 3). There was no significant relationship between HAI and age for either SR (R2 = 0.118, P = 0.1091) or HP (R2 = 0.004, P = 0.8047). Overall, SR brown bullheads exhibited a higher frequency of abnormal conditions than did HP brown bullheads (Figure 3). Brown bullheads from the SR had significantly more fin abnormalities (P = 0.0065), barbel abnormalities (P = 0.0005), and mouth abnormalities (P = 0.0324) than HP brown bullheads. Brown bullheads from the SR also had more skin abnormalities (P = 0.0714) than HP brown bullheads, although the difference was not significant at the α = 0.05 level. There were no significant differences between SR brown bullheads and HP brown bullheads for occluded eyes (P = 0.3675) or gross liver abnormalities (P = 0.8085). Three SR individuals showed abnormalities in three or more organs or tissues. Fin abnormalities took the form of hemorrhagic, frayed, or split. Abnormal barbels were bent, missing, bumpy, or forked, and skin deformities took the form of raised pigmented spots. These deformities were seen in only one HP brown bullhead that showed a bent barbel.

Frequency of abnormal conditions found in target tissues and organs in brown bullheads from Hopkins Pond, Camden County, New Jersey, and the Schuylkill River, Philadelphia, Pennsylvania
Discussion
The PAH contamination in Hopkins Pond and the Schuylkill River was surprisingly similar. Suburban HP, which received no industrial effluent, contained similar amounts of PAHs as the industrialized SR. Sources of contamination in the SR included several petrochemical plants along its banks, as well as various abandoned industrial sites. There were no obvious routes for contamination into Hopkins Pond except for runoff from nearby streets. No data were available for other contaminant concentrations from the lower reaches of the SR or from HP.
Incidence of parasitic infection was significantly greater in brown bullheads from HP than in brown bullheads from the SR. The relationship between pollution and parasite infection is unclear. Adult winter flounder Pleuronectes americanus exposed to oil-contaminated sediment for 6 months exhibited a higher myxozoan infection rate (80% of 20) than controls (28% of 21; Khan and Thulin 1991). Andrews et al. (1966) found an increased susceptibility to trematode infection in bluegills Lepomis macrochirus when they were exposed to low concentrations of heptachlor; the lowest prevalence of infection was in fish exposed to the highest concentrations of heptachlor. The authors suggested that lower levels of heptachlor impaired the fish's immune system, making them more vulnerable to parasitic infection, while higher levels of heptachlor caused a reduction in the number of intermediate hosts required by the parasite.
Several other studies have noted decreases in the incidence of parasitic infection in fish exposed to contaminants. Kussat (1969) found that white suckers Catostomus commersoni and longnose suckers C. catostomus taken from a region upstream from industrial and domestic discharge were more heavily infected with acanthocephalans than white and longnose suckers taken downstream of the discharge. The intermediate hosts of these parasites, believed to be ostracods and amphipods, were not present downstream of the discharge, thus interrupting the life cycle of these parasites in that area. Overstreet and Howse (1977) found that Atlantic croakers Micropogonias undulatus taken from polluted Gulf of Mexico waters had significantly fewer infections by an acanthocephalan than fish taken from cleaner waters. Reduced availability of an intermediate host, an amphipod, influenced the prevalence of parasites in the fish.
Brown bullheads had a higher parasitic lesion rate from the cleaner Black Creek site (98%) than from the contaminated Love Canal–102nd Street dump site (25%) along the Niagara River (Hickey et al. 1990). Parasites requiring intermediate hosts had a 7% incidence in the Love Canal–102nd Street dump site but a 49% incidence in Black Creek. Parasites with a direct life cycle (protozoans and monogenetic trematodes) had a 18% incidence in the Love Canal–102nd Street dump site and a 49% incidence in Black Creek. Fabacher and Baumann (1985) report multiple liver parasites, identified as Proteocephalus sp., in 6 of 27 brown bullheads collected from Buckeye Lake, a reservoir that receives no industrial effluent but does receive domestic effluents and small amounts of oil and grease from recreational boating. In the present study, all HP brown bullheads contained parasites identified as Proteocephalus sp.
Cestodes of the genus Proteocephalus found in the HP brown bullheads have two life cycle stages. Adults are found in the intestine and pyloric ceca of fish, while plerocercoids are found in the visceral organs and musculature. First stage larvae, procercoids, develop in hemocoel of crustacea. Hoffman (1967) notes that the adult (intestinal) form does not usually generate detectable tissue damage. However, numerous plerocercoids, especially P. ambloplitis in black bass, may generate damaging tissue adhesions, which may in turn cause impaired metabolism, decreased egg production, and sexual sterility. In HP brown bullheads, in some cases hundreds of plerocercoids were present in organs and musculature. This parasite load may be responsible for stunting the growth rate in young HP brown bullheads, which would lead to the observed difference in size-classes between HP brown bullheads and SR brown bullheads.
The differences in parasite incidence between polluted and nonpolluted sites presented in this and other studies suggest that fish parasites requiring intermediate hosts may be restricted from contaminated waters due to the sensitivity of the intermediate hosts to contamination. The incidence of parasite infection may be a useful indicator of contamination stress in an aquatic ecosystem if the distinction is made between parasites that require intermediate hosts (and what those intermediate hosts are) and those that do not.
We found epidermal lesions in SR brown bullheads (21.7%) but not in HP brown bullheads. The highest frequencies of lip and body papillomas reported in previous studies were from brown bullheads in Hamilton Harbor (30% and 29%, respectively; Smith et al. 1989). Lip and body papillomas in the Black River occurred in less than 1% of age-2 brown bullheads, while frequencies in age-4 brown bullheads were 32% for lip papillomas and 18% for skin papillomas. Brown bullheads from the Niagara River also exhibited a high lesion frequency; in 1985, 65.1% exhibited external lesions and 16.6% exhibited liver neoplasms (J. T. Hickey, U.S. Fish and Wildlife Service, and J. R. Spotila, Drexel University, unpublished data). In 1986, 76.8% of the brown bullheads from the Buffalo River and 65.2% of those from the Love Canal–102nd Street dump site exhibited external deformities. Hickey et al. (1990) reported that 40 of 101 brown bullheads collected from the Love Canal–102nd Street dump site in New York had skin lesions and that four of those had papillomas. Black et al. (1980) reported that two of nine brown bullheads (22%) collected from the Buffalo River displayed dermal tumors, one of which was highly malignant. Sediments in the Cuyahoga River were less contaminated than that in the Black River, and brown bullheads exhibited fewer liver neoplasms (9.4%) and potentially preneoplastic lesions (11.8%) but similar numbers of external neoplasms (8.9%; Baumann et al. 1991). Sediments from the Menominee and Fox rivers contained even lower levels of PAHs, and brown bullheads had no liver neoplasms but similar numbers of epidermal neoplasms (7.9%; Baumann et al. 1991).
The suspected causative agents for these neoplasms were contaminated sediments. Black (1983) painted sites on the integument of brown bullheads with Buffalo River sediment containing PAHs and established a cause and effect relationship between PAHs and neoplasms. Varanasi et al. (1986) demonstrated the metabolism of benzo(a)pyrene and the binding of its metabolites to hepatic DNA in two marine flatfish, establishing a possible route of hepatocarcinogenesis in fish exposed to PAHs. As levels of PAHs were similar between the SR and HP, the increased frequency of lesions found in SR brown bullheads cannot be attributed to PAH contaminants. It is possible that another contaminant (e.g., PCBs or metals) was responsible for the higher frequency of lesions in SR brown bullheads or that some other contaminant in the SR was acting synergistically with PAHs. Little is known about the interactions of xenobiotics and their implications for health, although Stein et al. (1987) found an increase in the accumulation of benzo(a)pyrene in English sole Pleuronectes vetulus in the presence of PCBs. The etiology of lesions in SR fish deserves further attention.
Population Characteristics and Indices of Health
Brown bullheads from the SR were larger than brown bullheads from HP in similar age-classes. The growth curves (age versus length) for both populations were similar, although the growth rates did not include age-0 brown bullheads and only few age-1 brown bullheads. There may be differences in initial growth rates due to differences in habitat or temperature regimes between the SR and HP. The SR is a warmwater river which rarely undergoes ice formation during the winter; HP typically experiences colder winter temperatures and often forms a thick ice cover. These differences in habitat may result in a shorter growth period for HP brown bullheads.
Older fish (5–7 years) were more prevalent in HP (N = 5) than in the SR (N = 1; Figure 3). Baumann et al. (1990) noted a similar difference in brown bullhead population age structures between Black River and the Old Woman Creek reference site and hypothesized that differential mortality was due to the high incidence of age-selective hepatic cancer in Black River fish. Other studies have also found that brown bullheads from less contaminated sites had an older age structure than brown bullheads from more contaminated sites (Hickey et al. 1990; Baumann et al. 1991). However, because PAH levels were similar between the SR and HP, differences in age structure between the sites were not due to PAH contaminants. Further studies of brown bullhead age structure at both sites, including studies with larger sample sizes, are needed to determine why these differences exist.
Hepatosomal index values were similar for HP brown bullheads and SR brown bullheads. However, the presence of Proteocephalus sp. in the livers of all HP brown bullheads make the data difficult to interpret. It is not known whether the cestodes displaced a portion of the liver mass or whether their presence actually increased the liver mass. Hepatosomal index in fish increases after exposure to certain types of contaminants (Poels et al. 1980). This increase may be due to hepatocellular hypertrophy in concert with the induction of the mixed-function-oxidase (MFO) detoxifying system in the liver (Poels et al. 1980), although the relationship between HSI and MFO induction is not always clear (Fabacher and Baumann 1985).
Hepatosomal index values from this and other studies support the positive correlation between HSI and the degree of aromatic hydrocarbon contamination. In previous studies, brown bullheads from more contaminated sites had higher HSIs than fish from less contaminated sites (Fabacher and Baumann 1985; Baumann et al. 1991). Brown bullheads from the contaminated Black River had a mean HSI of 0.0393, and brown bullheads from the less contaminated Buckeye Lake had a mean HSI of 0.0176 (Fabacher and Baumann 1985). The HSIs in this study for HP and SR (0.0246 and 0.0254, respectively) fell between those from Black River and Buckeye Lake. The HSIs from the present study were very similar to those in fish from Lake Trempealeau, (0.0239; Fabacher and Baumann 1985), Munuscong Lake (0.0255), and the Cuyahoga River, (0.0257; Baumann et al. 1991).
Coefficient of condition (K) was higher for SR brown bullheads (1.457) than for HP brown bullheads (1.343). Due to length-associated differences between the two populations, however, comparison of K was uninformative. The length–mass relationship for brown bullheads from the SR was described by log10(W) = log10(−1.677) + 2.883 × log10(L), where L = length and W = mass; for HP brown bullheads, the equation was log10(W) = log10(−2.463) + 3.448 × log10(L). In both of these cases, b, the slope of the line, was not equal to 3.0 and thus violated the assumption of isometric growth (Cone 1989). Hopkins Pond brown bullheads showed a significant positive relationship between K and L, indicating that K increased with fish length (P = 0.0016). There was no significant relationship between K and fish length for SR brown bullheads (P = 0.6699).
Brown bullheads from the SR had a higher health assessment index (HAI) than brown bullheads from HP, indicating more tissue and organ abnormalities in SR brown bullheads. There are few reports detailing types of barbel deformities in brown bullheads (Ovais 1974; Datta and Ghosh 1975; Hickey et al. 1990). Effects of barbel deformities on brown bullheads are unknown. However, because catfish use barbels as a chemosensory device for feeding, deformities in these organs may reduce food intake. Fin abnormalities in benthic fish, especially fin erosion, have been associated with contamination (Hickey et al. 1990). Sindermann (1982) reported that fin and skin abnormalities were commonly associated with areas of high petroleum contamination. Although no direct cause and effect relationship has been shown, Mearns and Sherwood (1974) suggested that direct contact of tissues with contaminated sediment initiated fin erosion.
Conclusions
Brown bullheads were a good bioindicator of industrial contamination. The absence of liver lesions in brown bullheads from the SR indicated that the SR was not as contaminated as some industrialized rivers, such as the Black River or the Niagara River. The lower incidence of lesions than in the past (Lucké and Schlumberger 1941) indicated that the lower Schuylkill River was less contaminated in 1991–1993 than in 1941. However, this study indicated that contamination should still be of concern to fishermen because brown bullheads in SR were less healthy than those from Hopkins Pond.
Brown bullheads taken from the industrialized SR had higher lesion frequency, higher HAI, and younger population age structure, which indicated lower overall health. The HSI values for SR and HP brown bullheads were between the values for other brown bullhead populations from moderately industrialized areas and fit into a continuum of generally increasing HSI values in fish from more contaminated areas. The high cestode parasite infection rate in HP brown bullheads indicated a healthier aquatic system for intermediate hosts of the parasites in HP than the SR. Thus, HP was less affected by the urban environment than the SR. However, PAH levels were only slightly higher in SR than in HP, so PAHs were not singularly responsible for differences in health status between the two studied populations.
Although substantial differences existed in the physical attributes of the two study sites (e.g., lentic versus lotic, temperature regimes, food base), health comparisons of the brown bullhead populations were still useful. Whereas growth rates may be expected to vary with habitat, frequency of lesions and characters examined with the HAI would not be expected to be dependent on habitat type. Histopathology, HAI, and population age structure were the most informative biomarkers, and we recommend the use of HAI as a general method for comparing the health of different fish populations. Size alone was not useful because larger bodies of water generally contain larger fish.
Acknowledgments
We thank J. Black for providing helpful comments on the initial design of the study, S. Kilham, A. List, Jr., and M. O'Connor for critically reviewing earlier drafts of the manuscript, and W. Baroletti, S. Kemp, and C. Salice for their help in the field. D. Arnold provided important help in the design of this study and the initiation of the field research. We thank Roy Weston, Inc., and especially Josie Edwards, who donated time and services for the sediment analysis of contaminants. The New Bolton Center, School of Veterinary Medicine, University of Pennsylvania, donated time and services in mounting the tissues for histopathological analysis. This study was supported by the Betz Chair Endowment of Drexel University.




