Migration Timing of Atlantic Salmon Smolts Relative to Environmental and Physiological Factors
Present address: Federal Energy Regulatory Commission, Office of Hydropower Licensing, 888 First Street, Northeast, Washington, D.C. 20426, USA.
The Unit is jointly supported by the U.S. Geological Survey, Biological Resources Division, the Vermont Department of Fish and Wildlife, the University of Vermont, and the Wildlife Management Institute.
Abstract
We determined the migration timing of fry-stocked smolts of Atlantic salmon Salmo salar relative to environmental and physiological factors, by using net weirs and counting fences in three tributaries of the West River, Vermont. Smolt migration began in late April and early May when water temperature was 5°C, peak movements occurred in early and mid-May at temperatures exceeding 8°C, and migration was complete by early June. Within this seasonal window, significant differences in migration timing and gill Na+,K+-ATPase activity occurred among tributaries. In both years of the study, smolts tended to migrate earlier and exhibit greater gill Na+,K+-ATPase activity in the warmest tributary than in the coolest tributary. Smolt migration timing differed most among tributaries in mid-May when (1) water temperatures were more than 8°C, (2) smolts peaked in gill Na+,K+-ATPase activity, and (3) discharge peaked, stimulating smolt migration. Smolts captured after the migratory period had lower gill Na+,K+-ATPase activity than migrating smolts. Relating smolt physiology to migration was crucial for explaining complex interactions among water temperature, discharge, and smolt behavior during both the onset and cessation of migratory activity. Because the period between onset of migration and loss of smolt physiological characteristics may be brief, delays in downstream passage that may occur at dams must be minimized to maximize the successful recruitment of smolts to the marine environment.
Atlantic salmon Salmo salar exhibit a highly developed smolt stage (McCormick 1994) typified by physiological events that prepare juvenile salmon for seawater entry and behavioral changes that facilitate their movement to the ocean (Saunders and Henderson 1978; McCormick and Saunders 1987; Hoar 1988). Physiological and behavioral processes of smolting are regulated by environmental changes and mediated and integrated by the pituitary–endocrine axis (Hoar 1988). Because smolting is seasonally labile, optimal periods for seawater entry and survival may be brief (Bilton et al. 1982). Therefore, determining the relation between smolt migration and physiology contributes to an understanding of what controls migratory success.
Photoperiod is the most important factor entraining juveniles to undergo the par–smolt transformation and thus be “primed” to migrate (McCormick et al. 1987; Saunders and Harmon 1990). Within the context of photoperiod, water temperature and discharge have concurrent effects on smolt migration and physiology. Increasing water temperature may initiate smolt migration (Youngson et al. 1983; Jonsson and Ruud-Hansen 1985) and either accelerate development (Solbakken et al. 1994) or regression (Duston et al. 1991; McCormick et al. 1997, in press) of smolt physiological characteristics. Peaks in discharge may affect thyroxine levels (Youngson et al. 1986, 1989) and stimulate smolts to migrate (Hesthagen and Garnås 1986; Dempson and Stansbury 1991). Given their interdependence, smolt migration and physiology must be studied simultaneously to understand how water temperature and discharge influence migration timing.
Physiological aspects of smolt migration have been addressed primarily on hatchery-reared Oncorhynchus smolts. Because smolt physiological development and subsequent migration are affected by presmolt development (Skilbrei 1991), hatchery-reared smolts may not be suitable surrogates for wild-reared individuals (McCormick and Björnsson 1994; Shrimpton et al. 1994). The relation between gill Na+,K+-ATPase activity (a common indicator of smolt status) and migration timing, migration rate, and timing of seawater entry is variable for Oncorhynchus smolts (Ewing et al. 1980, 1985; Rodgers et al. 1987). Because Oncorhynchus spp. demonstrate greater early life history plasticity, existing models of smolt physiology and migration may not be directly applicable to Atlantic salmon (McCormick 1994).
For Atlantic salmon, information on smolt physiology and migration is limited to comparisons of migrants and nonmigrants (Virtanen and Sovio 1985; Cunjak et al. 1990; McCormick and Björnsson 1994), and few studies have included physiology when quantifying how environmental factors influence smolt migration (Cunjak et al. 1990). Herein, we determine interactions among water temperature, discharge, smolt physiological development, and migration timing in three Vermont tributaries of the Connecticut River, USA. Because smolt age and size are related to migration timing (Jonsson et al. 1990) and gill Na+,K+-ATPase activity (Johnston and Saunders 1981), they too were quantified. We addressed the following questions: (1) How does timing of smolt migration and physiological development differ among tributaries? (2) Are these differences related to water temperature and discharge? (3) How do seasonal patterns in the development and loss of smolt characteristics relate to migration? By integrating smolt physiology with migration, we were able to explain complex interactions among water temperature, discharge, and smolt behavior during both onset and cessation of migratory activity.
Methods
Study area
We collected migrating Atlantic salmon smolts from three tributaries of the West River, Vermont: Rock River (155 km2), Wardsboro Branch (96 km2), and Utley Brook (65 km2; Gries et al. 1997). The West River flows from southern Vermont into the Connecticut River. These tributaries were third- to fourth-order systems with several subtributaries that contributed to habitat heterogeneity. Watersheds contained scattered residential development but were primarily forested with mixed deciduous and coniferous forests; logging and agricultural activities were relatively minor. Riffle habitats and coarse substrates were dominant in each tributary. Tributary gradients were near or greater than 1.0%, typical of streams routinely stocked with salmon fry in the West River basin (Rock River = 1.7%; Wardsboro Branch = 1.8%, and Utley Brook = 0.9%). Because the tributaries differed latitudinally by less than 0.5°N, we assumed photoperiod influences on migration were uniform. Elevations of tributary confluences with the West River ranged from 108 m (Rock River) to 340 m (Utley Brook). Smolt density in the three tributaries has ranged from 0.38 to 1.50 smolts/100 m2 for age-2 smolts and from 0.023 to 0.24 smolts/100 m2 for age-3 smolts (Whalen 1998).
Fry stocking
Age-2 and age-3 smolts analyzed (1994 and 1995) originated from fry stocked in 1991–1993 (J. McMenemy, Vermont Fish and Wildlife, unpublished data). Sources of fry are described in Whalen and Parrish (1999). Fry stocking densities were based on 100-m2 habitat units, defined as 100 m2 of wetted streambed area standardized to summer-low-flow conditions. Between 1991 and 1993, both unfed and fed fry were stocked in the three tributaries in April and May at densities ranging from 25 to 50 fry/100 m2. Yearling parr densities resulting from these stocking levels typically ranged from 3 to 10/100 m2, although higher densities have been observed (McMenemy 1995). The number of 100-m2 habitat units stocked with fry increased between 1991 and 1993, primarily through stocking fry into upper reaches of each tributary, including small subtributaries. Several sea-run adult Atlantic salmon have been observed in the West River and its tributaries, but we do not know whether wild fry or parr were present. We assumed the majority of smolt production is from fry stocking.
Smolt collection
Smolts were collected with net weirs in 1994 (Orciari et al. 1994) and with counting fences in 1995 (Anderson and McDonald 1978). Net weirs had 1-cm-mesh net wings, and counting fences had 2-cm spacing between the vertical conduits of the fence portion of the weir; hereafter, both are referred to as “traps.” A single collection box was used with each trap. A net trap also was installed on upper Utley Brook in both years to evaluate within-tributary migration.
Traps were installed in the lower portion of each tributary in early to mid-April, when water temperatures were near 4°C, and were fished through May in 1994 and through June in 1995, until 5–6 d of no catch occurred. Traps spanned the entire wetted width at each location. Counting fences were operated continuously for April–June, but all net traps in 1994 and the one on upper Utley Brook in 1995 were inoperable for 1–3 d several times during the migratory period due to high flows. Inoperable days in 1994 were Rock River: May 3, 11, 17; Wardsboro Branch: April 25–26, May 2, 9, and 17–18; and Utley Brook: May 2, 9, and 17–18.
In conjunction with trapping, we completed mark–recapture studies at each site to determine trap efficiency. Briefly, a portion of smolts collected daily in the traps were double-marked with a visual implant (VI) tag (Blankenship and Tipping 1993) and acrylic paint applied to the postocular adipose tissue or a partial caudal fin clip (Whalen 1998). Within 24 h after tagging, marked smolts were released about 75–100 m upstream of each trap. Releases of tagged smolts were stratified through the migration season, with intent to estimate trap efficiency across a wide range of conditions (Dempson and Stansbury 1991). Trap efficiency ranged from 10% to 33% for net weirs and from 15% to 46% for counting fences. Daily catch totals of unmarked smolts closely tracked the daily catch totals of the known, marked population and were positively related in all cases except in the Rock River during 1994 (linear regression, all: P < 0.05; R > 0.63). Thus, daily catch totals, unadjusted for trap efficiency, were used to calculate cumulative smolt catch during migration.
Traps were checked at least once daily, typically during hours of darkness between 2300 and 0500 hours. All smolts were counted; as many as 200 randomly selected smolts were anesthetized with buffered tricaine methanesulfonate (MS-222; 100 mg/L), measured (total length, TL, nearest 1 mm), and weighed (nearest 0.1 g). Length and weight were used to calculate condition factor as 105·body weight(g)/TL (mm)3. Scale samples were taken to determine age.
Gill sampling
Nonlethal gill biopsies were taken from 5–12 smolts collected in the traps at about 1-week intervals for determination of gill Na+,K+-ATPase activity, expressed as micromoles of adenosine diphosphate (ADP) per milligram protein per hour, as in McCormick (1993). Typically, smolts were processed (including biopsies) within 12 h of collection. Laboratory studies revealed that gill Na+,K+-ATPase activity changes minimally (5–10%) within 12 h even under stressful conditions (Carey and McCormick 1998).
In 1994, gill samples were collected on May 13 from the Rock River; on May 6, 11, and 13 from Wardsboro Branch; and on May 11 from lower and upper Utley Brook. In 1995, gill samples were collected on April 28 and May 11, 19, and 31 from the Rock River; on May 5, 13, and 22 from Wardsboro Branch; and on May 5 and 13 from lower Utley Brook. Because gill Na+,K+-ATPase activity changes minimally at sampling intervals shorter than 4 d (McCormick et al. 1989), gill biopsies collected on similar dates—for example, on May 11 and 13—were considered the same sampling interval for cross-tributary comparisons.
We determined seasonal patterns of gill Na+,K+-ATPase relative to migratory status by sampling gills from Rock River fish (1995) collected by electrofishing during premigration on April 21 and by angling and electrofishing during postmigration on June 7. As part of a companion study to track the smolt development of immature and mature parr, the 22 Atlantic salmon collected on April 21 were taken to the laboratory where they were marked individually with passive integrated transponder (PIT) tags (K. G. Whalen, S. D. McCormick, and D. L. Parrish, unpublished data). Gill biopsies were taken weekly for 4 weeks from the date of collection. Fish that achieved and maintained gill Na+,K+-ATPase activity greater than 3.0 μmole ADP/mg protein per hour were classified as smolts; others were considered parr (McCormick and Björnsson 1994).
Smolts sampled on June 7 were part of a group that had remained 200–300 m upstream of the Rock River trap. Because smolt migratory activity had effectively ceased by June 1 and water temperature had increased to 15–20°C (per Wedemeyer et al. 1980), we classified these smolts as residuals. Resident parr also were collected for comparison and we used phenotypic characteristics, such as silvering and darkened fins, to distinguish residual smolts from parr in the field (Cunjak et al. 1990).
Water temperature and discharge
We analyzed how water temperature, collected hourly by thermographs, influenced smolt migration timing. Degree-days were calculated by summing the mean daily water temperature between April 7 and May 20 in each tributary. For each site, we developed equations to predict daily discharge (Q, m3/s) from stadia rod water levels. Discharge was determined by measuring wetted width, water depth, and midcolumn water velocity (0.6·depth) at 0.5-m intervals across the river. We measured discharges ranging from 0.21 to 2.7 m3/s in the Rock River (N = 6), from 0.087 to 2.3 m3/s in Wardsboro Branch (N = 8), and from 0.084 to 2.5 m3/s in Utley Brook (N = 9). At each trap location, regressions related discharge (dependent variable) to the stadia rod level (independent variable). Stadia rod level was recorded each day and daily discharge was determined retrospectively with the predictive equation for each site.
We evaluated the effect of discharge on smolt catch. Peaks in discharge were categorized as medium (<100% increase in discharge) or high (≥100% increase in discharge). Smolt catch on the 1–2 d associated with discharge events was summed and then divided by the total smolt catch for each tributary. This “percent catch” for peaks in discharge was plotted by day-of-year to evaluate seasonal trends in the effect of peaks in discharge on smolt migration.
Statistical analyses
A Komolgorov–Smirnov (K–S) test of distributions was used to determine differences in cumulative relative frequency distributions of smolt migration timing (Sokal and Rohlf 1995). Analysis of variance (ANOVA) was used to test for differences in mean gill Na+,K+-ATPase activity among tributaries and sampling periods. Homogeneity of sample variances (Levenes test) was verified before ANOVA. We used the Tukey–Kramer studentized range test for multiple comparisons.
We used analysis of covariance (ANCOVA) to test for differences among tributaries in smolt total length and condition factor, including day-of-year as a covariate. Because the range of the covarying variable (day-of-year) was similar among tributaries, unadjusted sample means were reported for smolt total length and condition factor. We also used ANCOVA to determine if total length and condition factor covaried with gill Na+,K+-ATPase activity. We used a standard single-factor ANCOVA model and followed stepwise procedures in Sokal and Rohlf (1995).
We used regression to determine if percent smolt catch occurring with peaks in discharge was related to day-of-year. Differences among tributaries and years in smolt age were determined with χ2 analysis. Statistical analyses were completed with JMP software (SAS Institute 1996), and statistical significance was considered at α = 0.05.
Results
Smolt Migration Timing
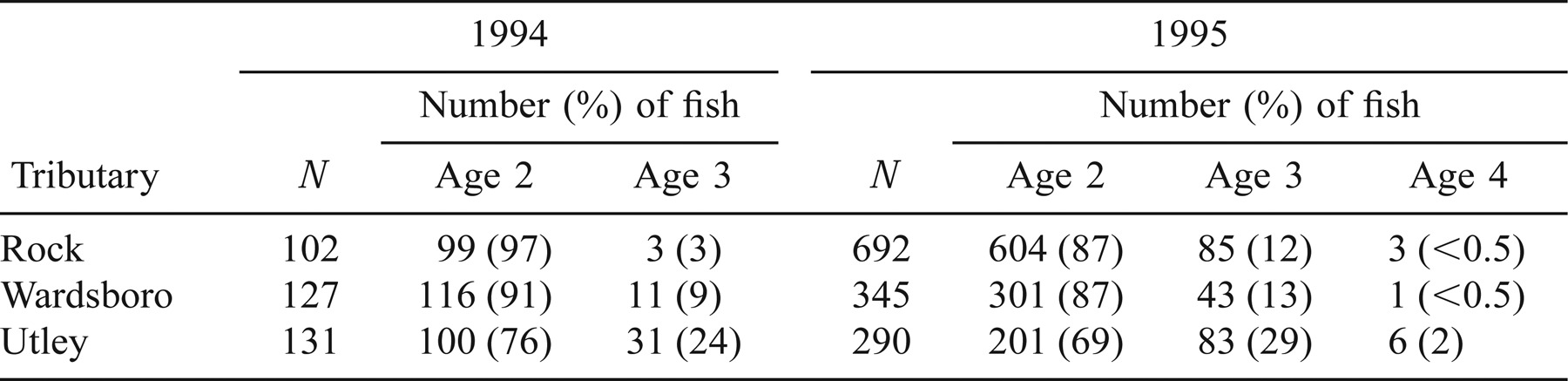
Smolt migration consistently began in late April or early May and was completed by late May or early June with most smolts migrating May 2–22 (Figure 1). Between-year differences in migration timing of smolts occurred at each trap location except for upper Utley Brook (K–S test; all trap locations: P < 0.05; Figure 1); however, no consistent trend for smolts to migrate earlier in either year was observed. The maximum difference in smolt cumulative catch between years within each tributary occurred between May 6 and 11.
Cumulative relative frequency plots for 1994 (solid line) and 1995 (broken line) timing of Atlantic salmon smolt migration for Rock River, Wardsboro Branch, Utley Brook, and upper Utley Brook, Vermont. Sample size is given along each catch curve. Arrows point to date of 50% cumulative catch
Between-tributary differences in smolt cumulative catch occurred in each year (K–S test; all tributaries: P < 0.05). In 1994, smolts migrated earlier from the Rock River and Wardsboro Branch than from Utley Brook, whereas in 1995, Rock River smolts migrated earlier than Utley Brook smolts and Utley Brook smolts tended to migrate earlier than Wardsboro Branch smolts (Figure 1). The maximum difference in smolt cumulative catch distributions occurred between May 2 and 9 in 1994 and May 6 and 15 in 1995. The timing of the smolt migration between the Utley and upper Utley trap locations did not differ significantly in either year.
Migration versus Water Temperature and Discharge
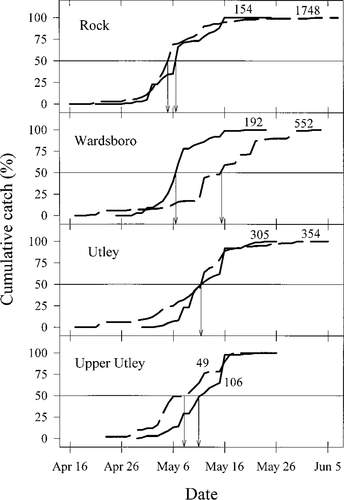
In 1995, smolt migration began when mean daily water temperatures increased beyond 5°C; peaks in smolt catch occurred after water temperatures had risen beyond 8°C (Figure 2). Through mid-May, mean daily water temperature ranged between 8°C and 12°C. From the week beginning on April 7 through the week ending May 20 the trend in water temperatures (with degree-days in parentheses) decreased from Rock River (336) to Wardsboro Branch (325) to Utley Brook (309; Figure 2).

Number of smolts collected by sampling date (histogram) from Rock River, Wardsboro Branch, and Utley Brook, Vermont, in 1995 in relation to daily discharge Q (m3/s; top line graph) and daily mean (lower graph, solid black line) and minimum–maximum (solid gray lines) water temperature, recorded at 1-h intervals with thermographs. Filled circles indicate sampling beginning and end dates. Asterisks (*) indicate discharge events used in the analysis of the effect of peaks in discharge on smolt migration. Inverted triangles point to dates gill biopsies were collected
Discharge was not quantified in 1994; however, increased catches of smolts occurred on several occasions when discharge was increasing before the sampling gear became inoperable. In 1995, the pattern of river discharge at each location was generally similar as discharge declined from a large peak on April 21 and 22 through mid-May, whereas thereafter, peaks in discharge were at times tributary-specific, reflecting the highly localized nature of several rainfall events (Figure 2). During the migratory period in 1995, five discharge events accounted for 24.9% of the smolt catch in the Rock River and 52.1% of the total smolt catch in Wardsboro Branch, whereas seven discharge events accounted for 50.6% of the total smolt catch in Utley Brook.
The 17 peaks in discharge recorded in the three tributaries in 1995 comprised 12 medium- and 5 high-discharge events. Smolt catch associated with discharge events was related to day-of-year (P < 0.01; R2 = 0.55; Figure 3). Peaks in discharge recorded before May 5 as well as after May 20 resulted in smolt catches that represented less than 10% of the within-tributary totals, whereas peaks in discharge occurring May 5–20 resulted in smolt catches that ranged as high as 30% of the within-tributary totals. Seasonally, high-discharge events did not cause greater smolt catches than medium-discharge events (Figure 3).

Seasonal trend in percent of total smolt catch mobilized on 17 discharge peaks for the Rock River, Wardsboro Branch, and Utley Brook, Vermont, combined in 1995. Unfilled symbols refer to medium-discharge events (<100% increase in discharge) and symbols containing a plus sign (+) refer to high-discharge events (≥100% increase in discharge). See also asterisks (*) in Figure 2
Gill Na+,K+-ATPase Activity
Cross-tributary comparisons
For migratory smolts, significant differences in smolt gill Na+,K+-ATPase activity occurred both among tributaries and sampling periods (Figure 4). On May 11 and 13 in 1994, the mean gill Na+,K+-ATPase activity of smolts collected at both Utley Brook locations was about half the mean gill Na+,K+-ATPase activity of Rock River smolts (ANOVA; P < 0.05). Mean gill Na+,K+-ATPase activity of Wardsboro Branch smolts fell between Rock River and Utley Brook smolts (Figure 4). Mean gill Na+,K+-ATPase activity differed in all cross-tributary comparisons in 1995 (ANOVA; all comparisons: P < 0.05) with trends among tributaries being very similar to those observed in 1994. On May 5 and 6, mean gill Na+,K+-ATPase activity of Utley Brook smolts was nearly half the mean gill Na+,K+-ATPase activity of Wardsboro Branch smolts. As in 1994, Utley Brook smolts also exhibited lowest mean gill Na+,K+-ATPase activity of the three tributaries on May 11 and 13, whereas the mean gill Na+,K+-ATPase activity of Wardsboro Branch smolts was intermediate to that of Rock River and Utley Brook smolts. Mean gill Na+,K+-ATPase activity of Rock River smolts also was significantly greater than that of Wardsboro Branch smolts in the May 19 and 22 sampling interval.
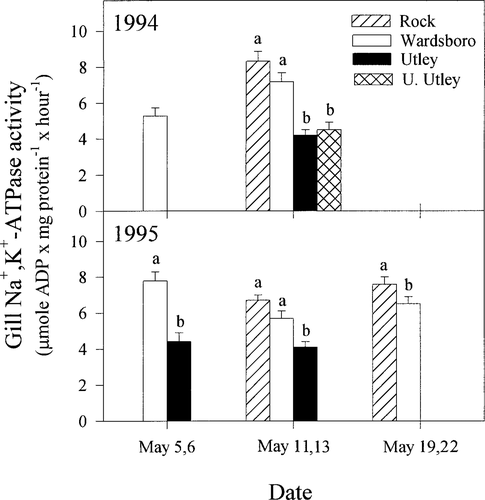
Mean (+SE) gill Na+,K+-ATPase activity of migratory Atlantic salmon smolts collected in downstream traps in 1994 and 1995 from the Rock River, Wardsboro Branch, and Utley Brook, Vermont; upper Utley Brook (U. Utley) trap location was sampled in 1994. Within each year and sampling interval, means not sharing a common letter differ significantly (ANOVA; P < 0.05)
Influence of smolt age and size
The effect of smolt age, either age-2 or age-3, on gill Na+,K+-ATPase activity in a three-way ANOVA including the effects of tributary and sample interval was not significant (P = 0.45). Mean ± SE gill Na+,K+-ATPase activity of age-2 smolts was 6.2 ± 0.2 μmole ADP/mg protein per hour (N = 50) and 5.5 ± 0.5 μmole ADP/mg protein per hour (N = 12) for age-3 smolts.
Total length did not covary with gill Na+,K+-ATPase activity for Rock River, Wardsboro Branch, or Utley Brook smolts (ANCOVA; all locations: P > 0.25). For Rock River and Wardsboro Branch migratory smolts, condition factor did not covary with gill Na+,K+-ATPase activity (ANCOVA; both locations: P > 0.50), whereas condition factor was a significant covariate of gill Na+,K+-ATPase activity for Utley Brook smolts (ANCOVA; P < 0.05). Gill Na+,K+-ATPase activity tended to increase with increasing smolt condition factor in Utley Brook; however, this relation was highly variable (R2 = 0.16).
Seasonal patterns
In 1995, gill Na+,K+-ATPase activity was highly seasonal in the Rock River (ANOVA; P < 0.01), experiencing near twofold increases from the premigratory period on April 21 to the seasonal peak on May 19 and then dropping back to premigratory levels from May 19 to June 7 (Figure 5). The increase in gill Na+,K+-ATPase activity between April 21 and May 19 was associated with rising water temperatures ranging from 5–8°C to 9–12°C, whereas the decrease in gill Na+,K+-ATPase activity between May 19 and June 7 was associated with rising water temperatures ranging from 9–12°C to 16–21°C (Figures 2, 5). Mean gill Na+,K+-ATPase activity of residual smolts collected on June 7 was significantly lower than mean gill Na+,K+-ATPase activity of migratory smolts sampled on May 31 but did not differ significantly from the mean gill Na+,K+-ATPase activity levels of premigratory smolts collected on April 21 (ANOVA; P > 0.05).
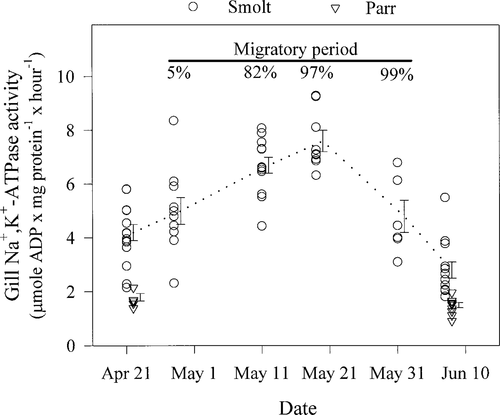
Seasonal trend in gill Na+,K+-ATPase activity of Atlantic salmon smolts and parr collected between April and early June in the Rock River, Vermont, in 1995. Gill Na+,K+-ATPase activity values on April 21 and June 7 are offset to reduce overlap. The vertical bars represent SE, and midpoint of a vertical bar is the sample mean. The darkened horizontal bar with percentages refers to smolt cumulative catch (see Figure 1)
No smolts were collected in the Rock River trap before April 21 (Figure 2); however, juvenile salmon with elevated gill Na+,K+-ATPase activity were collected by electrofishing (Figure 5). The increase in gill Na+,K+-ATPase activity from April 28 to May 19 was associated with a sharp increase in the smolt cumulative catch (Figure 5). By May 19, the observed peak in gill Na+,K+-ATPase activity, about 97% of the smolts in the Rock River had migrated. The smolt cumulative catch increased only 2% (97–99%) between May 19 and 31, when migratory smolts showed a significant decline in gill Na+,K+-ATPase activity. Concomitant with the continued decline in smolt gill Na+,K+-ATPase activity on June 7, the smolt migration had effectively ended.
Although mean gill Na+,K+-ATPase activity of premigratory smolts on April 21 and residual smolts on June 7 were the lowest recorded, on each date the gill Na+,K+-ATPase activity of smolts was greater than mean gill Na+,K+-ATPase activity of parr (t-test; both dates: P < 0.01; Figure 5). Differences in size and morphology also were observed between smolts and parr. Mean (±SE) total length of smolts was greater than that of parr on both April 21 (smolts = 151 ± 4 mm and parr = 121 ± 1 mm) and June 7 (smolts = 184 ± 4 mm and parr = 147 ± 2 mm; t-test; both dates: P < 0.01).
No difference in mean (±SE) condition factor occurred among premigratory (0.78 ± 0.09), migratory (0.76 ± 0.1), and residual (0.76 ± 0.2) smolts (ANOVA; P = 0.61). On April 21, condition factor of parr (0.77 ± 0.03) did not differ from smolts (t-test; P = 0.91), but condition factor of parr (0.97 ± 0.02) was greater than that of residual smolts on June 7 (t-test; P < 0.01). During daytime underwater surveys in early June, we observed that residual smolts, in contrast to parr, were highly silvered, had darkened fins, and swam in the upper water column, whereas parr were near the stream bottom.
Smolt Age
Smolt age differed among tributaries (both 1994 and 1995: χ2 > 25.0; P < 0.01) with about 25–30% of smolts in Utley Brook spending three winters in stream before migrating compared with 13% or fewer smolts in the Rock River and Wardsboro Branch (Table 1). The migration timing of age-2 and age-3 smolts within each tributary in 1995 did not differ (K–S test; all locations: P > 0.05); dates of 50% cumulative catch among ages differed by less than 1 d.
Smolt Total Length and Condition Factor
Differences in age-2 smolt mean total length among tributaries were small in each year (≤5 mm) but significant (ANCOVA; both years: P < 0.01). The trend in mean age-2 smolt length was a decrease from Rock River (156 mm) to Utley Brook (154 mm) to Wardsboro Branch (152 mm) in 1994 and a decrease from Wardsboro Branch (168 mm) to Utley Brook (164 mm) to Rock River (163 mm) in 1995 (sample sizes in Table 1). Age-3 smolts averaged 7 mm longer than age-2 smolts in 1994 (161 mm) and averaged 13 mm longer than age-2 smolts in 1995 (177 mm). Smolt condition factor differed among tributaries in both 1994 (ANCOVA; P < 0.01) and 1995 (ANOVA; P < 0.01). Mean smolt condition factor ranged from 0.71 to 0.75 in 1994 and from 0.69 to 0.73 in 1995, and in both years the trend across tributaries was a decrease in condition factor from Rock River to Utley Brook to Wardsboro Branch.
Day-of-year covaried with age-2 smolt length in 1994 (ANCOVA; P < 0.01) and with both age-2 and age-3 smolt lengths in 1995 (ANCOVA; both ages: P < 0.01). For age-2 smolts in each year and for age-3 smolts in 1995, total length tended to increase with time. This was most likely due to smolt growth, as indicated by heavy postwinter banding on scales of smolts migrating later in the season.
Discussion
We observed significant differences among tributaries in the timing of migration of Atlantic salmon smolts as well as in their physiological development. Smolt migration timing was inversely related to water temperature, whereas gill Na+,K+-ATPase activity was positively related to water temperature. Rock River was warmest during premigration and warmed first to temperatures where migration began (5°C) and where peak migration was observed (>8°C). Smolts migrated earliest from the Rock River in each year and generally had the greatest gill Na+,K+-ATPase activity. In contrast, Utley Brook, which was coldest during premigration, had smolts that migrated latest with significantly lower gill Na+,K+-ATPase activity. Previously, increasing water temperature has inversely affected the timing of smolt migration (Jonsson and Ruud-Hansen 1985; Greenstreet 1992) and positively affected the date of peak gill Na+,K+-ATPase activity (McCormick et al. 1997). Our results strongly support water temperature as influential in driving the timing of Atlantic salmon smolt migration and, potentially, the level of gill Na+,K+-ATPase activity of migratory smolts.
How water temperature influenced smolt migration timing and gill Na+,K+-ATPase activity was not without variability, however. Wardsboro Branch water temperatures were intermediate between those recorded in the Rock River and Utley Brook in 1995, yet Wardsboro smolts migrated latest. Migration variability, relative to water temperature, might have arisen because migration in Wardsboro Branch often depended on peaks in discharge after early May. More than half (52.1%) of the smolts in Wardsboro Branch in 1995 migrated during five peak discharge events, which represented less than 20% of the days we sampled. Peaks in discharge in the Rock River and Utley Brook were also important to smolt migration but not as much as in Wardsboro Branch.
This variability could have been due to avoidance of sampling gear. Fixed traps may provide only gross measures of migration timing, and as we observed, differences in migration timing among tributaries may need to be large—that is, on the order of weeks—to be recognized. Improved methods and systems that enable smolt movements to be detected without directly affecting behavior will need to be developed to resolve finer-scale patterns in smolt migration timing.
Large differences in mean daily water temperature of about 4°C are needed to cause a significant advance (2-weeks) in the date of peak gill Na+,K+-ATPase activity (McCormick et al. 1997). Thus, our finding of significantly greater gill Na+,K+-ATPase activity in smolts from warmer tributaries, although consistent with previous studies (Johnston and Saunders 1981; Solbakken et al. 1994; McCormick et al. 1997), was unexpected because water temperatures differed by a small amount (≤2.0°C) for only 3–4 weeks. It is therefore unlikely that water temperature alone could explain the near twofold differences in gill Na+,K+-ATPase activity we observed between Utley Brook and our other streams. Perhaps, large daily water temperature fluctuations (about ±5°C) that occur in natural systems during the rapid increase in water temperature after winter somehow influence smolt physiological development. Our analysis of wild-reared smolts suggests that the effect of water temperature on smolt physiological development is more complex than previously identified for hatchery-reared smolts (Johnston and Saunders 1981; Solbakken et al. 1994; McCormick et al. 1997) and requires further investigation.
Few studies on Atlantic salmon smolts have integrated physiology into the analysis of the effect of environmental factors on migration (Cunjak et al. 1990). The analysis of seasonal patterns in the development and loss of smolt characteristics helped explain complex interactions among water temperature, discharge, and smolt behavior during both the onset and cessation of migratory activity. In 1995, juveniles with elevated gill Na+,K+-ATPase activity (i.e., putative smolts) occurred in the Rock River on April 21. However, we captured no smolts in our trap between April 16 and 20, a period of high discharge but low water temperature (3–6°C), and a large peak in discharge on April 21 resulted in only a small catch of smolts. Comparatively small peaks in discharge in mid-May at 8–12°C, when smolt gill Na+,K+-ATPase activity was higher, caused migration of many smolts. Perhaps, peaks in discharge stimulated greater migration in mid-May because temperature thresholds were surpassed, more juveniles had undergone the parr–smolt transformation, and many were peaking physiologically. Our results suggest a hierarchy of cues for smolt migration with smolt physiology, as an influence on the onset of migration, depending upon water temperature and the effect of discharge depending on both water temperature and smolt physiology.
Peaks in discharge late in the migration season had varying effects on smolt behavior. Fewer smolts are left late in the year, so links between environmental stimuli and migration may be weak (Solomon 1978). Additionally, smolts may decline physiologically to a point where they are no longer responsive to environmental stimuli to migrate. In the Rock River in 1995, a decrease in smolt gill Na+,K+-ATPase activity between May 19 and 31 was associated with a sharp decline in smolt catch, whereas a further decline in gill Na+,K+-ATPase activity was associated with the cessation of migratory activity. The cessation of smolt migratory activity coupled with significant declines in gill Na+,K+-ATPase activity, in some cases to values comparable with parr, suggested they had lost smolt characteristics. The loss of smolt characteristics is temperature dependent (Duston et al. 1991; McCormick et al. 1997, in press). Rock River water temperatures had increased to 16–21°C when significant declines in gill Na+,K+-ATPase activity were observed and migration had ceased. We believe the late-season decline in smolt physiology, which was linked to the cessation of migratory activity, might have been caused by high water temperatures prevailing in late May and early June (McCormick et al., in press).
In some cases, presmolt Atlantic salmon may migrate downstream in the fall in lieu of (Riddell and Leggett 1981) or in addition to (Youngson et al. 1983) the normal spring smolt migration. How gill Na+,K+-ATPase activity relates to migration in these cases is unknown. However, for typical spring migrants, the result of the seasonally brief, but heightened, sensitivity to environmental stimuli is that Atlantic salmon smolt migration, including that observed in the three West River tributaries, is highly synchronous (Jonsson et al. 1990; Hvidsten et al. 1995). Synchronous migration facilitates communal transit of smolts to the ocean and improves survival of smolts (Hvidsten and Hansen 1988; Hvidsten and Johnsen 1993). Premigratory physiological priming and coupling of migration with elevated gill Na+,K+-ATPase activity may enhance a smolt's ability to acclimate to seawater (Johnston and Saunders 1981). Juvenile Atlantic salmon may grow in estuaries until they smolt (Cunjak et al. 1990), but smolts migrating from freshwater may make only brief use of the estuary before entering the ocean (McCormick et al. 1985; Hvidsten and Hansen 1988; Moore et al. 1995). The benefit of minimizing travel time from freshwater to the ocean may be that exposure to nearshore predators is minimized (Hvidsten and Møkkelgjerd 1987; Hvidsten and Lund 1988). However, some salmon (e.g., coho salmon Oncorhynchus kisutch) that may make greater use of estuaries before entering the ocean (Moser et al. 1991; McMahon and Holtby 1992), may have greater flexibility in the timing of development of hypoosmoregulatory ability and migration (Ewing et al. 1985). Differences in plasticity of migration and smolting between Oncorhynchus and Atlantic salmon may be due to different strategies for use of estuaries and adaptation to the marine environment.
We found no evidence that smolt length was related to gill Na+,K+-ATPase activity or that gill Na+,K+-ATPase activity of age-2 and age-3 smolts differed. Although smolts in the tributaries differed in age and size, these factors did not influence migration timing. The lack of a relation between smolt size and gill Na+,K+-ATPase activity may be a function of the threshold-like nature of the size criteria for the par–smolt transformation (Skilbrei 1991; Fängstam et al. 1993). Size determines which parr transform to smolts, but thereafter, size may be unrelated to smolt physiological development and migration timing.
Our results have implications for the management and restoration of Atlantic salmon to the Connecticut River. Physiologically primed smolts responded to water temperature as a cue for the initiation of migration. Many smolts may migrate during peaks in discharge occurring after temperature thresholds have been surpassed until smolt physiological status declines. The period between onset of migration and cessation of migratory activity or loss of smolt physiological characteristics may be brief. Smolts leaving Vermont tributaries encounter multiple hydrofacilities that may delay migration (McMenemy and Kynard 1988), resulting in exposure to water temperatures that accelerate the loss of physiological characteristics (McCormick et al. 1997, in press). Delays in downstream passage that may occur at dams must therefore be minimized to maximize the successful recruitment of smolts to the marine environment.
Acknowledgments
This research was supported by the National Marine Fisheries Service, Vermont Department of Fish and Wildlife, and Massachusetts Division of Marine Fisheries. Additional funding was provided by the Conte Anadromous Fish Research Center of the Biological Resources Division, U.S. Geological Survey. Logistical support was provided by Stephen Roy of the National Forest Service, Green Mountain–Finger Lakes, and by personnel from the Manchester, Vermont, Ranger District. We thank Jay McMenemy, Gayle Barbin, Michael Ross, Jay Hestbeck, William Bemis, and Peter MacNeil for their critical review of an earlier version of the manuscript and Todd Bennett, Peter Nicholson, Michael Cunningham, and Michael O'Dea for their efforts in the field and laboratory. The views expressed herein are those of the authors and do not represent the official views and policies of the Federal Energy Regulatory Commission.