Identification of Anadromous and Nonanadromous Adult Brook Trout and Their Progeny in the Tabusintac River, New Brunswick, by Means of Multiple-Stable-Isotope Analysis
Abstract
Multiple-stable-isotope analysis was used to infer anadromous and nonanadromous origins of adult brook trout Salvelinus fontinalis and maternal migration history of age-0 progeny in the Tabusintac River, New Brunswick. Forty-seven adults collected above head of tide displayed deviations (δ) from standard ratios of 13C/12C, 15N/14N, and 34S/32S of −30.3‰ to −16.0‰, 7.4‰ to 16.8‰, and 1.5‰ to 14.1‰, respectively; higher values (positive or less negative) denote relatively greater enrichment in the heavier isotope. Isotopically enriched brook trout exhibited isotope profiles typical of fish from marine environments, and those that were isotopically depleted were considered to be of freshwater origin. Age-0 brook trout from Home Camp Pool, the most downstream freshwater rearing site sampled, were more enriched (δ13C = −24.4 ± 2.7‰ (mean ± SD), δ15N = 12.5 ± 3.1‰, and δ34S = 7.4 ± 1.8‰) than those at two other sites, and were believed to be progeny of anadromous females. Age-0 brook trout from the Bathurst Highway site (δ13C = −27.9 ± 0.6‰, δ15N = 8.9 ± 0.8‰, and δ34S = 5.8 ± 0.8‰) and the Little Eskedelloc River (δ13C =−28.6 ± 0.5‰, δ15N = 8.1 ± 0.4‰, and δ34S = 2.9 ± 0.8‰), with less isotopic enrichment, were most likely from nonanadromous parents. Stable-isotope ratios varied with fork length; at Home Camp Pool, this relationship was thought to represent an “isotope dilution factor” as recently emerged juveniles assimilated new food from freshwater, grew, and masked the marine signatures of their maternal parents. This study suggests that stable-isotope ratios may be used to distinguish between sympatric anadromous and nonanadromous adult brook trout and their progeny as long as brook trout are collected before they dilute their maternal isotope signatures.
Although brook trout Salvelinus fontinalis once demonstrated “sea-run” or anadromous behavior in many streams of eastern Canada and northeastern United States (Mullan 1958; Ritzi 1959; Dutil and Power 1980; Scott and Scott 1988), few anadromous populations remain and most continue to decline (Ryther 1997). Anadromy enhances the intraspecific diversity of brook trout, and it is believed that this type of evolutionary development occurs to improve long-term reproductive success (Power 1980; Randall et al. 1987; Thorpe 1987).
Factors inducing anadromous behavior may include environmental conditions (Smith and Saunders 1958; Doyon et al. 1991), food availability (Nordeng 1983; Metcalfe and Thorpe 1992), and density-dependent behavior (Castonguay et al. 1982). However, few authors agree on the specific mechanism that initiates anadromy or on the relatedness of resident (i.e., nonanadromous) and sea-run brook trout in mixed populations. Many techniques have been used in efforts to identify individual salmonids that migrate to and from the marine environment, including morphometry (Wilder 1952); parasite loads (Black 1981; Frimeth 1986); analysis of allozymes (Stoneking et al. 1981) and mitochodrial DNA (Jones et al. 1996); carotenoid pigments (Youngson et al. 1997); spawning behavior (Maekawa et al. 1993); and strontium concentration patterns in scales (Moreau and Barbeau 1979) and otoliths (Kalish 1990; Rieman et al. 1994; Babaluk et al. 1997). To our knowledge, only otolith microchemistry (Kalish 1990; Rieman et al. 1994) has been used to distinguish between the progeny of sympatric anadromous and nonanadromous salmonids.
Stable-isotope analysis (SIA) may offer an alternative for identifying migratory origins of adult salmonids and their progeny. An SIA provides indirect assessments of food sources and energy pathways unencumbered by ambiguous visual interpretation of stomach contents. Detailed laboratory feeding experiments have demonstrated reliable relationships between the stable-isotope ratios of animals and their assimilated diets (DeNiro and Epstein 1978, 1981; Tieszen et al. 1983), and many field studies have confirmed the general utility of SIA in describing food web relations (reviewed by Fry and Sherr 1984; Peterson and Fry 1987; Rundel et al. 1989; Lajtha and Michener 1994).
Marine primary producers tend to have different stable-isotope ratios from freshwater producers, offering opportunities to delineate between animals feeding from these two environments. For example, chemical fractionation between atmospheric CO2 and bicarbonate in ocean water results in a 13C/12C ratio almost identical to a defined standard (i.e., δ13C/12C ∼ 0‰; Mook et al. 1974). Uptake of this dissolved inorganic carbon (DIC) into marine plankton gives δ values near −21‰ (Fry and Sherr 1984), meaning relative depletion of 13C. Plants in freshwater are generally much more depleted (δ = −28‰ or more extreme) than those found in marine areas because respiration of terrestrial organic matter causes 13C depletion in the freshwater DIC source (Rau 1978). Terrestrial inputs also are more depleted in 15N (relative to 14N) than marine plankton (Peters et al. 1978). As well, the 34S/32S ratio of seawater sulfate is significantly higher than that found in freshwater, so marine organisms are 10‰ to 20‰ more enriched than their freshwater counterparts (Peterson et al. 1985). As a result of these differences, SIA has been used successfully to distinguish between organisms feeding in marine and freshwater environments (Chisholm et al. 1982; Hobson 1990) and the method has also been used to identify the presence of anadromous fishes in freshwater (Kline et al. 1990; Bilby et al. 1996).
This study was undertaken to better understand coexisting anadromous and nonanadromous brook trout and their habitats in the Tabusintac River, New Brunswick. To do this, a stable-isotope survey was conducted to determine if there was isotopic evidence of marine and freshwater feeding in adult brook trout populations. If the river contained two distinct populations representing anadromous and nonanadromous life histories, the stable-isotope ratios in the adult sample should be bimodal. Evidence of anadromy was also looked for in recently emerged brook trout, because their initial stable-isotope ratios are derived from the yolk sac and should reflect the feeding environment of their maternal parents. If successful, SIA may provide fisheries managers with an inexpensive method of identifying spawning areas of anadromous fish and the relative abundance of their progeny within a mixed population. The ability to accomplish this task could benefit conservation efforts and may protect anadromous brook trout populations from further human exploitation.
Methods
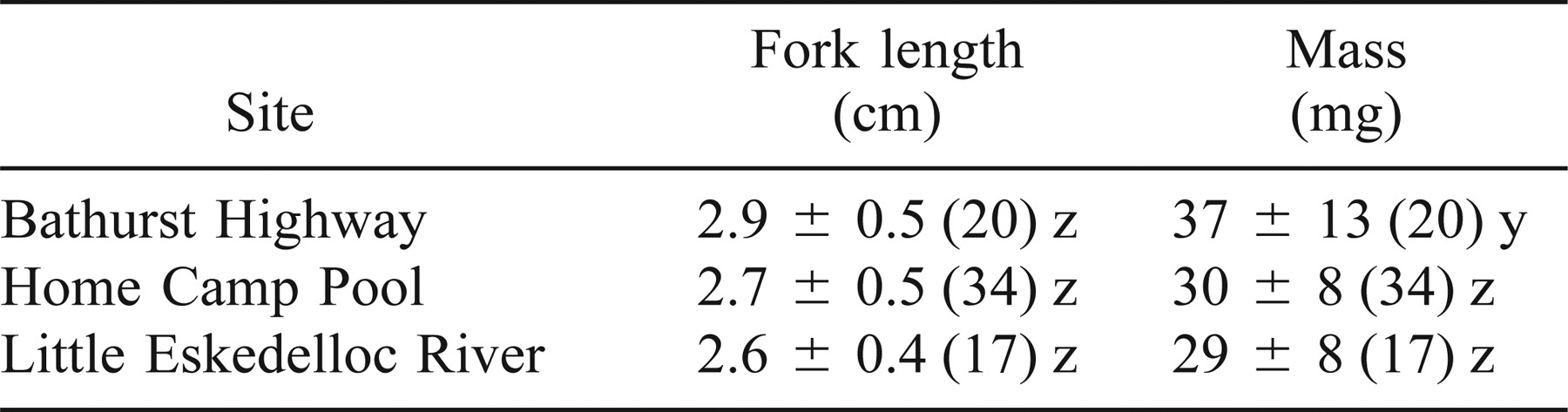
Adult brook trout were collected during September 1995 by using a seine net, a hoop net, and electrofishing gear in various holding pools along the Tabusintac River (47°20′N, 64°48′W) (Figure 1). In the interests of conservation, adipose fins were sampled rather than muscle tissue. Rounick and Hicks (1985) found no isotopic differences between fin and muscle tissue of New Zealand fishes, and we have presumed, for the purpose of our study, that muscle and fin tissue have comparable isotopic turnover times and fractionation factors. Age-0 brook trout were electrofished on 5 June 1996 from three locations along the Tabusintac River: (1) Home Camp Pool (HCP); (2) the Little Eskedelloc River (LER); and (3) the main Tabusintac River above the Bathurst Highway (BH). The LER site was considered inaccessible to anadromous brook trout because of a large long-standing dam created by beavers Castor canadensis just below the collection area (W.H., personal observation). Lethal sampling of age-0 brook trout was necessary to obtain the required amount of tissue for sulfur isotope analysis (about 6 mg dry mass); carbon and nitrogen isotope analysis required only about 0.6 mg of tissue. Other fishes (slimy sculpin Cottus cognatus; age-1 Atlantic salmon Salmo salar, and age-1 brook trout) and benthic invertebrates (Ephemeroptera: Ephemera sp., Odonata: Boyeria sp., and Trichoptera: Pycnopsyche sp.) were also collected from the electrofishing locations to verify site-specific stable-isotope compositions of resident fish and potential prey.
Map of the Tabusintac River drainage basin showing location of adult brook trout holding pools (heavy arrows) and age-0 collection sites. 1 = Home Camp Pool (HCP); 2 = Little Eskedelloc River (LER); and 3 = Bathurst Highway site (BH)
All samples were immediately frozen in the field, brought back to the laboratory, and freeze-dried for 24–48 h. Fish stomachs were removed before drying to eliminate the possibility of isotopic contamination of tissue samples. Whole dried individuals were pulverised to a fine powder with a mortar and pestle or a ball mill grinder. Prepared samples were stored in clean, precombusted (at 500°C for 2 h) glass vials and held in a desiccator to await isotopic analysis.
Stable-isotope ratios are expressed as delta (δ) values and are measures of a parts-per-thousand (or “per mil,” ‰) difference between the isotope ratio of a sample and that of an international standard according to the formula:

R = 13C/12C, 15N/14N, or 34S/32S. For simplicity, δ often is expressed in terms of the numerator (heavier stable isotope; e.g., δ13C), a convention used in this paper. Samples that are more negative or less positive contain less 13C, 15N, or 34S (relative to the lighter stable isotope) than samples with higher δ values and are termed “depleted” for an isotope. Samples with higher values are “enriched.” International standards are PDB (Pee Dee belemnite) carbonate (Craig 1957), nitrogen gas in the atmosphere (Mariotti 1983), and primordial sulfur from the Canyon Diablo meteorite (Rees et al. 1978).
Isotopic analyses were performed exclusively on a Micromass VG-Isochrom Continuous Flow Isotope Ratio Mass Spectrometer (CFIRMS) at the Environmental Isotope Laboratory (EIL), University of Waterloo. Replicate analyses of common laboratory standards yielded results that were both accurate and precise (International Atomic Energy Agency [IAEA] standard CH6: δ13C = −10.54 ± 0.15‰ [mean ± SD; N = 132]; IAEA standard N1: δ15N = 0.59 ± 0.30‰ [N = 225]; National Bureau of Standards (USA) standard 122: δ34S = 0.75 ± 0.45‰ [N = 25]). Replicate samples of a lipid-extracted fish standard (EIL-70) also produced reliable δ values for carbon, nitrogen, and sulfur (δ13C = −20.71 ± 0.21‰ [N = 38]; δ15N = 16.37 ± 0.29‰ [N = 38]; δ34S = 5.28 ± 0.95‰ [N = 31]).
Statistical analyses were performed with SYSTAT 7.0 (Wilkinson 1997). Maximum type-one error rates were set at α = 0.05. Normality and homogeneity of variance assumptions were checked via residual plots. Significant analyses of variance (ANOVA) were followed by among-site and among-species comparisons with Tukey's honestly significant difference (HSD) post hoc test (Sokal and Rohlf 1995). We used model-1 regression analysis to determine the significance of relationships between fork length and stable-isotope ratios for adult brook trout and power curves (e.g., Fry and Arnold 1982; Hobson and Clark 1992; Vander Zanden et al. 1998) to describe changes in stable-isotope ratios as a function of growth and turnover in age-0 brook trout.
Results
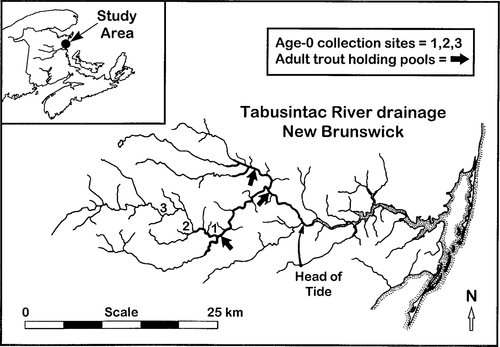
Adult brook trout caught in the Tabusintac River had stable-isotope ratios suggesting either diverse feeding locations, trophic levels, or diets. The δ13C, δ15N, and δ34S signatures of the adults ranged from −30.3‰ to −16.0‰, 7.4‰ to 16.8‰, and 1.5‰ to 14.1‰, respectively. Significant relationships existed between stable C, N, and S isotope ratios and fork length in adult brook trout (model-1 regressions, P < 0.001; Figure 2); smaller fish were more depleted in the heavier isotopes than larger fish.
Stable-isotope ratios of sulfur, carbon, and nitrogen (y), expressed as deviations (δ) from standards, as a function of fork length (cm, x) in adult brook trout collected from holding pools in the Tabusintac River in September 1995; N = 47 fish for each graph
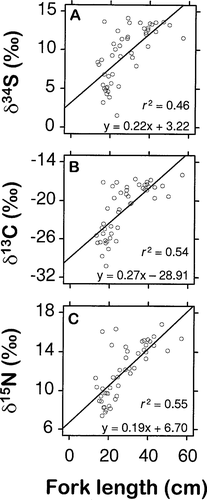
Dual isotope plots showed considerable similarity in isotope ratios between age-0 and adult brook trout (Figure 3). Age-0 brook trout from the HCP site were significantly more enriched in 13C, 15N, and 34S than those from either the BH or LER sites (Table 1) (Tukey's HSD, P < 0.001). The δ13C and δ15N values of BH and LER juveniles were not significantly different, but BH juveniles were more enriched in 34S than LER fish (P < 0.001).
Dual isotope ratio plots, expressed as deviations (δ) from standards, for age-0 and adult brook trout collected from the Tabusintac River. Adults were sampled in September 1995; age-0 fish were collected in early June 1996; N = 47 for adults and 71 for age-0 fish

Resident fishes and benthic invertebrates revealed little variation in stable C, N, and S isotope ratios at the BH, HCP, and LER sites (Figure 4). Except for age-0 brook trout, taxon- or age-specific δ13C and δ15N values did not differ significantly among sites (Tukey's HSD, P > 0.05). However, fish and invertebrates from the LER site were significantly more 34S-depleted than those from the HCP and BH sites (P < 0.05). As expected, invertebrate δ15N values were lower than those of fishes, which probably reflects the ability of δ15N to accurately reflect an animal's trophic position (Minagawa and Wada 1984).
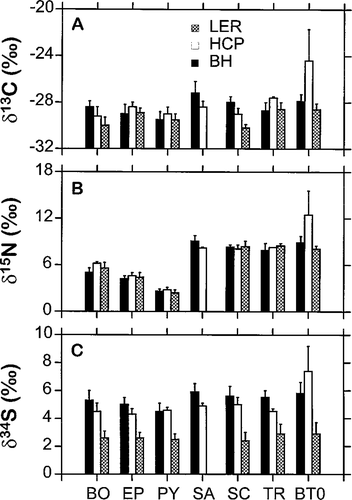
Delta values (means + SDs) for stable-isotope ratios of resident fishes and benthic invertebrates collected on 5 June 1996 from three sites in the Tabusintac River: BO = Boyeria sp.; EP = Ephemera sp.; PY = Pycnopsyche sp.; SA = age-1 Atlantic salmon; SC = slimy sculpin; TR = age-1 brook trout; BT0 = age-0 brook trout; BH = Bathurst Highway site; HCP = Home Camp Pool; and LER = Little Eskedelloc River. Sample sizes ranged from 3 to 20 individuals
Age-0 brook trout showed more variation in their isotope compositions at the HCP than at the other two sites (Table 1; Figure 4). These differences appeared to be related to fish size, because δ13C, δ15N, and δ34S values of HCP fish regressed well against fork length (FL) (nonlinear regression: δ13C = −14.46FL+0.53; δ15N = 43.77FL−1.30; δ34S = 18.22FL−0.92; all P < 0.001; Figure 5). Such regressions for age-0 fish from the BH and LER were not significant (P > 0.05). Mean fork lengths of age-0 brook trout did not differ among sites (ANOVA, P > 0.05), but BH fish were heavier than the others (Tukey's HSD, P < 0.05; Table 2).
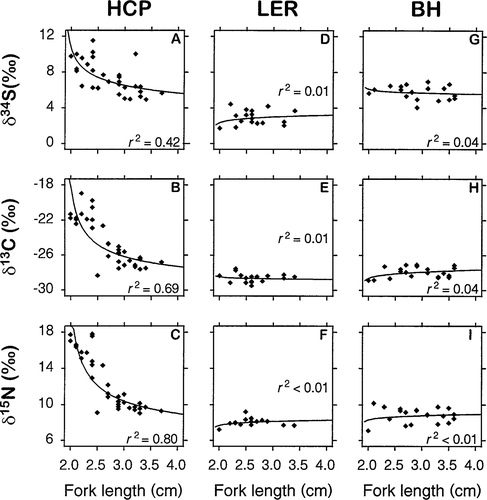
Nonlinear regression relationships between δ13C, δ15N, and δ34S and fork length for age-0 brook trout collected on 5 June 1996 from three sites in the Tabusintac River: HCP = Home Camp Pool; LER = Little Eskedelloc River; and BH = Bathurst Highway site. Sample sizes are in Table 1
Discussion
Stable C, N, and S isotope ratios of adult brook trout collected from holding pools in the Tabusintac River in autumn 1995 were divergent enough to warrant separation of the fish into two or more groups. Larger brook trout (> 30 cm) were typically the most isotopically enriched adults and probably were anadromous fish that had migrated back from the marine environment (Figure 2). Their delta values—−18 to −16‰ for 13C, 14 to 17‰ for 15N, and 10 to 15‰ for 34S—were similar to those reported for other marine-feeding fishes (Rau et al. 1981; Fry 1988; Jarman et al. 1996). Significant positive relationships between fork length and stable C, N, and S isotope composition (Figure 2) may suggest that size is an important indicator of anadromy for adult brook trout. It is reasonable that fish would grow much larger in productive marine environments than in the space- and food-limited habitats of streams and rivers.
The greater heavy-isotope depletion of smaller brook trout (< 20 cm) probably indicates that these fish had not migrated to the isotopically enriched marine environment to feed. Smaller brook trout were most likely residents of the holding pools in which they were caught and were reflecting the heavy-isotope depletion of their freshwater prey (Fry 1991). Although changes in δ15N values with fish size have been correlated with changes in trophic position (Cabana and Rasmussen 1994; Hobson and Welch 1995), similar patterns would not be expected for δ13C or δ34S (Peterson and Fry 1987). Isotopic enrichment in our study was correlated with increasing fish size for all three stable-isotope ratios, so the patterns we observed probably were related to recent feeding environments, not to changes in trophic status.
Brook trout of intermediate sizes (20–30 cm) exhibited intermediate δ values for the three elements (Figure 2). Isotope compositions of resident Tabusintac River fishes and invertebrates (Figure 4) suggest that intermediate δ values did not originate from a strictly freshwater diet. One possibility is that intermediate-size brook trout moved to coastal waters to feed but did not accumulate the same amount of marine-derived tissue as larger fish and thus did not fully acquire a marine isotopic signature. Seaward migration of anadromous brook trout occurs in spring in the northern part of the species' range (Montgomery et al. 1983; McCormick et al. 1985), but varies in timing to the south (Mullan 1958; Smith and Saunders 1958). The timing of anadromous migration has not been well studied in the Tabusintac River, and we do not know how much time medium-size brook trout would have to develop marine isotopic complements before migrating back to freshwater to spawn (Hesslein et al. 1991, 1993).
It may also be that medium-sized brook trout did not feed in the same marine locations as larger brook trout. Peterson et al. (1985) documented a gradient of increasing δ34S and δ15N in the ribbed mussel Geukensia demissa from freshwater to the sea in a New England marsh system. They explained the S and N gradients by sequential changes in the relative availability of terrestrial detritus, salt-marsh grasses, and marine plankton as primary food sources. Similar isotope gradients may exist in the Tabusintac system. Salinity tolerance of anadromous brook trout increases with fish size (McCormick and Naiman 1984), and many brook trout larger than 18 cm leave the estuary to feed in the marine environment (Montgomery et al. 1990). The isotopic variation we measured in adult brook trout may simply express where fish had fed along the freshwater—marine transition. The possibility that stable isotopes can help elucidate habitat associations among adult brook trout is very exciting and should be studied further. However, trophic gradients in isotope composition and variation in brook trout excursions along the freshwater—marine continuum offer complex analytical challenges. The use of stable-isotope analysis to reliably identify anadromous adults and their progeny must be tested with fish whose recent life history is known.
Age-0 brook trout were more easily separated into two distinct groups than adult brook trout (Figure 3). These groups, according to their isotopic similarities to marine and freshwater organisms (Peterson and Fry 1987), appear to represent progeny of either anadromous or nonanadromous females. Recently emerged fish typically were more depleted in 13C and 34S for a given δ15N than adults (Figure 3), which may have resulted from utilization of abundant yolk lipids. Lipids are 2–3‰ more 13C-depleted than whole bodies and other tissue components (DeNiro and Epstein 1977). Our age-0 fish appeared to be 2–4‰ more depleted in 13C than adults (Figure 3). However, analysis of covariance confirmed statistical differences between the slopes and intercepts (P < 0.001), and this result did not permit a quantitative assessment of this “lipid effect.” Depletion of heavier stable isotopes from parents to progeny may be expected of all oviparous animals and requires further investigation. Similar differences in δ34S values between age-0 and adult brook trout were also observed, but we are unable to interpret them at this time.
Stable-isotope analysis suggested that Home Camp Pool may be an important site for anadromous brook trout production, because 13C, 15N, and 34S in age-0 fish were significantly more enriched there than at two other freshwater sites (Table 1). The HCP emergents also were significantly more enriched in these isotopes than other fishes and benthic invertebrates at that site, whereas emergents did not differ isotopically from the resident fauna at the other two sites (Figure 4). Slight differences in the δ13C values of lotic organisms collected in this study likely reflected site-specific or functional feeding group differences (Doucett et al. 1996). In contrast, variations in freshwater δ15N values are most easily explained by differences in trophic position (Fry 1991).
Age-0 brook trout from Home Camp Pool showed depletion of heavier isotopes as fork length increased (Figure 5). Recent emergents did not show such patterns elsewhere, although mean fork length did not differ among sampling sites (Table 2). We believe the HCP relationships demonstrate an “isotope dilution process” as young fish shift in nutritional reliance from maternally conditioned yolk to locally available freshwater prey. From this perspective, all age-0 brook trout collected at the HCP site may have been from anadromous parents and all those from the Little Eskedelloc and Bathurst Highway sites were from nonanadromous mothers. The “dilution” hypothesis is plausible because Home Camp Pool is known to be a site of high anadromous brook trout production (W.H., unpublished data). Again, however, confirmation of this information awaits data on fish of known life history.
The absence of age-0 progeny of inferable anadromous parents at the Little Eskedelloc site was expected because the long-standing beaver dam halted upstream migration by spawning brook trout. This beaver dam also may have been responsible for the significantly more depleted 34S found in fishes and invertebrates at this site (Figure 4). Anoxic sediments, the accumulation of which is enhanced by beaver dams, provide good environments for bacterial reduction of sulfate to sulfide (Trust and Fry 1992), which can result in large isotope fractionations during S assimilation into plant tissues. Carlson and Forrest (1982) and Fry et al. (1982) showed that aquatic plants rooted in anaerobic soils have lower 34S/32S ratios than plants growing in aerobic soils. However, the relatively large error in sulphur isotope analyses (see Methods) prevents us from evaluating this biogeographical connection. We urge caution in the use of sulfur isotopes in freshwater studies, and we suggest that stable isotopes of carbon and nitrogen alone may allow identification of anadromous fishes.
Results from our study suggest that it is possible to use stable-isotope technology for identification of anadromous and nonanadromous fishes. Adult brook trout caught in the Tabusintac River had stable C, N, and S isotope ratios suggestive of marine, freshwater, and estuarine feeding locations. Transfer of these energy resources to progeny allowed us to suggest the maternal origins of age-0 brook trout. Home Camp Pool appeared to be a site of important anadromous brook trout production, and no marine isotope signatures were found at two upstream sites. Further research on the Tabusintac River might reveal other sites of anadromous brook trout production, as long as age-0 fish are collected soon after emergence, before their isotope signatures are diluted by assimilated freshwater energy sources. Consideration should also be given to the downstream movements of young salmonids (Randall and Paim 1982; Johnston 1997), because juveniles of anadromous parents may drift into areas where anadromous females did not spawn. Future conservation efforts to protect anadromous brook trout, other sea-run fish populations, and their in-stream habitats, are likely to be enhanced by using multiple-stable-isotope analysis to identify migratory histories and measure the reproductive success of anadromous females.
Acknowledgments
We thank Bob Drimmie and Bill Mark of the Environmental Isotope Laboratory in assisting R.R.D. with the stable-isotope analyses. Lisa Atwell, Bernie Dube, Lisa McCabe, and Chris Connell assisted in the collection of age-0 brook trout. We are grateful to the manager and guardians of the Tabusintac Club for their insight on the location of trout pools. Comments received from Allen Curry, Keith Hobson, and an anonymous reviewer significantly improved the quality of an earlier draft of this manuscript. This research was funded by a Natural Sciences and Engineering Research Council (NSERC) Individual Research Grant to G.P. and an NSERC Industrial Post-Graduate Scholarship to R.R.D., the industrial sponsor being Noranda Inc.