Swimming in Two-Vector Flows: Performance and Behavior of Juvenile Chinook Salmon near a Simulated Screened Water Diversion
Abstract
We used a large, annular flume equipped with a simulated fish screen to assess the swimming and behavioral responses of juvenile Chinook salmon Oncorhynchus tshawytscha to two-vector flows typifying habitats near water diversions, where small fish may become entrained. Groups of 20 fish (4.4–7.9 cm long, at 12°C or 19°C) were tested for 2 h at one of nine experimental flow regimes derived from combinations of three (approach) velocities perpendicular to the screen and three (sweeping) velocities parallel to the screen and a (0-cm/s) control during daytime (lighted) and nighttime (dark) conditions. In the high-velocity (resultant vector) flow regimes, all fish swam at velocities comparable to the critical swimming velocities measured for similar-sized conspecifics, suggesting that exposure to such flow conditions near a water diversion is energetically expensive. Although most fish exhibited strong positive rheotaxis, older (smolt-size) fish acclimated to the warmer temperature exhibited higher rates of negative rheotaxis (particularly in the intermediate-velocity flow regimes), a behavior consistent with downstream migration. Fish life stage (length), time of day (light level), and water velocity influenced swimming velocity; sweeping velocity, swimming velocity, and rheotaxis influenced screen passage velocity. Regardless of the flow regime, juvenile Chinook salmon contacted the screen most frequently at night, and nighttime contact rates were not affected by the velocity of either flow vector. During the daytime, screen contact rates were inversely related to sweeping velocity and independent of approach velocity. Injury rates were low and unrelated to either flow or screen contact rates, and survival rates were high (>99%) in these predator-free experiments. Fish screen designs that minimize screen exposure duration (e.g., via reduced screen length or increased sweeping velocities) should optimally protect valuable juvenile Chinook salmon, a species that encounters multiple water diversions along many of its migratory paths to the ocean.
Introduction
Traditional laboratory methods of measuring fish swimming performance include the confinement of individual fish in a linear swim tunnel in which the velocity of the water flow can be adjusted and unidirectional, locomotory activity can be observed (see reviews by Beamish 1978; Hammer 1995). Many fishes, however, exist in groups and in much less confining aquatic habitats with complex (i.e., multivector) flow regimes. While the traditional techniques permit useful measurements of single-vector, locomotory ability (e.g., critical swimming velocity, Hammer 1995; swimming endurance or stamina, Adams et al. 2000), the apparatuses do not allow for either the expression or the observation of behaviors commonly observed in natural settings, including group interactions (such as schooling) or individual behaviors (such as prolonged negative rheotaxis). With freshwater and estuarine habitats becoming increasingly characterized by altered flow regimes (whether small-scale anomalies such as those associated with in-channel water diversions or landscape-level modifications of instream flows), an improved understanding of these responses will likely be more useful in formulating environmental management strategies to mitigate the impacts of these alterations and consequently protect fishes.
In California, juvenile Chinook salmon Oncorhynchus tshawytscha emigrating from the Sacramento–San Joaquin watershed are potentially exposed to thousands of water diversions that extract more than 8 × 109 m3/year (>6.5 million acre-feet/year; CALFED 1999; Herren and Kawasaki 2001) for urban, industrial, and agricultural use. Within the watershed's large estuary where smolting juveniles may reside for weeks prior to entering the ocean (Kjelson et al. 1982), as much as 65% of the total inflow may be diverted (CALFED 1999). The loss of parr and smolts at these water diversions has been identified as a contributor to population declines suffered by the four seasonal runs over the past few decades (USFWS 1995; Moyle 2002). Presently, Sacramento basin winter- and spring-run Chinook salmon are listed as endangered and threatened, respectively, under the federal Endangered Species Act.
Throughout the watershed, fewer than half of large water diversions (>2.8 m3/s) are equipped with fish screens (Herren and Kawasaki 2001; P. Raquel, California Department of Fish and Game, personal communication). The installation of fish screens, including new screens at the massive state and federal water project diversions in the estuary, has been identified as a high priority task in the ongoing watershed-wide fish protection and habitat restoration effort (i.e., CALFED Bay Delta Program; CALFED 1999). Fish screens function to (1) physically exclude entrained fish from diverted water, (2) reduce intake (approach) velocity by increasing intake surface area and/or orienting the screen surface oblique to the intake flow, and (3) promote fish movement past the diversion and screen, usually by means of a sweeping velocity parallel to the screen surface (Clay 1995; Hayes et al. 2000). However, while the presence of a fish screen effectively prevents fish from being diverted from the habitat, the screen itself could be harmful to fish. In California, the efficacy of presently mandated fish screen design and flow criteria for the protection of Chinook salmon, and many other native fishes, is unknown. Fish swimming close to the screen may become impinged on the screen surface, rendering them unable to escape or vulnerable to predation. Even brief contact with the screen could result in stress or injury. Further, exposure to the screen, and thus the potential for contact or impingement, could be prolonged for fish swimming rheotactically against the sweeping flow. Many fishes, including young Chinook salmon (Healy 1991), are attracted to structure in their environment; the diversion, fish screen, and associated supports and cleaning devices could deceptively appeal to young fish as refuge. Downstream migrants may also be attracted to the artificial flow localized at the water diversion point.
Historically, fish screen flow velocity criteria have been based upon laboratory measurements of fish swimming capabilities and the presumption that, if the intake velocity does not exceed the fish's swimming ability, the fish is safe (Clay 1995). However, Hanson and Li (1978) reported that a number of fishes, including juvenile Chinook salmon, contacted a simulated fish screen or become impinged at velocities substantially below their measured swimming abilities. For another threatened California fish, delta smelt Hypomesus transpacificus, Swanson et al. (1998) demonstrated frequent behavior-related swimming failure and screen contact at flows substantially below the species' critical swimming velocity. These results, as well as a variety of field observations from operational screened diversions, suggest that this physiologically based approach to developing fish screen criteria may be inadequate. For optimal protection, fish screen design and operation should match both the capabilities and the behavioral tendencies of the fishes intended for protection. The objective of the current study was to quantitatively evaluate these factors for juvenile Chinook salmon.
Methods
Fish collection and care
Fall-run Chinook salmon parr (2–4 months posthatch, 2.5–3.5 cm standard length [SL]) were collected from the California Department of Fish and Game Nimbus Hatchery (Rancho Cordova, California) on the American River, a tributary to the Sacramento River, and transported to the University of California–Davis Center for Aquatic Biology and Aquaculture. Following a prophylactic disease treatment and subsequent thermal acclimation period (maximum temperature change = 8°C; rate of temperature change = 1°C/d; minimum acclimation duration = 10 d), experimental fish were transported 1 km to the J. Amorocho Hydraulics Laboratory at the University of California–Davis, where the studies were conducted. At both facilities, the fish were held identically in flow-through circular tanks (diameter = 1.0–1.6 m; maximum water velocity = 10 cm/s) supplied with nonchlorinated, air-equilibrated, temperature-controlled well water (pH = 7.9; total alkalinity = 320 mg/L; hardness = 290 mg/L; specific conductivity = 585 μS/cm). The fish were fed an artificial diet (2–4% body weight, depending on fish size; Silver Cup, Stirling H. Nelson & Sons, Murray, Utah) and maintained on a natural or simulated natural photoperiod (38°N latitude) at ±1.0°C of the target acclimation temperature.
Experimental apparatus
Experiments were conducted in the Fish Treadmill, a large, annular flume designed to test and observe fish exposed to spatially uniform, two-vector flow regimes near a fish screen (for a detailed description of the Fish Treadmill, see Hayes et al. 2000). Based on a similar design used for fish survival tests (Kano 1982), the annular test channel was large enough to allow relatively unrestricted and volitional movement and, at least for small fishes, the curvature of the 2.8-m-diameter inner screen was slight enough to simulate a flat surface, albeit of infinite length. Unlike fish confined in a traditional linear flume (e.g., a “Brett-type” flume; Brett 1964) that are effectively required to hold station against a flow, fish in the Fish Treadmill were able to select and vary their swimming velocity and direction as well as their lateral and vertical location in the channel.
Water for Fish Treadmill operation was stored in and recirculated through an underground sump (volume = 190,000 L), continuously filtered and temperature controlled (±1.0°C) with two heat pumps (Universal Marine Industries, San Leandro, California), and replaced with new, nonchlorinated well water every 3 months or sooner, as determined by water quality measurements (e.g., ammonia values >0.5 mg/L as total N). The annular test channel (Figure 1) was delimited by an outer rotating screen (4 m in diameter) and an inner fixed (simulated fish) screen (2.8 m in diameter, 2.3 mm vertical wedgewire screen, 50% porosity; Bixby Zimmer, Galesburg, Illinois). Within this channel, two velocity vectors could be controlled independently. Approach (perpendicular to the inner screen) velocity was controlled by the water inflow rate into the channel (measured with an ultrasonic flowmeter; Dynasonics, Inc., Naperville, Illinois); for example, at the high approach velocity (15 cm/s; see below), more than 566 L/s moved through the channel. Sweeping (parallel to the inner screen) velocities were generated and controlled by rotation of the outer screen. Water depth in the channel was held constant at 44 cm (±2 cm) at the inner screen. For each experimental flow regime, detailed, three-dimensional flow velocity and flow vector maps of the channel were generated from flow measurements at more than 50 different locations within the channel made using a three-vector, acoustic doppler velocimeter (SonTek, San Diego, California). To minimize variations in flow velocity and direction within the test channel that could provide flow refugia to the fish, a number of design features, including baffles behind the inner screen and the sloped floor of the test channel, were incorporated. Although the resultant velocities through the test channel were relatively uniform and laminar, measured approach and sweeping velocities did deviate from nominal values in some areas of the channel's 0.23-m2 cross-sectional area, with the greatest differences in flow regimes that combined a low approach velocity with a high sweeping velocity (see Hayes et al. 2000). In general, both approach and sweeping velocities were highest in locations close to the screen and near the bottom of the channel, and decreased with distance from the inner screen. Experimental velocities were based on measurements from the test channel's geometric center (31 cm from the inner screen and 18 cm from the bottom).
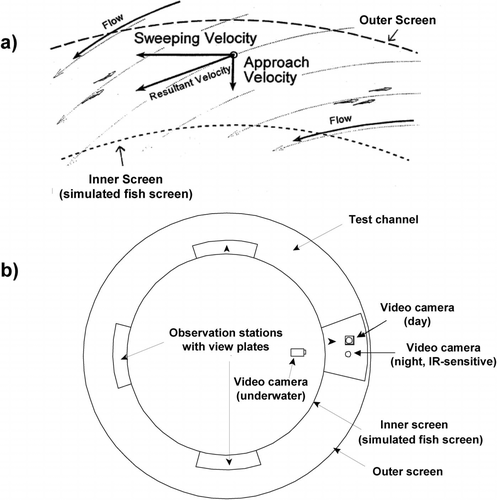
Top-view diagrams of the Fish Treadmill annular test channel: (a) inner and outer screens and the approach, sweeping, and resultant flow vectors controlled during the experiments; and (b) location of visual and video observation stations
For visual and video observations of fish behavior, four observation stations were established at evenly spaced locations around the annular channel (Figure 1b). At each of these stations, a clear acrylic “view plate” was fastened to the inner screen at the water surface in order to provide an undistorted image of fish–screen interactions. Each view plate covered approximately 9% of the inner screen's circumference. At one station, the view plate covered the entire width of the channel, and several video cameras were positioned to record fish behavior at this location. View plates did not significantly alter the three-dimensional flow field in the test channel. Two cameras, one equipped with an infrared-sensitive lens (LCEO, Waterford, New York), filmed the channel from above, and a third was positioned underwater and behind the inner screen to view the fish from the side. For daytime experiments, the swimming channel was illuminated from above with both direct and indirect fluorescent lights to produce a relatively uniform light level around the channel. During nighttime experiments, the channel was illuminated with infrared light produced by small emitters (Neward Electronics, Sacramento, California) attached to each view plate.
Experimental design and protocol
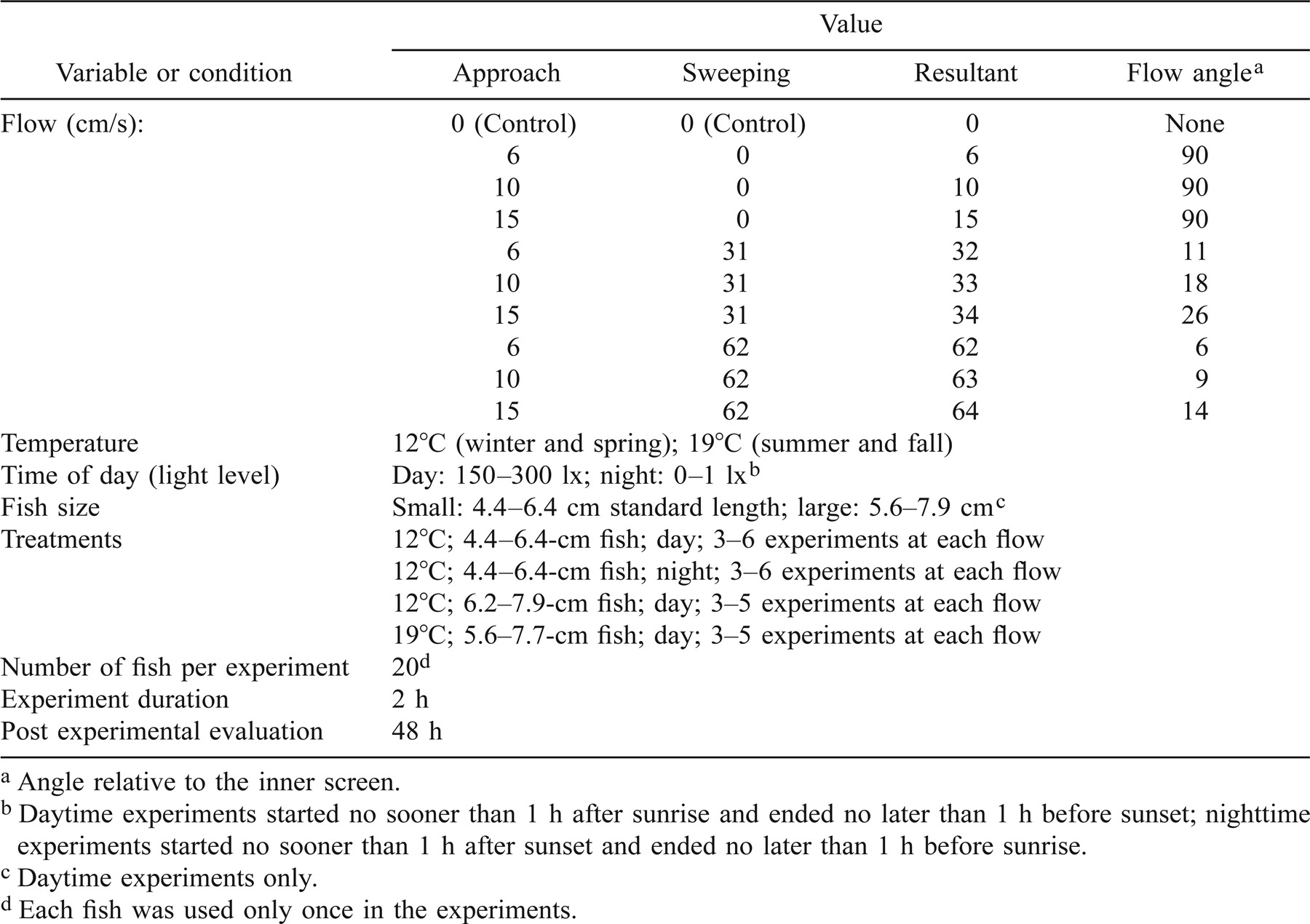
Experiments were conducted at nine experimental flow regimes derived from combinations of three approach velocities and three sweeping velocities and a control (0 cm/s approach and sweeping velocity) and at two time-of-day–light level combinations: daytime under light conditions (150–300 lx) and nighttime under dark conditions (0–1 lx; Table 1). Approach and sweeping velocities were selected based on presently mandated fish screen flow criteria for Chinook salmon and delta smelt in California and the northwestern United States (CDFG 2000; NMFS 1997).
Water temperature was held constant (±1°C) at one of two seasonal temperatures, 12°C (February–June) and 19°C (June–August). Thus, the experiments and the experimental temperatures selected coincided with the timing and environmental conditions of the parr–smolt transformation for fall-run Chinook salmon in the Sacramento River basin, which emerge in the winter and migrate to the ocean during their first spring and summer (Healy 1991; Moyle 2002). In the 5-month-long 12°C treatment, two fish size-classes were used: small (range = 4.4–6.4 cm; daytime experiment, 5.3 ± 0.1 cm [mean ± SE]; nighttime experiment, 5.6 ± 0.1 cm) and large (range = 6.2–7.9 cm; 7.0 ± 0.1 cm). For the shorter-duration 19°C treatment, only the large size-class (5.6–7.7 cm SL; 6.7 ± 0.1 cm) was tested. Only the 12°C, small-size fish were tested under nighttime conditions. Although there was some overlap in the two size-classes and our measurements did not include observations of silvering or the collection of gill samples for Na+,K+-ATPase analyses (the most commonly used assays for smoltification), we hypothesized that the small fish acclimated to the cold temperature corresponded to the parr stage (prior to out-migration) and the large fish in the two temperature treatments to older parr and smolts. A variety of studies using both wild and hatchery-reared Sacramento basin fall-run Chinook salmon indicate that out-migration and smoltification begin when the fish reach 50–70 mm in length (Castleberry et al. 1991; Healy 1991; Katzman 2001).
Twenty fish were used in each 2-h experiment. After the experimental flow regime was stabilized, fish were collected from their holding tank, transported to the Fish Treadmill in an opaque cylindrical container (water volume = 8 L) with a hinged bottom, and released directly into the channel from the partially submerged container (distance = 20 m; maximum duration of transport and handling prior to release < 5 min). After 2 h, the flow was suspended and the fish were collected using dip nets and beakers and held in a separate holding tank for 48 h. All fish were used only one time in the experiments. All experimental treatments were replicated at least three times (mean = four replicate experiments per flow; Table 1).
Measurements
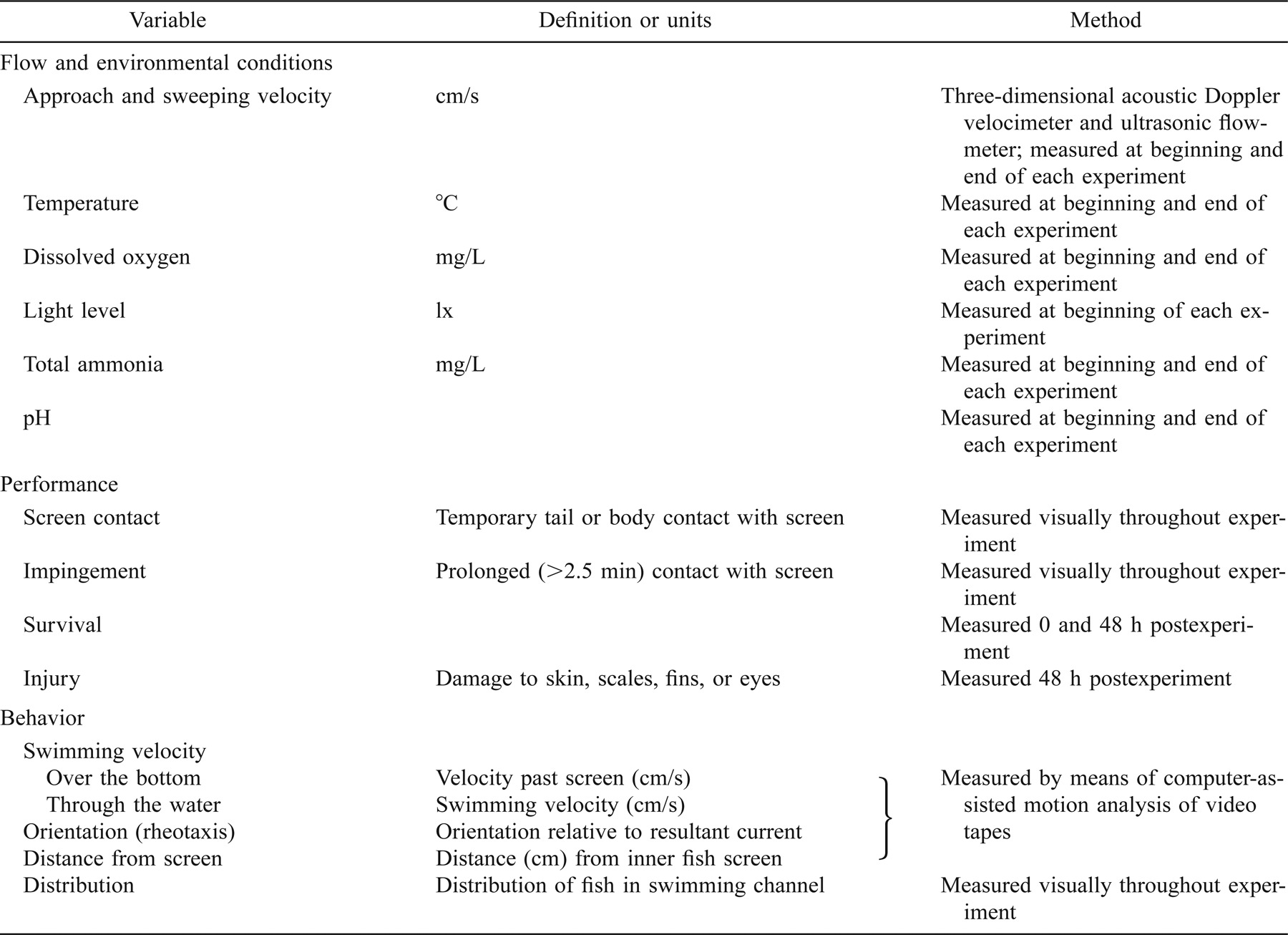
Measurements included water velocity, water quality, and fish performance and behavior (Table 2). Approach and sweeping flow velocities, water depth, water temperature, dissolved oxygen, total ammonia, and pH were measured immediately before the fish were released into the Fish Treadmill test channel and just prior to the end of the experiment and cessation of flow. Light level at the water surface was also measured before each experiment. Continuous visual observations of swimming behavior (e.g., swimming direction, distribution within the channel, loss of equilibrium, or other anomalous behaviors), fish–screen contacts, and impingements (prolonged screen contacts >2.5 min) were made throughout the experiment. The distribution of fish within the channel (e.g., random, dispersed, or clumped) was assessed visually as a simultaneous count of the number of fish present at each observation station every 10 min. Swimming behavior was quantified using computer-assisted motion analysis of videotape records from each experiment (Peak Performance Technologies, Englewood, Colorado). Velocity (cm/s over the bottom) and direction of travel (upstream or downstream relative to the sweeping flow), distance from the inner screen (cm), and angle relative to the screen (degrees) were measured for five fish at the beginning of the experiment and at 5, 10, 20, 40, 60, 80, 100, 110, and 119 min in the experiment. Measured fish were selected as the first fish to appear after the target time. Depth (cm from the bottom ± 2–6 cm depending on depth) was measured manually and simultaneously from the underwater videotape and/or from calibrated linear displacement of shadows cast by the fish on the channel floor. From these data and flow velocity and flow vector maps, swimming velocity (cm/s through the water), and angle relative to the flow (degrees; e.g., 0° for positive rheotaxis and 180° for negative rheotaxis) were calculated. Impingements were recorded for the entire test channel, and screen contacts (“tail” [caudal fin or <50% of the posterior body length] and “body” [contact by >50% of the fish's length]) were counted at two to four observation stations within sequential 5-min intervals. For each 5-min interval, screen contact rates (contacts/fish/min) were calculated for the entire test channel from the number of screen contacts observed, total screen area observed (i.e., number of stations observed), and the number of fish swimming (i.e., total number of fish less number of fish impinged).
After the 48-h postexperiment period, each fish was anesthetized with tricaine methanesulfonate (70 mg/L), weighed (wet weight ± 0.01 g), measured (SL ± 0.1 cm), and carefully examined for damage to fins and eyes, scale loss, and abrasions or hemorrhaging. Injury rates were calculated as the number of fish injured divided by the total number of fish. The severity of each injury type was also coded numerically using a five-point scale (e.g., 1 = no damage, 5 = severe damage) and indices created for each injury type and total injuries. Fish that died during the experiment or the postexperiment period were similarly examined. Regularly throughout the study, one or more groups of “preexperiment” fish (n = 10–20 fish per group) randomly selected from the holding tanks were also examined for injuries prior to use in the experiments. Survival was assessed at the end of the experiment (0 h postexperiment) and, for the small size fish, at the end of the 48-h postexperiment holding period. For the large size fish (12°C and 19°C), 48-h postexperiment survival was measured for 10 fish only (the remaining fish were used to assess physiological stress responses; P. S. Young, unpublished results).
Statistical methods
Data from individual experiments were analyzed (SPSS 1998) as either experiment means or as means of sequential 30-min time intervals (e.g., 0–30 min, 31–60 min, etc.). For some analyses, the results from similar flows (e.g., all approach velocities within a particular sweeping velocity treatment) were pooled. For some analyses, the effects of flow were examined with and without the results of control experiments. The effects of flow on various responses were also analyzed in terms of the resultant velocity, which was calculated as the square root of (approach velocity2 + sweeping velocity2), as the flow angle (degrees) relative to the inner screen (calculated as the arctangent of {[approach velocity in cm/s]/[sweeping velocity in cm/s]}; e.g., 90° for flow perpendicular through the inner screen), and as actual approach and sweeping velocities measured for specific channel locations where the fish were swimming. Screen contact rates are presented as total contact rates (the sum of tail and body contact rates) unless otherwise specified. Results expressed as rates (e.g., screen contact rates), proportions (e.g., survival), or indices were log10 transformed for statistical analyses but are presented untransformed in tables, figures, and equations. Fish velocities (i.e., velocity over the bottom) in the 0 cm/s sweeping velocity treatments are expressed as absolute values.
Comparisons among appropriate treatment groups (e.g., temperature, light level) were made by means of analysis of variance (ANOVA) and two-tailed t-tests. Linear and multiple regression analysis, analysis of covariance (ANCOVA), and correlation analyses were used for examining the effects of continuous variables (e.g., time, flow velocity, swimming velocity) and categorical covariates (e.g., temperature). For all general linear models presented, all coefficients are significant at the same level (P < 0.05) unless otherwise noted.
Results
Behavior
Swimming behavior of the young Chinook salmon differed among the four treatment groups and was significantly affected by flow in all of them (Figure 2). In daytime experiments, all fish generally swam steadily around the channel. In the low- and moderate-velocity regimes, the fish consistently swam in groups, but in the 62-cm/s sweeping velocity regimes, the fish tended to be more dispersed. During nighttime, fish did not swim in groups but were distributed regularly or randomly around the channel in all flows.
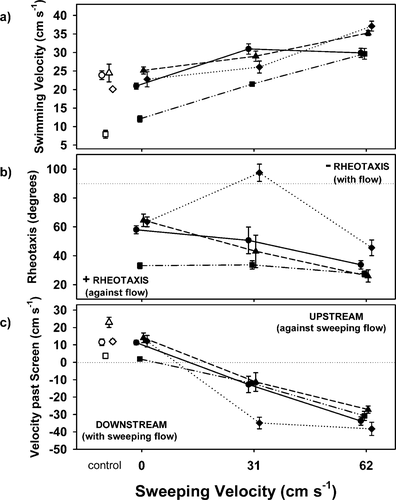
Effects of sweeping velocity on the swimming behavior of juvenile Chinook salmon. Panel (a) shows swimming velocity through the water; panel (b) shows rheotaxis, defined as swimming orientation relative to the resultant flow (0° = positive rheotaxis, 180° = negative rheotaxis; results from control experiments with no flow are not shown); and panel (c) shows screen passage velocity, defined as velocity over the ground. Positive values are for upstream movement relative to the sweeping flow; negative values are for downstream movement relative to the sweeping flow. Results for 0-cm/s sweeping flow treatments are absolute values. Within each sweeping flow treatment, the results from the three approach velocities are pooled. Each point is the mean (±SE) of the pooled results from replicate experiments. Symbols are as follows: circles = 12°C, small size-class, daytime; squares = 12°C, small size-class, nighttime; triangles = 12°C, large size-class, daytime; and diamonds = 19°C, large size-class, daytime. Closed symbols are for experimental flow regimes and open symbols for the corresponding controls. Points from the four treatment groups have been offset relative to the x-axis for visual clarity
Swimming velocity increased significantly with increases in flow (expressed as either sweeping, resultant, or measured velocity; regression, P < 0.05 for all tests) in all groups (Table 3), although during the daytime, the swimming velocities of the small 12°C fish were maximized at the intermediate sweeping velocity and did not increase with further increases in flow (Figure 2a). During nighttime, Chinook salmon swam significantly slower (mean difference = 8.6 cm/s) than daytime fish at comparable water velocities (ANCOVA, P < 0.05), with the swimming velocity difference decreasing with water velocity increases. Approach velocity did not significantly affect swimming velocity (P > 0.05). Swimming velocity also increased with increases in fish size; at comparable flows, larger fish swam faster than smaller fish in all groups (ANCOVA, P < 0.05 for all tests). Among the large fish, swimming velocities at comparable flows were not significantly affected by temperature (ANCOVA, P > 0.1).
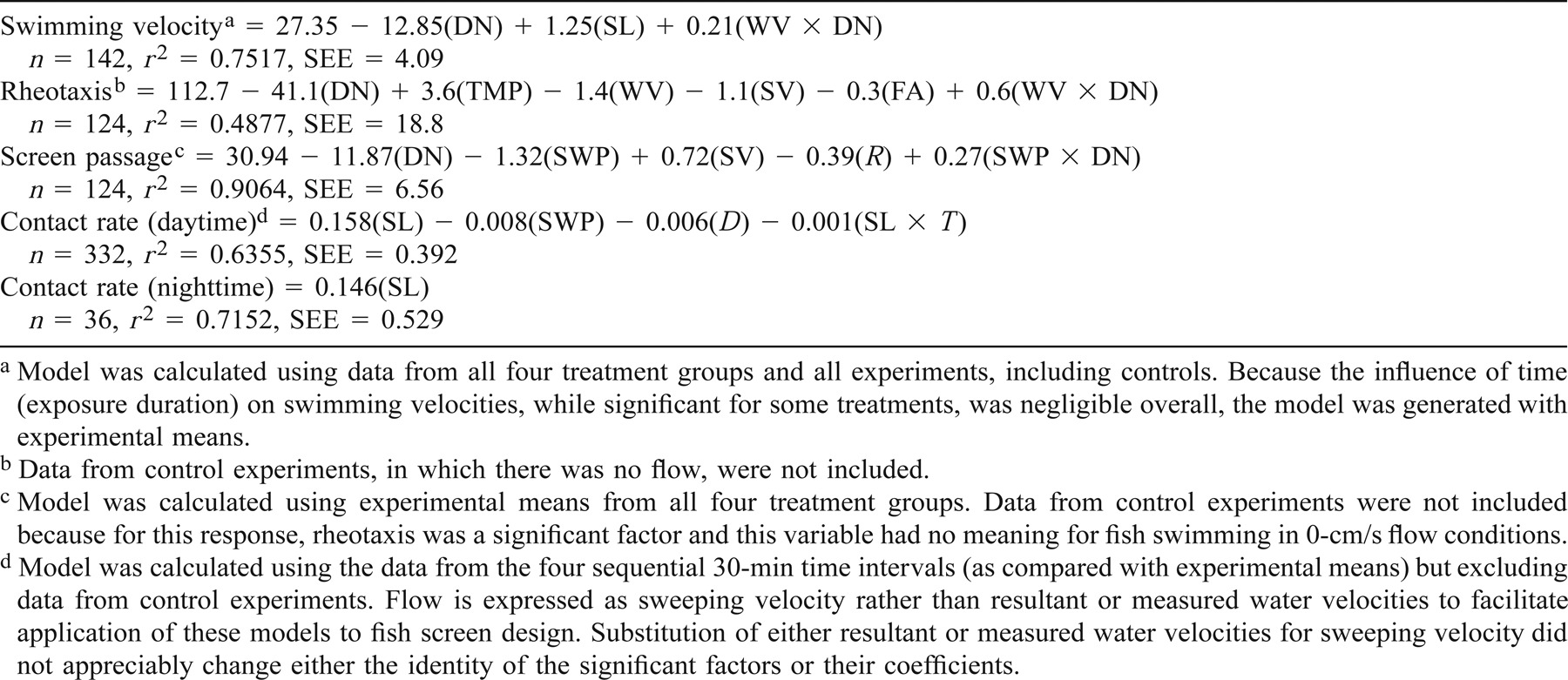
Juvenile Chinook salmon generally exhibited positive rheotaxis (Table 3), swimming against the resultant flow in all treatments and flow regimes with the exception of the large, 19°C fish (when swimming in moderate and, to a lesser extent, high flows), which tended to swim downstream (Figure 2b). Positive rheotaxis increased with increases in water velocity for all groups (regression, P < 0.05, all tests) except the large, 19°C fish (P > 0.05). For all groups, positive rheotaxis became stronger as the fish swam faster (regression, P < 0.05 for all tests). The strongest positive rheotaxis was exhibited by the small, 12°C fish during nighttime experiments. The poor rheotaxis of the large, 19°C fish in the 31-cm/s sweeping velocities reflected large proportions of the fish swimming downstream, an average of 69%, compared with 14–33% of the fish from the other groups at intermediate velocity flows. Rheotaxis did not differ significantly among the two size-classes tested that were acclimated to 12°C (ANOVA, P > 0.05). Within the large size-class, fish acclimated to 19°C exhibited significantly reduced positive rheotaxis compared with the 12°C fish (ANOVA, P < 0.01).
Screen passage velocity (velocity over the bottom) was strongly related to sweeping velocity (Figure 2c). For all fish, their velocity past the screen depended on sweeping velocity, swimming velocity, and rheotaxis (regression, P < 0.01 for all tests) and, except for the 19°C fish that exhibited reduced positive rheotaxis, was roughly equal to the difference between the sweeping velocity and the fish's swimming velocity (Table 3). Screen passage velocities for the 19°C fish were significantly higher, particularly at intermediate flow velocities in which these fish tended to swim downstream (ANOVA, P < 0.001 for 31-cm/s sweeping velocity and P < 0.01 for 62-cm/s sweeping velocity). In flow regimes without a sweeping flow, daytime-tested Chinook salmon tended to swim around the channel and, although the direction of travel was unpredictable, it was usually consistent throughout the 2-h test, thus resulting in net movement relative to the screen. In contrast, at night under these flow conditions, the fish showed no net movement along the screen.
While the fish utilized the entire channel, significant differences in preferred swimming locations among flows and treatment groups resulted in small but significant differences in water velocities to which the groups were exposed. In general, most fish swam within the inner half of the 62-cm-wide channel and 1–5 cm above the bottom. During daytime experiments, the distance from the inner screen increased with increases in sweeping velocity (regression, P < 0.01 for all tests), with fish swimming an average of 4.6–13.2 cm farther from the screen at the high sweeping velocity. The preferred swimming location of fish tested at night was not affected by flow, averaging 30.1 ± 1.1 cm from the screen (regression, P > 0.05). The approach velocity did not significantly affect swimming location relative to the screen (ANCOVA, P > 0.3 for all tests). Large fish swam significantly closer to the screen (ANCOVA, P < 0.001) than small fish, (e.g., 16.9 ± 1.3 cm from the screen compared with 28.4 ± 2.2 cm for small fish, in 62-cm/s sweeping velocities). Among the large fish, temperature did not affect swimming location (ANCOVA, P > 0.2). As a consequence of their closer proximity to the screen, large fish experienced significantly higher water velocities than the small fish in equivalent flow regimes (e.g., 57.5 ± 0.9-cm/s resultant velocity in the high sweeping velocity treatments compared with 52.7 ± 0.8 cm/s for the small fish; ANCOVA, P < 0.05).
Throughout the 2-h experiment, the swimming behavior of the fish was generally consistent, with few significant variations among the sequential 30-min intervals. However, for all groups except the small 12°C fish tested at night, swimming velocities in the 62-cm/s sweeping velocity treatments decreased significantly with exposure duration (regression, P < 0.05 for all tests; mean difference = 6.5–7.5 cm/s for the 12°C fish and 14.9 cm/s for the 19°C fish), with most of the decrease occurring in the first 60 min. Swimming velocities in the 0 and 31 cm/s sweeping velocities did not vary with time (regression, P > 0.05 for all tests). All fish in the 12°C treatments exhibited consistent rheotaxis throughout the 2-h experiment. In contrast, large fish acclimated to 19°C and tested in the 31-cm/s sweeping velocity, after displaying initial positive rheotaxis, reversed swimming direction after the first 30-min of the experiment (regression, P < 0.001). In the high sweeping velocity treatment, these fish also showed a trend of reduced rheotaxis as the experiment proceeded (regression, P = 0.07). For these fish, reduced rheotaxis and, in the high sweeping velocity, reduced swimming velocity, corresponded to significant increases in downstream screen passage velocity (regression, P < 0.01 for both tests). Preferred swimming locations generally did not change with time, and the water velocity to which the fish were exposed remained constant throughout the experiment (ANCOVA, P > 0.4 for all tests).
Screen Contact and Impingement
Chinook salmon experienced frequent temporary contact with the screen but rarely became impinged on it and unable to escape; in 164 experiments, only eight fish in six different experiments were impinged (<0.3% of all Chinook salmon tested). The impingements occurred at both temperatures, in daytime and nighttime experiments, and in 5 of the 10 flow treatments. There were no detectable relationships between incidences of impingement and any experimental variable.
Screen contact rates were significantly affected by sweeping velocity (regression, P < 0.01), time of day–light level (ANOVA, P < 0.05), and fish size (ANCOVA, P < 0.01), but not temperature (ANOVA, P > 0.05; Table 3; Figure 3a). Increases in approach velocity resulted in small but significantly higher contact rates in only one treatment group, the large 12°C fish (ANOVA and regression, P < 0.05, with significant differences in only the 31-cm/s sweeping velocity); therefore, for all subsequent analyses, approach velocities were pooled within sweeping velocity treatments. In all daytime treatments, Chinook salmon contacted the screen more frequently in the absence of a sweeping flow (mean = 0.62–0.93 contacts/fish/min for 0-cm/s sweeping velocity flow regimes, compared with 0.10–0.21 contacts/fish/min in the 62-cm/s sweeping velocities) when water passed through the screen perpendicularly rather than at an oblique angle. Screen contact rates at the 31-cm/s sweeping velocity were intermediate, and the decreases in screen contact frequency with increases in sweeping velocity were generally linear. At night under dark conditions, screen contact rates in the experimental flow regimes were consistently high (mean = 0.81 ± 0.05 contacts/fish/min) and unaffected by either approach or sweeping velocity (regression, P > 0.2). However, even within the small size range tested (4.4–6.4 cm SL), nighttime screen contact rates increased significantly with increases in fish size (regression, P < 0.001). Among fish acclimated to 12°C and tested during the daytime, larger fish contacted the screen more frequently than smaller fish in comparable flow regimes (ANCOVA, P < 0.001). Screen contact rates in all control experiments with no flow were consistently low (mean = 0.06 ± 0.01 contacts/fish).

Effects of sweeping velocity on fish–screen interactions for juvenile Chinook salmon: Panel (a) shows the screen contact rate; panel (b) shows the screen contact severity as a proportion of total contacts that were body contacts; and panel (c) shows postexperiment injuries expressed as an injury index that combines injury rates and injury severity (an injury index of 10 = no injuries). See the caption to Figure 2 for additional details.
Most screen contacts were tail contacts, only 20% (range = 2–66%) of total contacts being body contacts among all experiments. For all 12°C groups, the relative proportions of body contacts increased significantly with increases in sweeping velocity (regression, P < 0.05, all tests; Figure 3b) although, for daytime treatments, the corresponding reduction in total contact rates at higher sweeping velocities (Figure 3a) resulted in no increases in the frequency of body contact with the screen. During nighttime experiments, the proportion of body contacts also decreased slightly with increases in approach velocity (regression, P < 0.01), but the proportion of body contacts was significantly higher in nighttime experiments than daytime experiments (ANCOVA, P < 0.001).
Screen contact rates decreased significantly for the larger fish in both temperatures (ANCOVA, P < 0.05) as the experiment progressed but not for the small, 12°C fish during the daytime (P > 0.2 [although the contact rate did show a significant decrease {regression, P < 0.05} in the 62-cm/s sweeping flow treatments) or nighttime (P > 0.4). On average, the contact rates for the large fish were 92% higher during the first 30 min of exposure than during the final 30 min.
Injury and Survival
The injury rates of both preexperiment and experimental fish were generally high but most injuries consisted of minor damage to fins and scales. Among the four treatment groups (Table 1), significant differences in injury indices were apparently related to the duration of laboratory holding, with larger, older fish exhibiting more damage (Figure 3c). Within treatments, the injury index was not significantly affected by either flow regime or screen contact rate (regression and correlation, P > 0.3, all tests) and, in general, preexperimental indices were similar to those measured for fish after exposure in the Fish Treadmill.
Survival in all experiments was high. Of the more than 3,200 fish tested, only five fish from four experiments died during the experiment and one fish, from a fifth experiment, during the 48-h postexperiment period. Two of the mortalities were from daytime experiments and four were from nighttime experiments. All mortalities were from flow treatments with a sweeping flow component, but the small number precluded the detection of significant flow effects on survival. The death of these fish did not appear to be related to observed impingements.
Discussion
Swimming Behavior and Fish–Screen Interactions
In response to a flow stimulus, young Chinook salmon were competent and enthusiastic swimmers. Under all flow conditions except the nighttime control (0 cm/s), most fish swam relatively steadily and in a directed manner, usually exhibiting strong positive rheotaxis. Variations in swimming behavior were attributable to both environmental and biological factors. Increases in water velocity stimulated juvenile Chinook salmon to swim faster and, with the exception of the large fish acclimated to the warmer water temperature, to focus their efforts on swimming upstream and, perhaps, holding station. This is consistent with the expected behavior of the smaller, parr-size fish that, at this life stage and in these seasonal environmental conditions, would likely still be resident in tributary stream habitats (Healy 1991; Moyle 2002). This behavior was also evident at night when, despite significantly slower swimming velocities, enhanced positive rheotaxis resulted in no net change in the downstream movement of the fish in comparable flow regimes. The downstream directed swimming of the larger (and older) smolt-sized fish in 19°C, particularly in the intermediate water velocities, probably reflects the behavioral shift towards out-migration, in this case associated with elevated water temperature and, perhaps, larger size.
At high water velocities (i.e., flow regimes with a 62-cm/s sweeping velocity), juvenile Chinook salmon achieved and sustained velocities comparable to the critical swimming velocities measured for conspecifics of similar size in other studies (Castleberry et al. 1991; Katzman 2001; authors' unpublished results). The significant decline in swimming velocities as the exposure progressed, observed only in these high velocity flow regimes, suggested initial velocities may, in fact, have exceeded sustainable (for 2 h) levels. This interpretation is further supported by the results from a separate study with these fish (12°C and 19°C, large size-class) that showed significantly elevated plasma lactate levels, an indication of anaerobic metabolic activity, at 0- and 30-min postexposure (P. S. Young, unpublished results). This suggests that exposure to a screened water diversion situated or engineered to function in moderate-to-high velocity flow conditions could be energetically expensive for young Chinook salmon.
The movement of young Chinook salmon along the length of the screen was controlled by the sweeping velocity and their swimming behavior. Despite generally strongly directed, velocity-dependent swimming, none of the fish were able to hold station in the face of the moderate sweeping flow (31 cm/s), a velocity demonstrably within their swimming capabilities (see Figure 2), and all were rapidly swept downstream at higher flows. Assuming the downstream movement of the positively rheotactic fish was involuntary, the failure to hold station in the intermediate flows was probably related to two factors. First, juvenile Chinook salmon did not exhibit perfect positive rheotaxis, particularly during the daytime. During the day, under lighted conditions, their swimming orientation was typically intermediate between the direction of the resultant flow and the fish screen surface, suggesting the fish were supplementing their rheotactic response to flow with a visual response to the fish screen to guide their swimming behavior. At night, when visual cues were absent, positive rheotaxis was enhanced, but the concomitant reduction in swimming velocity resulted in no net differences in downstream movement rates. Second, unlike most natural stream habitats, hydraulic conditions in the Fish Treadmill were relatively uniform and laminar; the test channel was specifically designed to eliminate (or to at least minimize) the types of velocity refugia, eddies, and marginal low-velocity areas that young Chinook salmon regularly exploit (Healy 1991; Moyle 2002). Therefore, even momentary reductions in swimming effort by the fish resulted in displacement downstream (as well as an increased likelihood of screen contact). While screen passage velocity (and, inversely, exposure duration at a screened water diversion) was strongly related to sweeping velocity, the results do not support the assumption generally held by fish screen designers and fisheries resource managers that the two are equivalent. Rather, they underscore the importance of diversion and fish screen designs that incorporate screen lengths and sweeping velocities calibrated to satisfy desired or mandated exposure durations and that minimize low-velocity conditions near the screen that could attract and entrain passing fish, as well as increase the likelihood of those fish contacting the screen.
The ability of juvenile Chinook salmon to avoid contact with the fish screen was not apparently related to their swimming capabilities but rather to their “level of effort” and visual acuity. During the day, when the fish relied on visual cues (as evidenced by consistent schooling behavior and preferred swimming locations within the channel), the highest screen contact rates occurred at water velocities substantially below the species' known swimming capacities as well as their swimming velocities measured at the time. In the low-velocity, “approach-flow-only” treatments, when the fish swam relatively slowly, close to the screen, and with less precise orientation to the flow, the probability of a fish contacting the screen was relatively high. Increases in water velocity and the corresponding increases in swimming effort, orientation, and distance from the screen resulted in less frequent contact. However, the greatest diurnal differences in screen contact rates—a four- to eight-fold increase in contact frequency at night—occurred in high-velocity flow regimes in which the fish exhibited nearly identical swimming behavior, strong positive rheotaxis, and near maximal swimming velocities. Nighttime observations of the fish indicated that, while the fish detected and responded to flow even in the relatively laminar flow field of the Fish Treadmill, they were unable to avoid the screen or even other fish; on several occasions, fish were observed bumping into each other. The inability of the fish to detect the screen in the dark could relate to the porous nature of the structure and a reduced (or absent) turbulent boundary layer near its surface that might alert the fish to its presence. Fish screens such as the type used in the Fish Treadmill are specifically designed to facilitate uniform flow conditions at and near the screen surface (Clay 1995; Hayes et al. 2000), in part to avoid exceeding regulatory approach velocity standards. Thus, for a fish, a properly designed fish screen could represent an anomalous and, under conditions of low visibility (including turbid waters), possibly undetectable structure to which they are incapable of either responding appropriately or avoiding.
On the other hand, although juvenile Chinook salmon experienced frequent contact with the screen under a wide range of flow velocities, such contact was apparently not harmful or lethal, at least in the short term (i.e., within 48 h) under the conditions used in the experiments. Further, injury rates and severity were not correlated with either contact frequency, contact severity, or flow velocity conditions, suggesting that the types of physical contacts the fish experienced in the Fish Treadmill were not immediately injurious. Given that in some experiments (e.g., most nighttime experiments) a single fish (on average) contacted the screen as many as 100 times during the 2-h exposure, this result may indicate that the smooth surface and narrow bar spacing features of the vertical wedgewire screen used in the Fish Treadmill is effective protection for fishes drawn too close to a water diversion. However, the high survival measured in these studies could also reflect the relatively benign environmental conditions in which the fish were tested and held postexperiment, particularly with respect to the absence of predators that might more successfully prey upon fish entrained in an artificial flow regime or disoriented by an involuntary screen contact. These results may also provide insight regarding the effectiveness of fish screens for preventing the loss of fish at unscreened diversions. Assuming that young Chinook salmon respond similarly to artificial flows in the wild as they did in the Fish Treadmill and that a screen contact event observed in the apparatus could correspond to lethal entrainment into a diversion without a screen, the screen contact rates measured for the fish under a range of flow, environmental, and biological conditions and described in the general linear models presented in Table 3 may approximate the degree to which the fish are vulnerable to entrainment loss. For example, for a group of 6-cm Chinook salmon swimming within 31 cm of an unscreened diversion for a period of 1 min in 12°C water flowing at 31 cm/s past the diversion (i.e., a 31-cm/s sweeping velocity), on average, 3 in 10 fish (33% of the group) could be entrained. At night, the rate of lethal entrainment would be higher, nearly 9 of 10 fish (88%).
Applications for Fish Screen Design and Operation
The studies described here were designed to examine the swimming performance and behavior of young Chinook salmon as related to two-vector flows, life stage, temperature, and time of day (light level). The results are applicable to address fish screen design and operation questions, especially regarding the calibration of water diversion fish screens within Chinook salmon habitat to optimize the protection of this threatened species. The results clearly suggest that, for this species, installation of fish screens to prevent the loss of juveniles at diversions located in their rearing and migratory habitat is effective and, probably, safe for the fish.
Contrary to most expectations (as well as current screen flow criteria for Chinook salmon; CDFG 2000; NMFS 1997), approach velocities within the range tested had no detectable effects on the behavior, performance, or survival of juvenile Chinook salmon exposed to a simulated fish screen. Further, periodic contact with the screen, hypothesized to be a function of approach velocity, exposure duration, or both but based on these results, in fact dependent on sweeping velocity and time of day–light level, was apparently not harmful, at least with the type of screen used in the Fish Treadmill. However, while screen contact was not injurious (or lethal) in the laboratory, for optimal protection, such potentially disorienting events should certainly be minimized for fish in the wild. Manipulation of the sweeping flow component of screen flow criteria appears to offer an effective strategy for facilitating the passage of exposed fish by the screen as well as minimizing the probability of screen contact. For example, using the general linear models developed from Fish Treadmill results (Table 3), a 6-cm Chinook salmon exposed during the day to within 31 cm of a 20-m-long fish screen in 12°C water and operated at the present screen flow criteria required by the California Department of Fish and Game (approach velocity ≤10 cm/s; sweeping velocity >2× approach velocity; CDFG 2000) would, on average, require more than 5 min to pass the screen, during which the fish would likely contact the screen three times. Doubling the sweeping velocity to 42 cm/s would cut exposure duration to less than 2 min and expected contacts during the exposure to less than one per fish. For nighttime exposure, the increased sweeping velocity decreases predicted exposure duration and contact probability by roughly 50%. Federal screen criteria, which require only that the sweeping velocity be greater than the maximum 10-cm/s approach velocity (NMFS 1997), appear to be less protective: for the same 20-m screen operated with a 15-cm/s sweeping velocity, a juvenile Chinook salmon would be expected to contact the screen eight times during the nearly 15-min exposure. Higher sweeping velocities (e.g., 62 cm/s) further decrease exposure duration and contact probability, but with the possible trade-off of higher energetic cost to the fish because these flow conditions stimulate high-intensity swimming activity that may be fueled by anaerobic metabolism. For young Chinook salmon subjected to prolonged exposure at a single large screened diversion or repeated exposures to multiple screens in their habitat or along their migratory route, the cumulative energetic costs could be substantial. Finally, with the exception of the marked difference in downstream passage behavior, the responses of the two (presumably) physiologically different life stages were very similar, particularly with respect to screen contact, injuries, and survival, suggesting that fish screen criteria for this species need not vary geographically or temporally. Collectively, the results indicate that, for juvenile Chinook salmon, optimal fish screen design should be guided by the objective of minimizing screen exposure duration, largely through balancing screen size (or length) with prevailing or engineered sweeping velocities.
Acknowledgments
The biological studies using the Fish Treadmill are a collaborative effort with many people from several departments and agencies. We would like to thank the University of California, Davis, J. Amorocho Hydraulics Laboratory, Department of Civil and Environmental Engineering, and M. L. Kavvas and his students, research, and technical staff for the design, construction, and operation of the Fish Treadmill; the California Departments of Fish and Game and Water Resources; NOAA Fisheries; the U.S. Bureau of Reclamation; N. Willits and H. Zhou for statistical assistance; and our student, postgraduate, and postdoctoral research assistants from the University of California, Davis, Department of Wildlife, Fish, and Conservation Biology. We are grateful for the helpful suggestions and comments of K. Bates, Washington State Department of Fisheries and Wildlife, and N. Taft, Alden Research Laboratories. This project was funded by the California Department of Water Resources, U.S. Bureau of Reclamation, CALFED Bay-Delta Program, and the Anadromous Fish Screen Program (U.S. Bureau of Reclamation and the U.S. Fish and Wildlife Service).