Relationship among Fish Assemblages and Main-Channel-Border Physical Habitats in the Unimpounded Upper Mississippi River
Abstract
Large rivers worldwide have been altered by the construction and maintenance of navigation channels, which include extensive bank revetments, wing dikes, and levees. Using 7 years of Long-Term Resource Monitoring Program (LTRMP) data collected from the unimpounded upper Mississippi River, we investigated assemblages in two main-channel-border physical habitats—those with wing dikes and those without wing dikes. Fishes were captured using daytime electrofishing, mini-fyke netting, large hoop netting, and small hoop netting. Our objectives were to (1) assess associations among fish species richness, physical measurements, and main-channel-border physical habitats using stepwise multiple regression and indicator variables; (2) identify abundant adult and young-of-year (age-0) families in both physical habitats to further investigate assemblage composition; and (3) calculate standardized species richness estimates within each physical habitat for adult and age-0 fishes to provide additional information on community structure. We found species richness was greater at wing dikes for both adult and age-0 fishes when compared with main channel borders. Stepwise multiple regression revealed significant relationships between adult species richness and passive gear deployment (e.g., hoop nets and mini-fyke nets), physical habitat type, and river elevation, as well as interactions between physical habitat and passive gears, and physical habitat and transparency (i.e., Secchi depth). This model explained 56% of the variance in adult species richness. Approximately 15% of the variation in age-0 species richness was explained by the sample period, sample date, transparency, physical habitat, and depth of gear deployment. Long-term impacts of river modifications on fishes have not been well documented in many large river systems and warrant further study. The findings from this study provide baseline ecological information on fish assemblages using main channel borders in the unimpounded upper Mississippi River, information that will aid managers making channel maintenance decisions in large river systems.
Introduction
Many large rivers worldwide are regulated and the development of these riverine systems has caused a decline in spatial and temporal habitat heterogeneity (Dister et al. 1990; Shields 1995). In the past 100 years, the number of waterways modified for navigation has grown from 9,000 to almost 500,000 (Abramovitz 1996). Large rivers in the United States have been managed and altered for navigation since the early 1800s, and the effects have been significant for many systems (Carlander 1954; Koebel 1995). For example, the Kissimmee River in Florida was channelized in the late 1940s, creating a straight canal from a formerly braided river (Koebel 1995). This system experienced a loss in available dissolved oxygen because of the buildup of organic matter and decreased floodplain wetlands (approximately 14,000 ha), a decrease in both habitat and fish diversity, and an increase in the colonization of exotic vegetation (Dahm et al. 1995; Koebel 1995; Toth et al. 1995). Because habitat degradation was significant, large-scale restoration projects are being implemented in an attempt to restore prechannelization water flow and reestablish floodplains to increase vegetation and wildlife production (Koebel 1995). The Missouri River system has been altered by dams and channelization (Hesse and Mestl 1993). These alterations have affected the hydrograph, sediment transport, and floodplain connectivity, aiding in the decline of native species such as the pallid sturgeon Scaphirhynchus albus, paddlefish Polyodon spathula, flathead chub Platygobio gracilis, and blue sucker Cycleptus elongatus (Hesse and Mestl 1993). A restoration plan has been developed for this system and altering discharge to create a more “natural” hydrograph is being considered (Hesse and Mestl 1993).
Channelization of the upper Mississippi River (UMR) began in 1878 when the U.S. Congress authorized the creation of a 1.8-m navigation channel. Manmade structures (such as wing dikes, closing structures, and levees) were constructed to maintain channel depth (Rasmussen 1979). Congress decided to further modify the UMR by creating a low-water navigation channel in the unimpounded UMR (that portion of the UMR extending south from the confluence with the Missouri River to the confluence with the Ohio River). This was achieved by extending existing wing dikes and building new dikes. The reduction in the width between the distal ends of the wing dikes further restricted water flow into secondary side channels and reduced access to these backwater physical habitats by aquatic organisms (Rasmussen 1979). Eight hundred wooden pile dikes were constructed in the unimpounded UMR reach and most have been replaced with rock (Mueller 1977). The U.S. Army Corps of Engineers (USACE) has Congressional authority to maintain the 2.7-m navigation channel through dredging, closing structures, and the creation/maintenance of wing dikes (Rasmussen 1979; Farabee 1986).
Wing dikes alter main channel width, depth, and direction, and may be the most significant human impact to river systems (Conner et al. 1983). Wing dikes and levees also alter hydrologic regimes, degrade aquatic environments, restrict natural river meandering (Rasmussen 1979; Yin and Nelson 1995; Pitlo 1998), and degrade the main channel bed (Mueller 1977). For example, the main channel bed in the unimpounded UMR is approximately 3.4-m deeper than it was in the 1800s before wing dikes were constructed (Mueller 1977). Additional damage reported from wing dike construction includes the reduction of natural lentic habitats, the loss of many deep holes associated with snags in unchannelized river systems, and the disjunction of low-velocity water associated with side channels from the main river channel (Rasmussen 1979; Beckett et al. 1983). Few studies have assessed the impact of wing dikes on fishes in riverine systems, and many large river studies have focused on guilds (e.g., populations of a few species) or single-species instead of multispecies assemblages (but see Farabee 1986; Poizat and Pont 1996). A shift from the population-level approach to an approach viewing community units as multispecies assemblages is necessary to address questions regarding biodiversity, understand biological processes, and successfully restore large river systems (Wiens 1989; Poizat and Pont 1996).
Using Long-Term Resource Monitoring Program (LTRMP) data, we investigated fish assemblages in main-channel-border physical habitat (naturally occurring but altered by channelization maintenance) and wing dike physical habitat (artificial rock structure) to understand better the impact of wing dikes on fishes. The objectives of this study were to (1) assess associations among fish species richness (i.e., number of species), physical measurements, and main channel border physical habitats using stepwise multiple regression and indicator variables; (2) identify abundant adult and young-of-year (age-0) families for each physical habitat to further investigate assemblage composition; and (3) calculate standardized species richness estimates within each physical habitat for adults and age-0 fishes to provide additional information on community structure. We hypothesized that fish species preferring low-velocity physical habitats would seek refuge in the low-velocity water and/or scour holes created by wing dikes because access and the availability of offshore areas have been reduced in the unimpounded UMR system (Rasmussen 1979; Barko and Herzog 2003). The findings from this study provide baseline ecological information on fish assemblages using channel borders in the unimpounded UMR that will aid managers making channel maintenance decisions in large river systems.
Methods
Study area
The unimpounded UMR reach is located between the confluences of the Missouri and Ohio rivers (see Figure 1 in Pitlo 2002). Our study was conducted in this reach between river kilometers (RKM) 48 and 129, as measured from the confluence of the Ohio River. Two hundred and eleven dikes occur in this section of river monitored by the LTRMP (D. Ostendorf, unpublished data). This section of the UMR lacks submerged and floating-leaf vegetation and is turbid because of suspended sediment loads (Rasmussen 1979; Yin and Nelson 1995). River channel meandering has been restricted by channelization and the construction of levees and wing dikes (Simons et al. 1975). The extensive levee system built along this reach has resulted in aquatic changes, such as a gradual loss of depth heterogeneity throughout the river (Theiling 1999) and increased turbidity (Sparks et al. 1990). In addition, increased sedimentation between wing dikes has reduced river width (Chen and Simons 1986) and contributed to the loss of side channels (Simons et al. 1975).

Identification of wing dike (MCWD) and main channel border (MCBU) physical habitats sampled in the unimpounded upper Mississippi River (UMR) from 1994–2000
Sampling design
Fish sampling and physical habitat measurements were conducted in wing dike and main-channel-border locations using a monitoring protocol developed by the LTRMP (Gutreuter et al. 1995). Main-channel-border physical habitat was defined as the zone between the margins of the main navigation channel and the nearest shoreline without wing dikes. Wing dike physical habitat was defined as main channel border with a wing dike as the main physical structure (Figure 1). All gears set downstream of wing dikes were within 50 m of the structure, and gears set upstream of the structure were along the bank within 10 m of the structure. The area defining wing dike physical habitat varied in size relative to the size of the downstream scour and the geographic location of the individual dike relative to other dikes in the dike field.
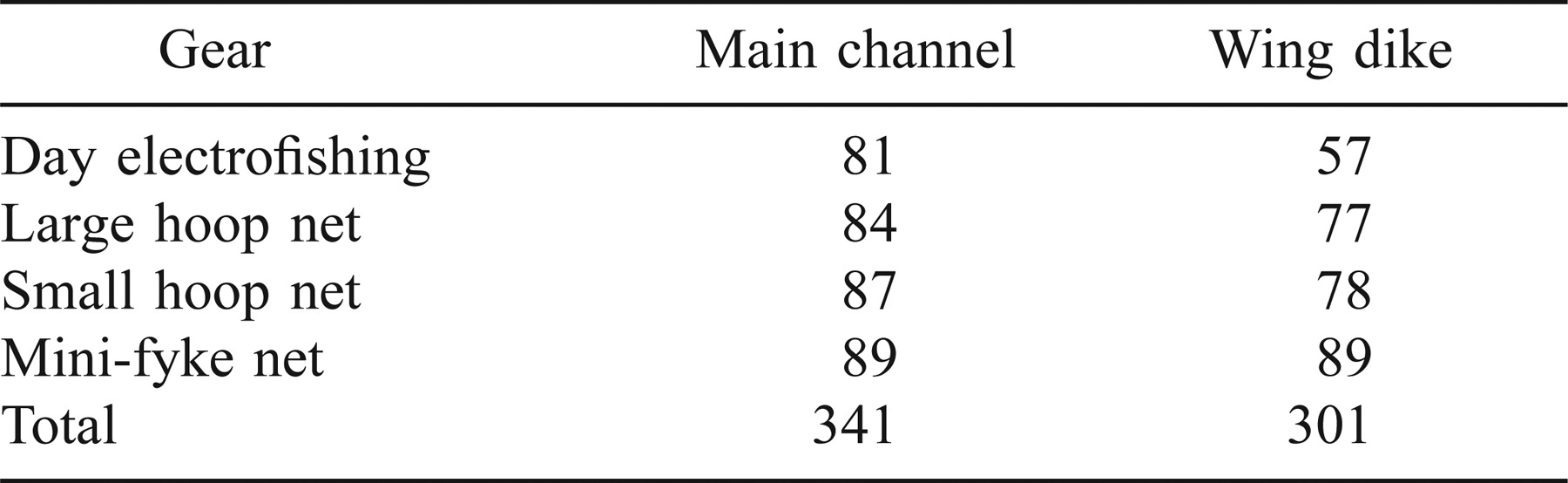
Fishes were sampled from 1994 to 2000 during three annual sampling periods (June 15–July 31, August 1–September 15, and September 16–October 30; Gutreuter et al. 1995). Only four sampling gears (daytime electrofishing, mini-fyke nets, large hoop nets, and small hoop nets) were consistently fished in both physical habitats and included in the analyses (Table 1). An array of gears was used because no single gear captures all species and size categories (Gutreuter et al. 1995; Hayes et al. 1996). Daytime electrofishing was conducted from a 5.5-m flatbottom aluminum boat, usually between 1000 and 1600 hours, using pulsed DC set at a 60 hz pulse frequency and a 25% duty cycle. Each sampling session lasted 15 min and covered an area approximately 200 m long and 30 m wide (see Figure 1 in Gutreuter et al. 1995). All electrofishing was conducted parallel to the shore in main-channel-border physical habitat. At wing dikes, electrofishing began on the downstream, nearshore side of the dike and continued around the dike until the 15-min time period expired (see Figure 1 in Gutreuter et al. 1995). Wisconsin-type mini-fyke nets with lead, frame, and cab sections were used, and all netting material had a 3-mm diameter mesh. Mini-fyke nets were set for approximately 24 h (i.e., 1 net-day) and were positioned approximately perpendicular to the shore or dike (see Figure 3 in Gutreuter et al. 1995). These nets were set along the shoreline on the upstream side of wing dikes. Large and small hoop nets were deployed as pairs (i.e., parallel sets), baited with 3 kg of soybean cake, and fished with the open end of the net facing downstream (see Figure 2 in Gutreuter et al. 1995). The bar mesh size of the small hoop nets was 1.8 cm, with the first hoop being 0.6 m in diameter. The bar mesh size of the large hoop nets was 3.7 cm, with the first hoop being 1.2 m in diameter. Both nets were set for 48 h. At wing dikes, sampling was conducted within the scour holes. For all gears, only nonsubmerged wing dikes were sampled. All captured fishes were identified to species, enumerated, and measured to the nearest millimeter.
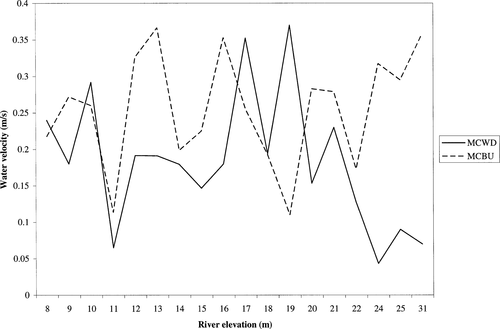
Relationship between average water velocity and river elevation measured at wing dike and main channel border physical habitats in the unimpounded UMR from 1994–2000
Sample sites were determined for each physical habitat (main channel border and wing dike) prior to the sampling season using a geographic information system (GIS) to overlay a 50 × 50-m grid on the study area. Site locations were randomly chosen for each sampling gear within each physical habitat for each period. Alternate sites were also chosen and used if the targeted site was inaccessible (i.e., dry). Because of the random sampling design, the sites could be unique or duplicate. If two gears were randomly selected for the same site, 24 h had to expire between the sampling episodes. LTRMP protocol required four sites to be sampled within each period for each gear type (e.g., 16 samples per period). Because of logistic or environmental constraints, this was not possible each year, hence the different number of samples between the physical habitats across gears (Table 1). At each site, measurements of water temperature, Secchi transparency (e.g., Secchi depth), the depth of gear deployment, water velocity, and conductivity were made prior to fish sampling. These variables were chosen because they are often reported as factors that influence fish–habitat associations (Jones and Hoyer 1982; Hayes et al. 1996). Water temperature was measured to the nearest 0.1°C, and conductivity was measured in μS/cm using a Labcomp digital conductivity meter. A Marsh–McBirney meter (model 201 D) was used to measure water velocity (e.g., current speed) to the nearest 0.01 m/s. The depth of gear deployment was measured to the nearest 0.1 m using boat-mounted sonar. River elevation (measured at Cape Girardeau, Missouri) was obtained from the U.S. Geological Survey for each day of sampling.
Statistical analyses
Because we were interested in assessing assemblage structure and little information is known about large river age-0 fishes, we separated age-0 fishes from adult fishes using reported lengths for each species (see Carlander 1969, 1977; Becker 1983; Etnier and Starnes 1993; Morrow and Kirk 1997; Pflieger 1997; Gido et al. 2000). We took a conservative approach and separated adults from age-0 individuals using the smallest adult length reported for each species. Stepwise multiple regression (SAS 1989) was used to assess associations among both adult and age-0 fish species richness, physical measurements, and main-channel-border physical habitats. We investigated species richness because this variable is the most widely used diversity measure and has been reported to be a crucial concern when conserving and managing biodiversity (May 1988; Stirling and Wilsey 2001). A Shapiro–Wilk test was performed on each measured variable (SAS 1989) and revealed no significant deviations from normality (P < 0.001). Therefore, the underlying distribution was approximately normal and the sample mean should converge on the true mean with increasing sample size. We used stepwise multiple regression with indicator variables in an effort to produce a parsimonious and unbiased model describing the relationship between the predictor and response variables. Three indicator variables (Neter and Wasserman 1974; Kullberg and Scheibe 1989; Scheibe and Robins 1998) were used to characterize the four gear types. Thus, X1 = 1 if the gear type used was mini-fyke, and 0 otherwise; X2 = 1 if large hoop nets were used, and 0 otherwise; and X3 = 1 if small hoop nets were used, and 0 otherwise. Active daytime electrofishing was therefore the default condition (X1, X2, X3 = 0). Similarly, physical habitats were coded as X4 = 1 if sampling occurred within wing dikes, and X4 = 0 if sampling occurred in the main channel border.
The indicator variables used as main effects and interaction terms in the regression model permitted a direct analysis of both the significance and magnitude of that component. For example, consider an indicator variable X2 in the model
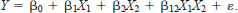
If X2 = 0, the model becomes

whereas if X2 = 1, the model is
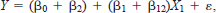
where significance of the parameter associated with X2 provides a new intercept (β0 + β2) and a new slope (β1 + β12) for the model. We deemed it necessary to code data based on gear type because sampling effort differed among methods, and active and passive techniques probably did not sample fishes equally (Hayes et al. 1996). This approach obviated the need for multiple pairwise comparisons, and enabled a determination of gear type effects and physical habitat differences. Furthermore, the use of stepwise regression with type II sums of squares (SAS 1989) provides relatively unbiased and parsimonious models. We used the default significance parameters of 0.15 for entry and removal from the models because we were more interested in relationships than statistical significance.
The regression model for adult fishes had the form
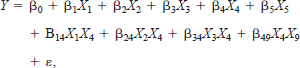
where X1 represents mini-fyke netting, X2 represents large hoop netting, X3 represents small hoop netting, X4 represents the physical habitat types, X5 represents river elevation, X6 represents Secchi transparency (e.g., depth), and the remaining terms represent interactions. The regression model for age-0 fishes had the form
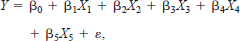
where X1 represents sample date, X2 represents physical habitat types, X3 represents Secchi transparency (e.g., depth), X4 represents depth of gear deployment, and X5 represents sample period.
Sample date was analyzed separately from sample period to test for autocorrelation of error terms using the Durbin–Watson d statistic (SAS Institute, Inc. 1989). A d statistic close to 2 represents uncorrelated error terms (SAS 1989).
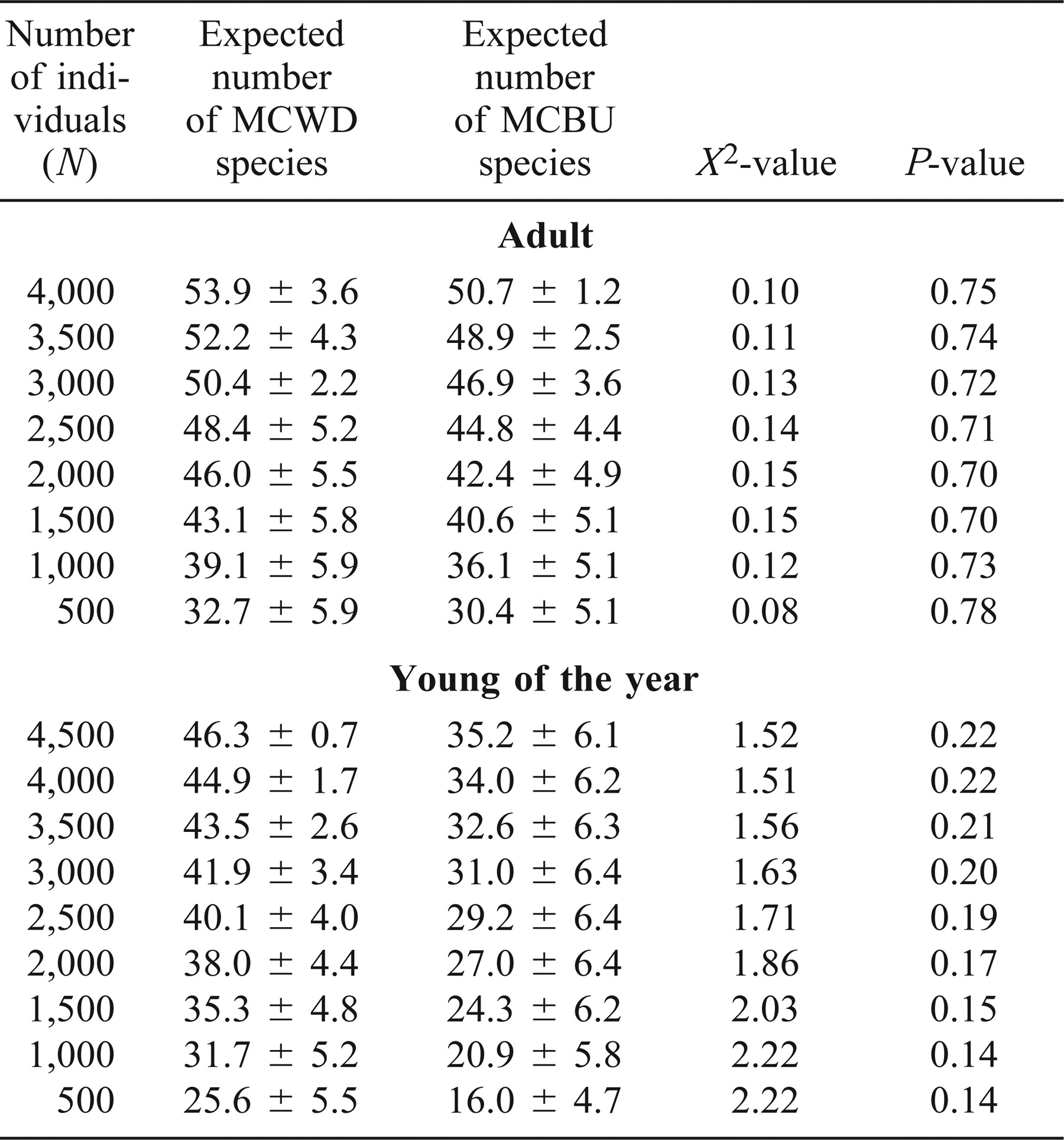
Because sample size varied between physical habitats, we used rarefaction to compare species richness between main channel border and wing dikes for both adult and age-0 fishes (Sanders 1968; Hurlbert 1971; Simberloff 1972). Before using rarefaction, samples were standardized to correct for differences in effort allocation between the physical habitats. We randomized and resampled to the smallest sample size (effort allocation) across years per gear and age-class. Rarefaction is a statistical method used to estimate the number of species one would expect to capture if the sample contained a user-defined number of individuals (N; Sanders 1968; James and Rathburn 1981; Krebs 1999). This method was appropriate because the fish assemblages being compared were taxonomically similar, and fishes were captured using the same sampling techniques (Sanders 1968; Simberloff 1979). A chi-square statistic was calculated to test for significant differences in species richness between main channel borders and wing dikes for both adult and age-0 fishes (Steel and Torrie 1980).
We identified the most abundant adult and age-0 families (>10% of overall abundance), and chi-square statistics were calculated to test for differences between main channel borders and wing dikes (Steel and Torrie 1980). Differences in sample size were corrected by determining the percentage of samples taken in each physical habitat (i.e., 53% in MCBU and 47% in MCWD), then multiplying this percentage by the total number of fish captured for a family. This conversion gave us the “expected” number of individuals, which was compared with the actual number of individuals captured (“observed”).
Results
Three hundred and one wing dike samples and 341 main channel border samples were taken over a 7-year period (Table 1). At wing dikes, we captured a total of 5,949 adult fishes representing 59 species and 15 families, and 4,855 age-0 fishes representing 47 species and 14 families. In main channel borders, we captured 5,971 adult fishes representing 54 species and 14 families, and 19,769 age-0 fishes representing 51 species and 15 families (Table 2). Rarefaction methods consistently revealed a greater species richness of adult fishes in wing dike physical habitat when compared with main-channel-border physical habitat (Table 3). However, chi-square tests did not reveal any differences for any value of N. The species richness of age-0 fishes was consistently greater in wing dike physical habitat when compared with main channel border physical habitat, yet chi-square tests did not reveal any significant differences for any value of N.
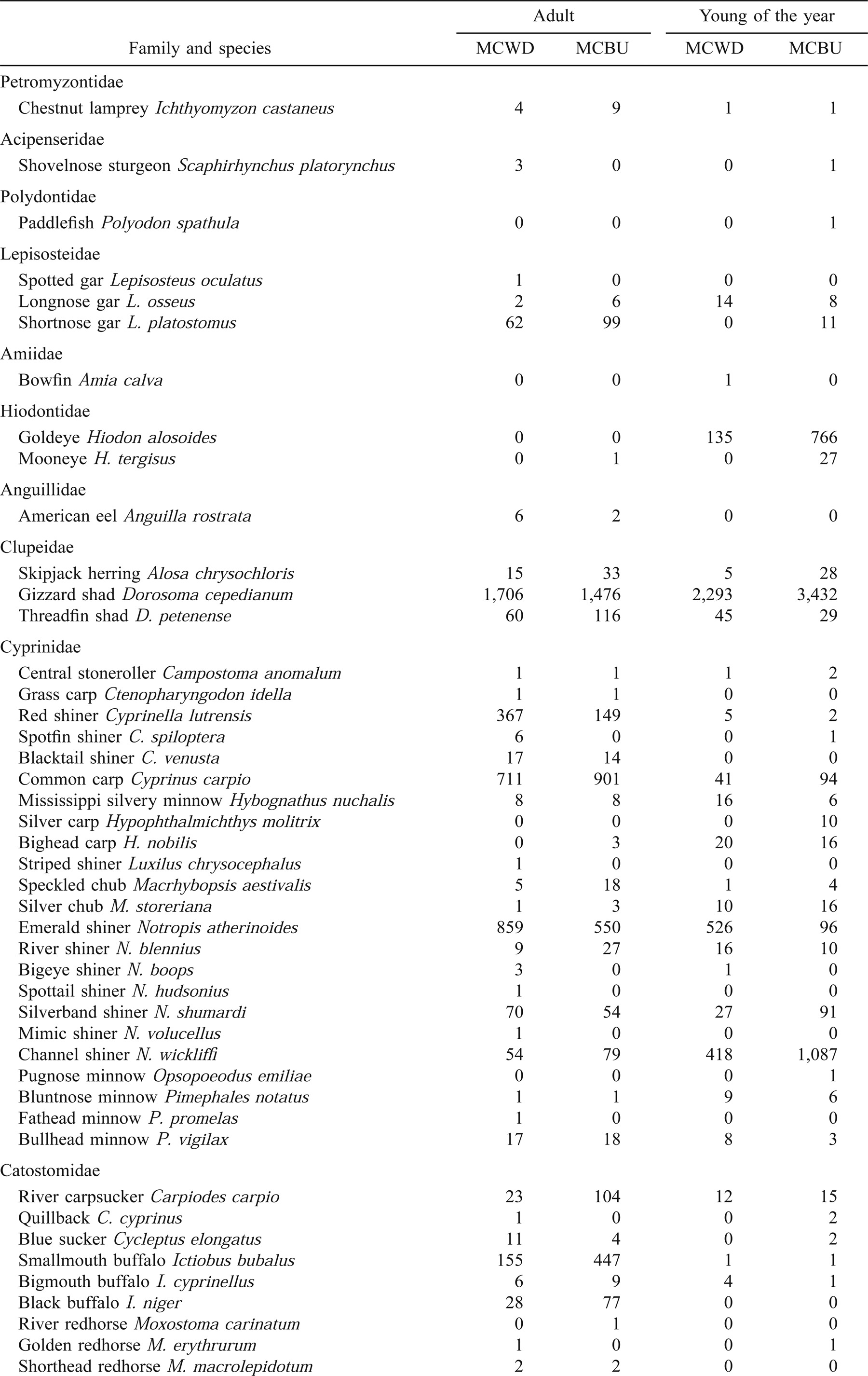
The most abundant families of adult fishes associated with wing dikes (MCWD) and main channel borders (MCBU) were Cyprinidae (MCBU = 30% and MCWD = 36%), Clupeidae (MCBU = 27% and MCWD = 30%), Centrarchidae (MCBW = 17%), Catostomidae (MCBU = 11%), and Ictaluridae (MCBU = 17% and MCWD = 12%; Table 2). The abundance of adult Cyprinidae, Clupeidae, and Centrarchidae were significantly greater at wing dikes (X2 = 75.1, P < 0.0001; X2 = 38.2, P < 0.0001; and X2 = 586, P < 0.0001, respectively), while the abundances of adult Catostomidae and Ictaluridae were greater in main channel borders (X2 = 153, P < 0.0001; and X2 = 20.3, P < 0.0001, respectively).
The most abundant families of age-0 fishes included Clupeidae (MCBU = 18% and MCWD = 48%), Cyprinidae (MCWD = 14%), and Sciaenidae (MCBU = 67% and MCWD = 13%; Table 2). The abundances of age-0 Clupeidae and Cyprinidae were significantly greater at wing dikes (X2 = 109.0, P < 0.0001; and X2 = 144.0, P < 0.0001, respectively), while the abundance of age-0 Sciaenidae was significantly greater in main channel borders (X2 = 0.10 × 105, P < 0.0001). Some age-0 fishes were captured in the same physical habitats as the adults. For example, in the family Clupeidae, the young of the ubiquitous gizzard shad were very common in both main channel borders and wing dikes, but the young of threadfin shad were twice as common at wing dikes.
Approximately 56% of the variation in adult species richness was explained by nine independent variables that entered the stepwise regression model (F = 71.46, df = 9,507, P < 0.0001; Table 4). There was greater species richness per sample at wing dikes for all four-gear types, but species richness declined as transparency (Secchi depth) increased within wing dikes. Adult species richness per sample at wing dikes using daytime electrofishing and mini-fyke netting was always greater than at main channel borders, even when sampling wing dikes with high transparency (Secchi depth). However, the species richness observed using large hoop nets and small hoop nets at wing dikes with greatest transparency (i.e., Secchi depth = 51 cm) was less than the species richness observed in main channel borders using the same gears. Fish species richness was positively influenced by river elevation (F9,507 = 8.96, df = 1,507, P = 0.003). This model did not reveal any significant effects of conductivity, water temperature, water velocity, depth of gear deployment, period, or sample date. The error terms associated with these data were uncorrelated (d = 1.816).
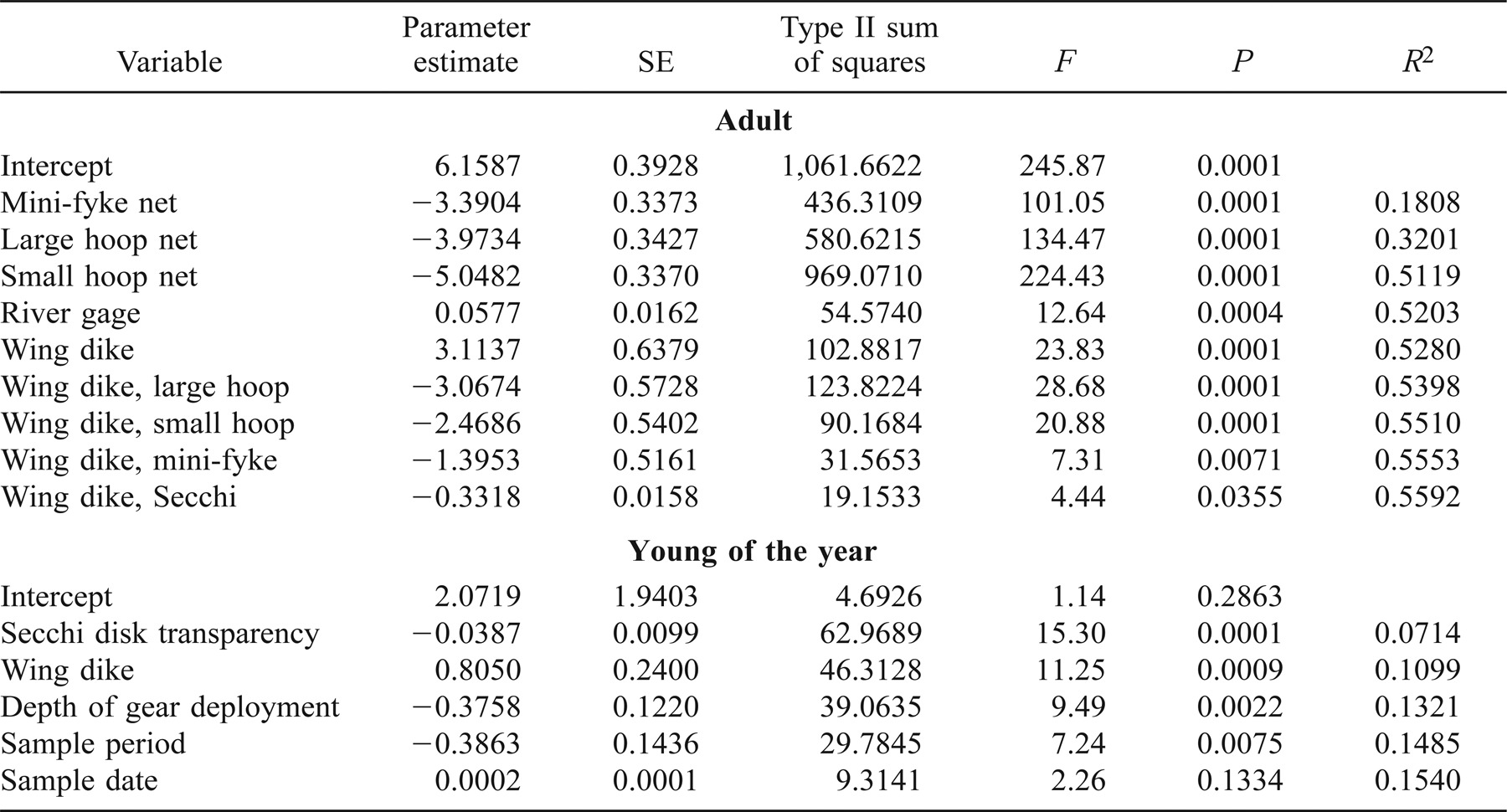
The stepwise multiple regression on age-0 fishes explained approximately 15% of the variation in species richness (F5,349 = 12.71, df = 5,349, P < 0.0001; Table 4). Species richness for age-0 fishes was lower in main channel borders, and was reduced by transparency (e.g., Secchi depth), depth of gear deployment, and sample period. This model did not reveal any significant effects of temperature, water velocity, conductivity, gear, or river elevation. The error terms associated with these data were uncorrelated (d = 1.835). Over 99% of age-0 fishes were captured using daytime electrofishing or mini-fyke netting. We found average water velocities measured at main-channel-border physical habitat to be greater approximately 72% of the time when compared with wing dike physical habitat (Figure 2).
Discussion
Wing dikes in the unimpounded section of the UMR contained more species of adult and age-0 fishes than main channel borders. As discussed below, this may be a result of gear bias, reduced current velocities, presence of scour holes, the existence of conditions similar to naturally occurring lentic habitats, or other factors known to influence fish recruitment that were not measured in this study (Matthews 1971; Beckett et al. 1983; Mills and Mann 1985; Schlosser 1985). The reduction in species richness of adult fishes in hoop net samples when Secchi disk transparency was high may be a gear bias and a function of water velocity. Higher Secchi disk readings occur at lower elevations when the velocity behind the wing dikes may approach zero. Hoop nets may function as a refugium for benthic fishes that seek velocity breaks. When water velocities approach zero, fish species may not seek velocity breaks, which decreases gear effectiveness.
Reduced current velocity may benefit fishes by decreasing energy expenditure and increasing growth rates through hover feeding (Bachman 1984; Todd and Rabeni 1989; Putman et al. 1995). Because some side channels are isolated from the main channel by wing dikes and/or other closing structures during low river elevations (Barko and Herzog 2003), some species may use wing dikes as a substitute for inaccessible offshore areas with lower water velocities. Logsdon (1993) compared water velocities between wing dike and main-channel-border physical habitats in the UMR, and the velocities behind wing dams were consistently lower. Our findings support Logsdon (1993) because we found that average water velocities measured at main channel borders tended to be greater when compared with wing dikes (Figure 2). Velocities at both upstream and downstream sections of wing dikes are reduced when compared with main channel borders. However, the degree of upstream velocity reduction varies based on the dike field, dike configuration, and dike location relative to other dikes in the field.
Scour holes created behind wing dikes may function like deepwater holes associated with snags in unchannelized river systems. In unchannelized systems, these holes are often used by fishes to hide from terrestrial predators and aquatic piscivores, while snags provide refuge from high velocity (Benke et al. 1985; Matthews et al. 1986; Lobb and Orth 1991). Also, piscivorous fishes may hide in the shadows produced by the physical structure of wing dikes while waiting to ambush prey (Helfman 1981). Deepwater scour holes may simulate lentic conditions (see Beckett et al. 1983) and attract Centrarchidae. This may explain the higher relative abundance of adults in this family at wing dikes (17% compared with 4% in the main channel border). Species in this group feed on invertebrates, inhabit pools, and often spend time in deepwater physical habitats (Pflieger 1997). Invertebrates often attach themselves to the rocks used to create wing dikes, which could provide food for some Centrarchidae (Beckett et al. 1983), including orangespotted sunfish and green sunfish. Deepwater, low-velocity pools are present at wing dikes during low river elevation (i.e., late summer–early spring) when the dikes are exposed. These pools provide refuge to fishes during unfavorable main channel conditions (e.g., high velocity and waves from barge traffic) and spawning activities, and functions as nursery areas for some fishes (Holland 1986; Harvey 1987). However, we presume these deep, low-velocity pools were historically associated with side channels and oxbow lakes that were periodically flooded. Many fishes no longer have reliable access to offshore aquatic areas because the river is now disjunct from the floodplain because of levees and closing structures (Barko and Herzog 2003). Although it may appear that wing dikes provide needed low-velocity refugia for Centrarchidae, these fishes may simply be seeking the next best physical habitat available because naturally occurring low-velocity aquatic areas have been reduced in this river reach. Wing dikes are relatively new, in ecological time, to the system and seem to provide structure that was likely more abundant in the unimpounded UMR before channelization.
The positive relationship between species richness of adult fishes and river elevation is likely a function of behavior. In our study reach, river elevations are usually higher in late spring–early summer when fishes are seeking sites for reproduction. Obligate riverine fish species use high water as a cue for feeding and reproduction (Dillard et al. 1986; Copp 1989; Jurajda 1995). Therefore, we believe this relationship may merely be an ecological function of fishes moving in search of flooded terrestrial areas for reproduction, food, or cover. The low variation in species richness of age-0 fishes explained by the stepwise multiple regression model occurred because many of the factors known to influence age-0 recruitment were not measured, such as climatic conditions, discharge, water quality, and food availability (Mills and Mann 1985; Schlosser 1985). Matthews (1971) and Schlosser (1985) found that combinations of these factors influence variability in species richness and abundance in age-0 fishes more than in adult fishes.
Although overall adult assemblage structure was similar, adult fish families appear to use the physical habitats differently. Cyprinidae, Clupeidae, and Centrarchidae were more abundant in wing dike physical habitat, while Catostomidae and Ictaluridae were more abundant in main-channel-border physical habitat. Smallmouth buffalo, river carpsucker, and channel catfish occurred in high numbers in main channel borders and strongly influenced our results. However, flathead catfish and freckled madtom were relatively more abundant at wing dikes. Flathead catfish are known to use cover when resting and move to riffles or rocky areas to feed (Pflieger 1997). Freckled madtoms are often found in woody cover over silty gravel (Pflieger 1997), but may also be found in swift water in rocky or gravelly riffles (Burr and Mayden 1982). Wing dikes contain cover in the form of logs and spaces between riprap on the dikes, making this type of habitat desirable for these species. Blue sucker, which is a swift water species (Pflieger 1997), was relatively more abundant at wing dikes and was often collected near the tips of wing dikes in high water velocities. Members of the Clupeidae and Centrachidae families were more abundant at wing dikes likely because of their body forms and habits (see Pflieger 1997). Of the Centrarchidae, only the ubiquitous bluegill was relatively more abundant than its conspecifics in main channel borders.
The most abundant families of age-0 fishes captured at wing dikes were Clupeidae and Cyprinidae, while the most abundant family of age-0 fishes captured at main channel borders were Sciaenidae. We know little about the life histories of most large river fish species, especially the young. However, some interesting dichotomies emerged in these data. For example, emerald shiners were common at wing dikes as young (47.9% of the total minnow catch compared with 6.6% in the main channel border), but as adults were common at both the wing dikes (40.3%) and main channel borders (30.1%). Conversely, the channel shiner was the most common age-0 minnow collected in the main channel borders (75.2%), yet was also found at wing dikes (38.0%). Adult channel shiners were much less common in both physical habitats (4.3% in the main channel border and 2.5% in wing dike physical habitats). In the Clupeidae, age-0 threadfin shad were more common at wing dikes, but as adults were relatively more common in the main channel borders. Unlike Floyd et al. (1984) and Mills and Mann (1985), we did not find the young of year of most fish species using the shoreline as a channel nursery. Instead, this physical habitat was dominated by age-0 freshwater drum. In summary, species richness for both adult and age-0 fishes was higher within the wing dikes relative to main channel borders. Wing dikes may create microhabitats suitable for some fish species and provide substrate that encourages macroinvertebrate colonization.
However, alterations made to the Mississippi River channel for navigation and flood control have likely had a negative impact on aquatic organisms (see Barko and Hrabik, in press). Channel maintenance activities have altered the main channel border by reducing woody debris, shoreline sinuosity, and depth diversity (Conner et al. 1983; Yin and Nelson 1995; Pitlo 1998). Floodplain rivers are directly dependent on the connectivity of the main channel to backwater areas and periodic flooding (Amoros and Roux 1998). Unlike many of the modified rivers in Europe (Copp 1989; Schiemer and Spindler 1989), the unimpounded UMR is almost completely isolated from backwater areas by wing dikes and is completely isolated from the floodplain by an extensive levee system. These alterations have likely affected the reproduction and recruitment of some fish species because of lost spawning and nursery physical habitat (Copp 1989; Junk et al. 1989). In addition, river modifications have been found to negatively affect native fishes, but positively affect introduced habitat generalists, such as the common carp (Gehrke et al. 1995). Long-term impacts of river modifications on fishes have not been well documented in many large river systems and warrant further study. Understanding the biotic and abiotic factors structuring aquatic assemblages and evaluating the effects of river regulation on aquatic organisms are essential before mitigation and conservation efforts can be successful in large riverine systems. The findings of this study and others conducted on channelized rivers in North America and Europe provide researchers, engineers, and politicians in countries with unchannelized flood plain rivers information needed to make informed decisions regarding river alteration and development to avoid the damage (such as increased flooding and the loss of habitat diversity and fish resources) that has occurred in many channelized systems.
Acknowledgments
This study was supported by the U.S. Army Corps of Engineers/U.S. Geological Survey Long-Term Resource Monitoring Program and by the State of Missouri through the Missouri Department of Conservation. We thank M. Petersen, D. Ostendorf, J. Ridings, J. Crites, C. Beachum, G. Lowes, J. Scott, D. Smith, K. Tilley, B. Spane, and J. Lopez for assistance with data collection. We thank M. Roell, B. Johnson, D. Ostendorf, D. Galat, J. Pitlo, B. Vondraak, and A. Buchanan for providing a review of this manuscript. L. Williams and B. Ickes provided discussions that greatly improved this manuscript.