Survival Estimates for Juvenile Fish Subjected to a Laboratory-Generated Shear Environment
Abstract
Juvenile rainbow trout Oncorhynchus mykiss and steelhead (anadromous rainbow trout), fall (age-0 and age-1) and spring Chinook salmon O. tshawytscha, and American shad Alosa sapidissima were exposed to shear environments in the laboratory to establish injury–mortality thresholds based on estimates of strain rate. Fish were exposed to a submerged jet having exit velocities of 0 to 21.3 m/s, providing estimated exposure strain rates up to 1,185/s. Turbulence intensity in the area of the jet where fish were subjected to shear was minimal, varying from 3% to 6% of the estimated exposure strain rate. Injuries and mortalities increased for all species of fish at strain rates greater than 495/s. American shad were the most susceptible to injury after being subjected headfirst to a shear environment, while steelhead and rainbow trout were the most resistant. There was no apparent size-related trend in susceptibility to high shear except that age-0 fall Chinook salmon were more resistant to shear environments than age-1 fall Chinook salmon. All groups of test fish exposed headfirst to high-shear environments had higher injury–mortality rates than fish introduced tailfirst at similar strain rates. These results document the relationship between fish injury and a fluid force present at hydroelectric facilities and provide biological specifications for improving fish passage and survival.
Introduction
It is well-established that the physical stresses associated with hydroelectric turbine passage (i.e., pressure changes, shear and turbulence, or striking the turbine blade or other mechanical structures) cause injury or mortality to downstream migrant juvenile fish (Turnpenny 1998; Čada 2001). For example, turbine passage is estimated to kill 5% of entrained fish in the best existing turbines (RMC and Skalski 1995), and mortality may exceed 30% in other less efficient designs (Bell 1981). Consequently, a wide variety of downstream passage and protection measures have been used to prevent fish from becoming entrained in turbine intakes, ranging from spill flows to pass fish over the dam to complicated physical screening and light- or sound-based guidance measures. However, no downstream passage protection system has yet been demonstrated to be 100% effective, practical to install and operate, and/or acceptable to regulatory agencies under a wide range of site conditions (Čada and Sale 1993; Francfort et al. 1994; Čada et al. 1997). Even well-designed screening and bypass systems may protect only a portion of the fish susceptible to entrainment (Ferguson 1992); the remainder will pass around the screens and through the turbines. Thus, it is also desirable to maximize the survival of turbine-passed fish.
High values of shear are known to occur where rapidly flowing water passes near fish passage structures, including over spillways and through the turbines of hydroelectric dams (Čada 2001). The effects of shear on turbine-passed fish are poorly understood because of difficulties in understanding this flow phenomenon, determining its magnitude and distribution within hydroelectric turbine systems, and then recreating it in a controlled setting. Ideally, fluid shear and/or turbulence could be reduced through operational changes or physical modifications to existing hydroelectric projects.
The objective of our study was to quantify the response of fish to a fluid shear environment. We created a shear environment in a controlled laboratory setting using a high-velocity submerged jet. Fish were exposed to hydraulic conditions thought to occur during passage through a hydroelectric turbine system, and their injury−mortality thresholds were described in terms of strain rate (i.e., the change in water velocity over distance). The data from these studies can be used to determine whether levels of shear and turbulence occurring in turbines are injurious to fish, and it will contribute to an improved understanding of relative risk when compared with other passage routes (e.g., spillways or bypass outfalls).
Methods
Test fish
We tested rainbow trout and steelhead Oncorhynchus mykiss, spring and fall Chinook salmon O. tshawytscha, and American shad Alosa sapidissima. Rainbow trout (147–173 mm fork length [FL]) were raised at the aquatic laboratory at the Pacific Northwest National Laboratory (PNNL), Richland, Washington. Steelhead (Skamainia strain; 175–232 mm FL) were from the Beaver Creek Hatchery, Longview, Washington; spring Chinook salmon (135–154 mm FL) were from the Leavenworth National Fish Hatchery, Leavenworth, Washington, and fall Chinook salmon (age-0: 85–95 mm FL; age-1: 123–152 mm FL) were from the Priest Rapids Hatchery, Mattawa, Washington. American shad (85–115 mm FL) were collected from the McNary Dam juvenile passage facility at Columbia River km 469. All fish had minimal scale loss, and sizes were representative of anadromous fish populations migrating downstream past hydroelectric dams. With the exception of hatchery-reared rainbow trout, test fish were either transitioning from the parr to smolt life stage or were active migrants.
Test system
A fiberglass flume (Figure 1), 9 m long × 1.2 m wide × 1.2 m deep when filled with water, was plumbed to receive a submerged water jet that created a quantifiable shear environment. Water was pumped into the flume via a conical, stainless steel nozzle 25.4 cm in diameter constricting to a circular 6.4 cm diameter over 50.8 cm in length. This reduction in diameter accelerated flow and reduced nonuniformity in velocity distribution. The jet exit was submerged about 0.6 m below the water surface during all tests. Fish were introduced immediately above the jet and in front of the nozzle through a Plexiglas deployment tube at an angle of 30° (Figure 1). A 1-cm separation width prevented fish from exiting the tube without being subjected to the shear environment. Viewing windows were located on the side and bottom at the nozzle end of the flume to allow for recording of fish reaction to flow fields.
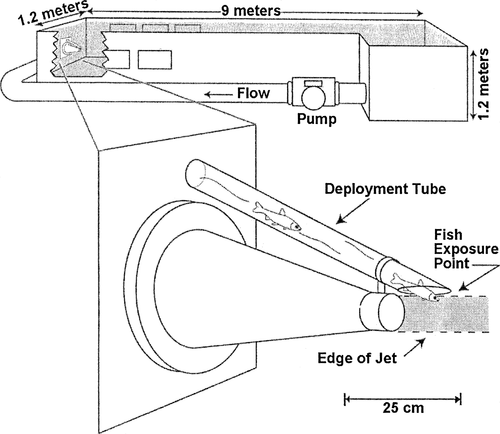
Plan view of the shear test facility, including a closeup of how test fish entered the shear environment via a deployment tube
An electric pump capable of pumping up to 9,464 L/min of water and controlled by a variable-speed motor drive was used to circulate water from a storage reservoir through the flume. The system was capable of creating exit velocities in excess of 20 m/s. We developed a relationship between pump volume and controller settings using velocity measurements at the exit of the nozzle and programmed the operating frequency (hertz) to achieve desired jet velocities.
Characterization of flow fields
To characterize the flow field and assure consistent exposure conditions, velocity measurements were taken using a heavy duty Pitot tube connected to a pressure differential sensor. Measurements in the zone of flow establishment were taken on a 1-mm grid covering a radial slice 1.5 diameters in the radial direction and 3 diameters downstream of the jet exit. Velocity fluctuations were measured in three directions using a two-component Dantec Laser Doppler Velocimeter (LDV) positioned and traversed with a three-axis step motor gantry.
Detailed velocity measurements were made for mean jet exit velocities of 3.0, 6.1, 9.1, 12.2, 15.2, 18.3, and 21.3 m/s. The corresponding exposure strain rates for each velocity were 168; 341; 517; 688; 852; 1,008; and 1,185/s, respectively (Gordon et al. 1992). Exposure strain rate (e) was estimated using the equation

where ū is the mean water velocity (cm/s) and y is distance (cm) perpendicular to the force.
It should be noted that the distance over which velocities change has an important influence on the calculated rate of strain. For example, a decrease in velocity from 1800 to 0 cm/s, occurring over a distance of 50 cm, results in an exposure strain rate of 36 cm/s/cm. If that same change in velocity occurs over a distance of 4 cm, the calculated exposure strain rate would equal 450 cm/s/cm. The units of exposure strain rate (cm/s/cm) simplify to per second (/s). We used a change in distance (Δy = 18 mm) to calculate the exposure strain rate. This provided a measurement scale at the width of the test fish or the assumed response distance for hydraulic forces encountered in the test apparatus. We refer to this value as the exposure strain rate, a rate not equivalent to the local fluid-exposure strain rate computed at a finer or coarser scale.
Characterization of injury and mortality
Test fish were exposed to the edge of the submerged water jet moving at velocities of 0, 3.0, 6.1, 9.1, 12.2, 15.2, 18.3, and 21.3 m/s. Fish were transferred from the holding trough to the exposure system in a water-filled Lucite tubing (cartridge), transferred to a deployment tube (Figure 1), and allowed to voluntarily enter the shear environment. Two orientations were tested. While most headfirst test fish readily passed down the introduction tube within a few seconds, some tailfirst fish had to be gently guided down the tube with a plunger. Following exposure, fish were captured with a dip net. Potential handling effects were determined by releasing fish through the deployment tube while the pump was not running.
We assessed the type and extent of injuries (tissue damage) and direct mortality (initial and delayed). After initial examination, surviving fish were placed in small net-pens adjacent to the flume at about 12°C. Test fish were held for 48 h to monitor delayed mortality or other effects indicative of stress or injury (i.e., discoloration, lethargy, or loss of equilibrium).
The occurrence and severity of injuries was recorded for each fish. Minor injuries were defined as those that were visible, but not life-threatening, and that disappeared over the 48-h postexposure observation period (e.g., small bruises ≤0.5 cm in diameter). Injuries that resulted in a prolonged loss of equilibrium, appeared life-threatening, or persisted throughout the postexposure observation were rated as major. Examples of major injuries include large bruises (≥ 0.5 cm in diameter), spinal fractures, cuts with visible bleeding, injured eyeballs (e.g., bulged, hemorrhaged, or missing), or gill damage (e.g., inverted gill arches, or isthmus tears). Descaling was evaluated based on the methods used to monitor juvenile salmonids at Columbia River dams (Basham et al. 1982).
Data analysis
Logistic regression was used to analyze the effect that strain rate had on injury or mortality levels for various test groups. The test groups were defined by a specific species and placement, either headfirst or tailfirst. The response variable used in each analysis is binomial, hence the use of logistic regression. Three different response variables were analyzed: (1) the proportion with a minor injury or worse; (2) the proportion with a major injury or worse; and (3) the proportion mortality. Strain rate was used as the independent variable. For most test cases the fish were tested at strain rates of 0 (control), 168, 341, 517, 688, 852, and 1,008/s. A logistic regression model was fit for each test group, using the following equation:
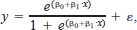
where y is the response variable, x is the strain rate, β0 and β1 are coefficients in the model, and ϵ is the error term. A quadratic equation was also evaluated to determine if it improved the fit. When the quadratic term was significant, it was added to make the equation
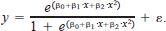
The number of test fish needed to estimate biological response at the desired significance level (P < 0.01) involved up to 180 fish per test series. Up to six treatment levels and a reference (i.e., no effect) were tested for each of the six groups of fish. A detailed description of the overall experimental design and analysis is provided in Neitzel et al. (2000). Video images of the test fish were acquired using high-speed cameras positioned to view the fish as they exited the deployment tube and entered the shear zone created by the underwater jet. These images were reviewed to verify fish orientation and injury mechanisms.
Results
Water Jet Characteristics
Our measurements showed that velocities in the high-speed, submerged jet were nonuniform and turbulent, especially at the edge of the jet. Immediately downstream of the nozzle exit, the vertical profile of the axial velocity showed a sharp decrease at the shear layer edge (Figure 2). The sharp decline in axial velocity at the jet edge created the region of fluid strain (velocity gradient) and shear to which fish were exposed. The maximum exposure strain rate occurred just downstream of the nozzle exit near the jet edge.

Vertical profiles of velocity vectors (shown by arrows) at five locations downstream of the nozzle exit. The edge of the shear layer is shown where the velocity transitions to zero. The computed strain rates were highest where fish entered the shear zone (fish entry zone) as indicated by the abrupt increase in velocity at the edge of the shear layer
Computed strain rates were nearly constant where a test fish initially intersected the jet, but the magnitude of the fluid strain decreased with increasing distance downstream from the nozzle as the jet expanded into the ambient fluid (Figure 2). The strain rate experienced by the test fish varied by about only 3–6%, based on measured axial velocity turbulence intensities (root mean square of the velocity fluctuations) within the jet.
Injury and Mortality
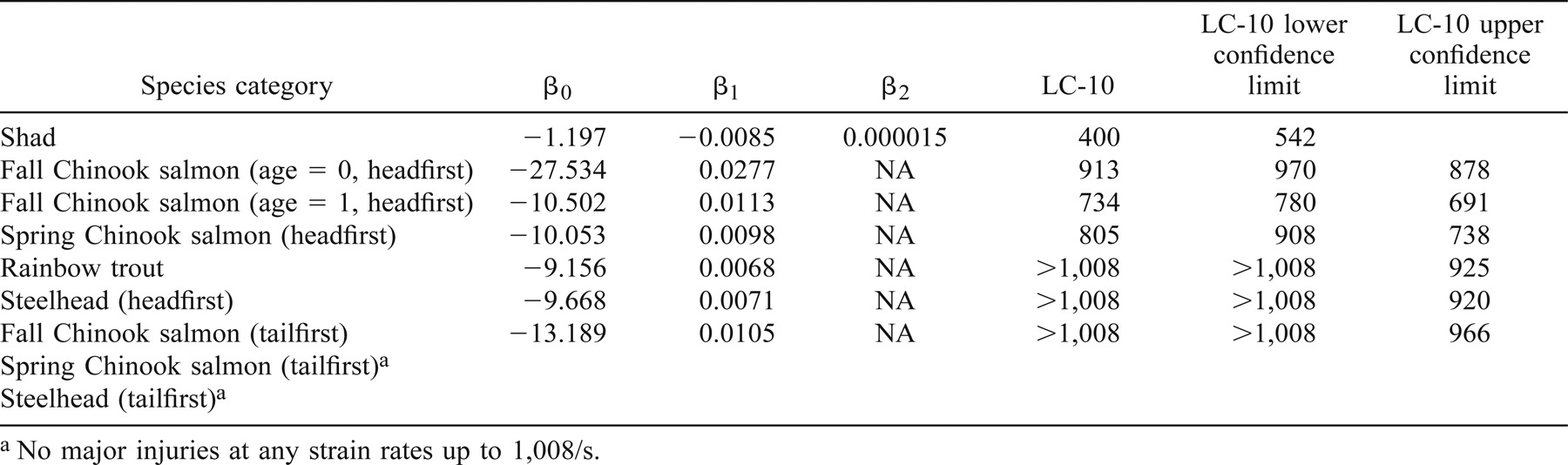
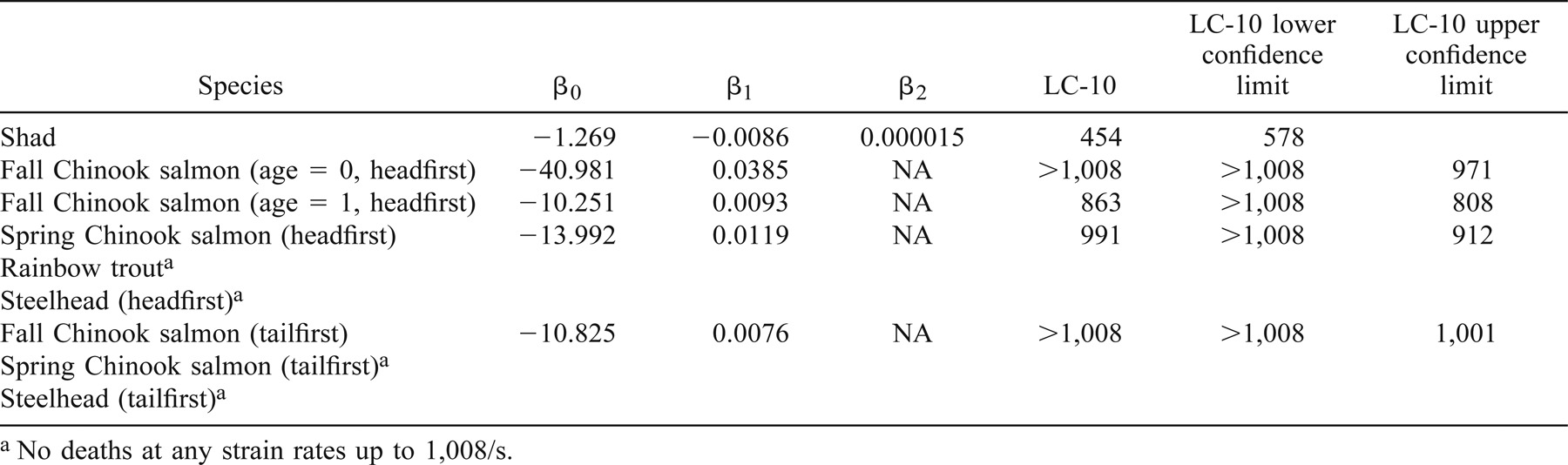
The effects of entering a shear environment were determined for six groups of test fish in a headfirst orientation and three groups in a tailfirst orientation. We estimated the strain rates at which 10% of the population would be affected (LC-10) for each test group and response variable.
Fall Chinook Salmon
The LC-10 for minor injury of age-0 (mean FL = 8.5 cm) and age-1 (mean FL = 14.0 cm) fall Chinook salmon occurred at a strain rate of 607 and 495/s, respectively, when the fish were introduced headfirst into the shear environment (Table 1; Figure 3). The LC-10 for major injury occurred at 913/s (Table 2), and death was predicted above 1,008/s (Table 3). The LC-10 for major injury and death of age-1 fall Chinook salmon exposed headfirst occurred at strain rates of 734 and 863/s, respectively (Tables 2, 3).
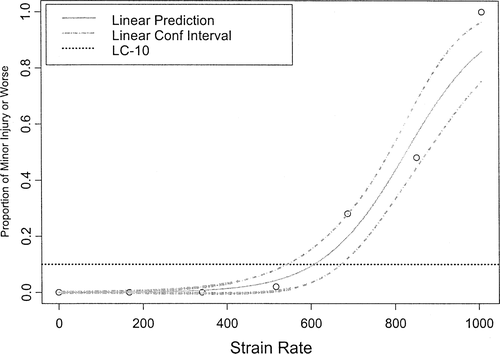
Proportional plot showing strain rates resulting in major injury or worse for age-1 fall Chinook salmon across the range of strain rates tested. Upper and lower 95% confidence limits about the LC-10 are shown by the dashed line
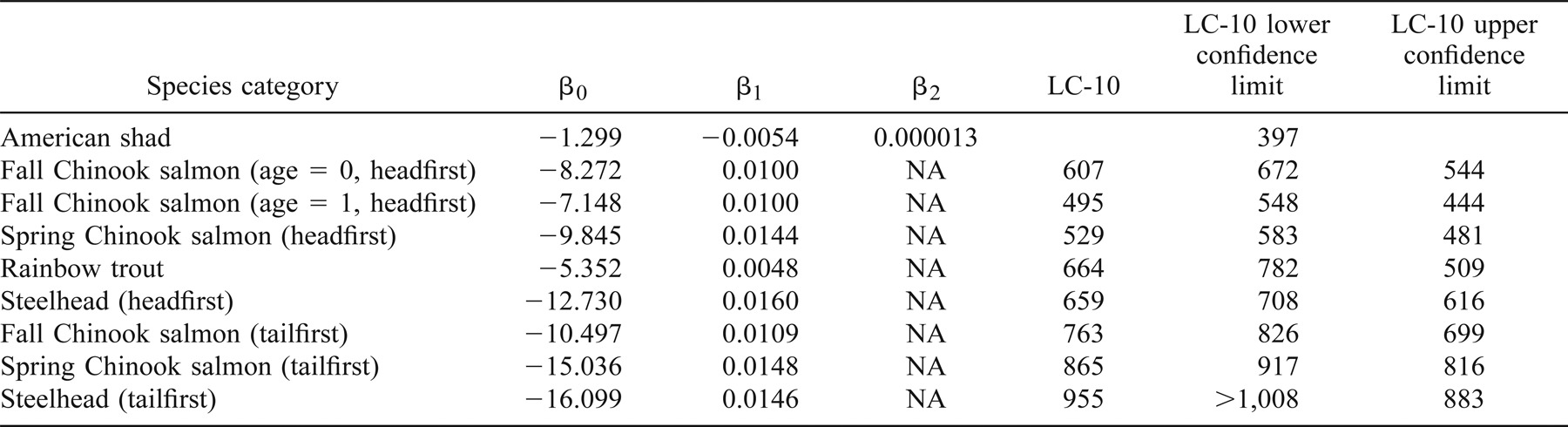
The LC-10 for minor injury or worse for age-1 fall Chinook salmon introduced tailfirst into shear environments was 763/s (Table 1). The LC-10 for major injury or mortality during tailfirst exposures was estimated to occur at strain rates above or equal to 1,008/s (Tables 2, 3).
Spring Chinook Salmon
The LC-10 for juvenile spring Chinook salmon (mean FL = 14.5 cm) exhibiting a minor injury (or worse) was detected when fish were introduced headfirst into a shear environment at an estimated strain rate of 529/s (Table 1). The LC-10 occurred at strain rates of 805 and 991/s for major injury and death, respectively (Tables 2, 3).
The LC-10 for minor injury in spring Chinook salmon occurred at a strain rate of 865/s (Table 1). No major injuries or mortalities were observed in spring Chinook salmon introduced tailfirst into the shear environment at strain rates up to 1,008/s (Tables 2, 3).
Rainbow Trout
The LC-10 for minor injury (or worse) occurred at a strain rate of 664/s for juvenile rainbow trout (mean FL = 15.5 cm) exposed headfirst to a shear environment (Table 1). The LC-10 for major injury or mortality was predicted to occur at strain rates above 1,008/s (Tables 2, 3).
Steelhead
The LC-10 for juvenile steelhead (mean FL = 21.5 cm) during headfirst exposure to a shear environment occurred at a strain rate of 659/s (Table 1). The LC-10 for steelhead sustaining major injuries was predicted to occur at strain rates above 1,008/s. No steelhead died as a result of headfirst exposure to the highest strain rate tested (1,008/s).
Tests of tailfirst orientation were conducted at only at the two highest strain rates due to steelhead tolerance in the headfirst orientation tests. The LC-10 for minor injury was 955/s (Table 1). No major injuries or mortalities were observed for steelhead exposed to the highest strain rate tested (1,008/s) in a tailfirst orientation.
American Shad
Juvenile American shad (mean FL = 10.0 cm) were the fish species most sensitive to the effects of exposure to shear environments in a headfirst orientation. Shad were also very sensitive to the effects of handling, as evidenced by a 20% mortality rate in control fish within the first 48 h posthandling. All American shad exposed to the highest exposure strain rate tested (1,008/s) died within 48 h of exposure. The LC-10 for injury and mortality in American shad occurred at strain rates of 400 and 578/s, respectively (Tables 2, 3).
Discussion
Our tests showed that an estimated 10% of the test population of juvenile salmonids sustained minor injuries when exposed to shear zones estimated to have strain rates equal or greater than 495/s. American shad were the most susceptible, and steelhead and rainbow trout were the most resistant to high-shear conditions. Typical injuries to test fish included torn opercula and missing eyes—injuries indicative of exposure to localized, severe forces. Similar injuries have been reported for salmonids passing turbines at the Elwha dam (Schoeneman and Junge 1954) and during recent tests at McNary Dam (Normandeau Associates 1999), suggesting extreme hydraulic events may be the causal mechanism.
There was no apparent size-related trend in susceptibility to high-shear environments among the six groups of fish, based on major injury or death. However, the larger age-1 fall Chinook salmon were more susceptible to injury and death at lower exposure strain rates than age-0 fall Chinook salmon. This difference cannot be explained by the response distance used to define the rate of strain. For instance, using a Δy of 9 mm (i.e., the width of an age-0 fall Chinook salmon) would result in a strain rate exposure twice that for a larger fish.
The response of juvenile salmonids entering the shear environment was influenced by their exposure orientation. For example, the LC-10 or response threshold for all three fish groups where comparative tests were run was lower when the fish entered the shear zone headfirst versus tailfirst (i.e., 529/s versus 865/s for spring Chinook salmon, and 659/s versus 955/s for steelhead). Because of a fish's streamlining and fusiform shape, they would seem to be more resistant to injury when the fluid force is directed from the head to the tail. In contrast, a force directed toward the head could pry scales off because they imbricate in a backward direction. The same directional force could also lift the operculum and bend or tear it or expose the gills to damage. Our review of video records suggested that most headfirst injuries occurred immediately after contact with the shear zone, and that bending or twisting action facilitated damage to the operculum and isthmus.
Direct comparisons between the exposure strain rates estimated during our tests and the values reported by other investigators are not straightforward because previous studies did not describe their velocity fields. For example, Turnpenny (1998) reported fluid shear stress values based on computational fluid dynamics estimations of their water jet. He found that juvenile salmonids exposed to a shear stress of 1,920 N/m2 or greater experienced pop-eye and torn opercles, but no immediate mortality. His exposure scenario, based on reported jet velocities, provides an exposure strain rate value equal to or greater than 628/s.
Groves (1972) reported that juvenile salmon were unaffected by exposure to jet velocities of 11.9 m/s, but that rate of disorientation, visible injury, and mortality increased as jet velocities increased above this value. Johnson (1970) reported that juvenile salmonids subjected to jet velocities of 17.5 m/s were not killed. However, he noted mortality at exposures to velocities greater than 20 m/s or at an estimated strain rate greater than900/s. Both studies can be compared with our no-effect strain rate value of 517/s for a jet velocity of 9.1 m/s.
This study and those of Groves (1972) and Johnson (1970) focused on fish entering a shear environment where the fish is in slow-moving water and the shear is created by a submerged jet. However, fish passing through turbines could be entrained in high-velocity streamlines and enter a shear environment by moving into slower or nonmoving water. This exposure might be simulated by ejecting fish from a nozzle into a pool of water. Johnson et al. (2003) recently conducted tests with rainbow trout in this manner and found that fish were injured at lower rates when tested at jet velocities equivalent to our test scenario.
The secondary effects of exposure to fluid forces should also be considered when specifying safe conditions. For example, fish may be weakened and thus subjected to death indirectly, even though there is no observable injury. Groves (1972) reported that fish exposed to a shear environment were disoriented but regained “normal capacities” within 5–30 min. Neitzel et al. (2000) subjected rainbow trout to predators after they were exposed to strain rates of 688/s and found they were more susceptible to predation. Collectively these data suggest that exposure to strain rates causing disorientation could result in indirect mortality.
To develop specifications that reduce or eliminate harmful shear environments for fish passing hydroelectric projects, we must know where shear environments occur and their associated strain rates. Modeled data indicates that exposure strain rates of 400–600/s may occur in a boundary layer near turbine structures such as the stay vane, wicket gate, and runner blade leading edge (Voith Hydro 1997). However, the bulk of the flow through a turbine likely contains smaller exposure strain rates, perhaps in the neighborhood of 50/s (Fisher et al. 2001). Based on this modeled data, less than 1% of the passage volume contains potentially injurious or lethal shear environments. While this seems low, actual impacts will depend on the total number or proportion of fish passing through areas having lethal or injurious strain rates. The biological specifications provided here are one step towards assessing the risks of harmful shear environments. Defining injury thresholds for juvenile fish that migrate downstream can be used to improve the design and operation of hydroelectric turbines or other facilities creating severe hydraulic conditions.
Acknowledgments
We thank Peggy Brookshier, Manager of the U.S. Department of Energy's Advanced Turbine System Program, for her leadership and support of this research. We also thank John Flynn, U.S. Department of Energy; Ben Rinehart and Garold Sommers of the Idaho National Engineering and Environmental Laboratory; and Mike Sale of the Oak Ridge National Laboratory for their active participation and support. Thomas Carlson and Geoffrey McMichael of Pacific Northwest National Laboratory (PNNL) provided advice during methods development. We thank Russ Moursund and Joanne Duncan of PNNL for laboratory assistance and data analysis. Georganne O'Conner provided an editorial review. The work was conducted at PNNL in Richland, Washington, under a Related Services Agreement with the U.S. Department of Energy, Contract DE-AC06-76RL01830. PNNL is managed by the Battelle Memorial Institute for the U.S. Department of Energy.




