Photoreceptive Systems in Ascidians†
This paper is part of the Proceedings of the 12th International Conference on Retinal Proteins held at Awaji Island, Hyogo, Japan on 4–8 June 2006.
Abstract
The brain vesicle of the tadpole larva of ascidians, simple basal chordates, contains an eye-spot (ocellus), which is responsible for the photic swimming behavior. Ascidian adults also exhibit several types of light-responsive behaviors. Molecular phylogenetic studies have suggested that ascidians are the closest living relatives of vertebrates, and therefore, understanding the photoreceptive systems in ascidians is a key to uncover the origin and evolution of the vertebrate eyes. The ocellus of the ascidian larva has ciliary photoreceptors resembling those of the retina and pineal eye of vertebrates. Recent studies have indicated that the ascidian larva has phototransduction and visual cycle systems similar to those of vertebrate eyes. Comparative studies on photoreceptor systems between ascidians and vertebrates provide us clues to reconstructing the evolutionary pathway leading to the lateral and median eyes of vertebrates.
Introduction
Recent molecular phylogenetic studies have suggested that tunicates, including ascidians, are the closest living relatives of vertebrates (1). Although the adult ascidian is a sessile organism with little resemblance to the vertebrate, the ascidian larva is similar to a frog tadpole and shares basic body structures with vertebrates (Fig. 1). The ascidian tadpole larva has a central nervous system derived from the dorsal neural tube and consisting of about 330 cells, of which only about 100 cells are neurons (2). The brain vesicle of the ascidian larva contains two sensory organs with a pigment cell: an eyespot (ocellus) and a gravity sense organ (otolith). The ocellus consists of three lens cells, one pigment cup cell, and a group of photoreceptor cells. The draft sequence of the ascidian genome has been generated in a widely studied species, Ciona intestinalis (3). The small genome size and easiness of molecular genetic manipulation of embryos of ascidians allow us to study the mechanisms that are common between ascidians and vertebrates. Thus, the ascidian provides us a unique opportunity to study the evolution and basic mechanisms of visual systems of vertebrates. In this review, we summarize the recent progress in studies on photoreceptive systems in ascidians. We also discuss the possible evolutionary relationships of photoreceptor organs in ascidians with those of vertebrates.
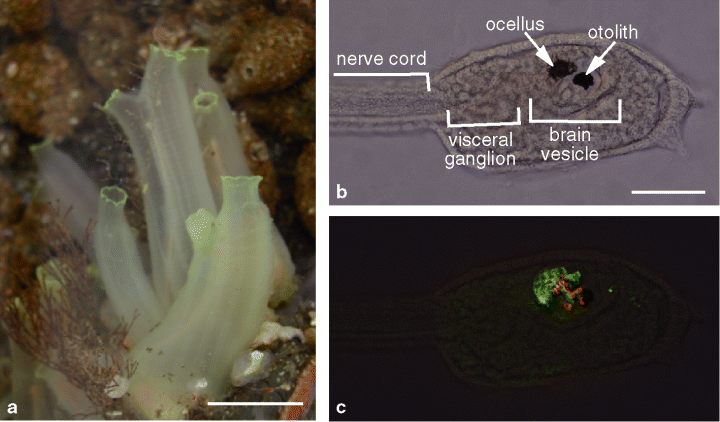
(a) Mature adults of the ascidian Ciona intestinalis. (b) A C. intestinalis larva. Sensory organs and regional subdivisions of the central nervous system of the larva are indicated. (c) Outer segments (red) and cell bodies (green) of photoreceptor cells of the ocellus are visualized in the same larva shown in (b) by immunofluorescent staining with anti-Ci-opsin1 and anti-Ci-Arr, respectively. Scale bars: 20 mm in (a) and 50 µm in (b). See the text for detail.
Swimming behavior of ascidian larvae
The ascidian larva has a characteristic swimming pattern consisting of an initial period when it swims upward followed by a period when it swims downward to settle (4–6). In nature, the initial phase serves to disperse the larvae, and in the second phase the larvae seek out suitable sites for the attachment and metamorphosis. In C. intestinalis, gravity sensing is responsible for the upward swimming behavior during the initial period of larval life (7,8). Ciona intestinalis larvae start to show photoresponse behavior at around 4 h after hatching, and they continue to show the photic response during the latter half of larval life (7,9–11). The larvae start swimming when light intensity decreases and stop swimming when light intensity increases. Thus, light is the only determinant for the downward swimming behavior of the C. intestinalis larva. Different ascidian species may exhibit different photic behavior of larvae (4–6,12). For example, larvae of the ascidian Aplidium constellatum switch from positive to negative phototaxis during a brief dispersal phase (12).
Sensory organs responsible for the larval swimming behavior
The sensory organs such as the ocellus and otolith are thought to guide the swimming behavior of the larva. Function of the otolith and ocellus as gravity sensor and photoreceptor, respectively, had long been predicted based on their morphology. Until recently, however, their sensory roles had not been directly demonstrated by behavioral analysis. Upward swimming behavior and photic swimming behavior were examined before and after laser ablation of the otolith and ocellus pigment cells in C. intestinalis larvae (7). The results suggest that the otolith is in fact responsible for the gravity orientation required for upward swimming. The otolith-ablated larvae retained the photic response, but ocellus pigment-ablated larvae lost this response. In the ocellus-pigment ablation experiments, the outer segments of ocellus photoreceptor cells in the pigment-cup were also disrupted. Larvae lacking melanin pigment were generated by either treatment with 1-phenyl-2-thiourea (8), an inhibitor of vertebrate tyrosinase, or knockdown of the tyrosinase gene (M. Tsuda et al., unpublished data), and subjected to the behavioral analysis. Melanin pigment itself is shown to be dispensable for photoresponse behavior of the larva. Results of these behavioral studies strongly suggest that the photoreceptor cells are responsible for their photic swimming behavior.
The action spectrum of photic behavior of ascidian larvae (λmax ≈ 500 nm) was similar to the absorption spectrum of human rhodopsin (9). Photoreceptor cells of the ascidian ocellus show morphological and electrophysiological properties that are similar to those of the vertebrate photoreceptor cells (13). The outer segment of the photoreceptor process is a modified cilium, consisting of many lamellae. The lamellae are thought to be homologous to photoreceptor disks of vertebrates. The ocellus of the C. intestinalis larva uses a vertebrate-type visual pigment, Ci-opsin1, as the photoreceptor molecule (14,15). Ci-opsin1 localizes in outer segments of the photoreceptor cells (Fig. 1c) and knockdown of Ci-opsin1 with antisense morpholino oligonucleotide inhibited the photic behavior (15). This experiment provides another line of evidence that the ocellus is the photoreceptor organ responsible for the photic swimming behavior of the ascidian larva.
Phototransduction
Morphology and electrophysiological response of photoreceptors are different between vertebrates and invertebrates. Vertebrate retinal photoreceptors are ciliary photoreceptors and they hyperpolarize in response to light. On the other hand, most photoreceptors of invertebrate eyes are rhabdomeric and depolarize in response to light. There are a number of important differences in phototransduction between ciliary photoreceptors and rhabdomeric photoreceptors (16–18). Ciliary photoreceptors and rhabdomeric photoreceptors use different types of opsins, which are clearly distinguished by their primary structures.
Ci-opsin1 is the visual pigment of the ocellus of the C. intestinalis larvae (14,15). The molecular phylogenetic analysis clearly showed that Ci-opsin1 is a member of the vertebrate ciliary opsin subfamily (Fig. 2) (14). Like most vertebrate opsins, Ci-opsin1 has a glutamate counter ion in the third transmembrane helix. The intron positions are also conserved between Ci-opsin1 and vertebrate visual pigments. Seven introns (codons 23, 104/105, 159, 194, 268/269, 317/318 and 347/348) interrupt the coding region of Ci-opsin1. Among them, three introns (codons 159, 268/269 and 347/348) are present at positions identical to those of vertebrate opsin genes. The three positions correspond to introns I, III and IV of the human rhodopsin gene. They are conserved among most vertebrate opsin genes, including genes encoding rod and cone opsins, pinopsin, VA opsin, parapinopsin and encephalopsin, but not conserved in genes for melanopsin and invertebrate rhabdomeric opsins. Thus, both the amino acid sequence and the gene structure show a close relationship between Ci-opsin1 and vertebrate visual pigments.
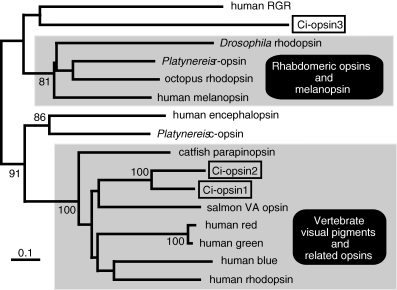
Molecular phylogenetic tree of the opsin family. Ciona opsins are boxed. A phylogenetic tree was inferred from amino acid sequences by using the neighbor-joining method. Bootstrap values based on 1000 replications are shown only when they are greater than 80%. Scale bars indicate 0.1 amino acid replacements per site.
Ciliary and rhabdomeric rhodopsins couple different types of G proteins. Vertebrate ciliary rhodopsin couples with Gt (transducin), while invertebrate rhabdomeric rhodopsin couples with Gq and Go (16,18,19). Five isoforms (Ci-Gαi1a, Ci-Gαi1b, Ci-Gαi2, Ci-Gαq and Ci-Gαx) of G protein α subunits were identified by cDNA cloning in Ciona intestinalis (20). Ci-Gαi1a and Ci-Gαi1b are generated by alternative splicing of the CiGαi1 gene. Although the Ciona genome contains several more genes encoding G protein α subunits, none of them seems to be a clear orthologue of vertebrate transducin (T. Kusakabe et al., unpublished data). The Ci-Gαi1 gene is specifically expressed in particular neurons of the brain vesicle and visceral ganglion, including the ocellus photoreceptor cells (20,21). Therefore, Ci-Gαi1 may couple with Ci-opsin1 in the ocellus of C. intestinalis larvae.
Arrestin is an important protein for the termination of active state of rhodopsin in vertebrate photoreceptor cells (22). Arrestin is also found in protostome invertebrate photoreceptors (23,24). Vertebrate arrestins can be divided into two distinct groups: visual arresin, which is expressed in photoreceptor cells, and β-arrestin, which is expressed in many different tissues. In the retina, cones and rods express different arrestin genes. The ascidian genome contains only one arrestin gene (Ci-arr) (25). Like Ci-opsin1, Ci-Arr is more closely related to vertebrate arrestins than to invertebrate arrestins. Molecular phylogenetic analysis suggests that β-arrestin and visual arrestin of vertebrates appeared by gene duplication after the split between the vertebrate and ascidian lineages. Interestingly, Ci-Arr has a clathrin-binding domain, which is a characteristic of β-arrestin and not found in vertebrate visual arrestins. Ci-arr mRNA are predominantly expressed in the photoreceptor cells of the ocellus (25). Consistently, immunostaining with the Ci-Arr antibody specifically visualizes the cell bodies and axons of photoreceptor cells (Fig. 1c) (26). Thus, Ci-Arr has the feature of both visual and β-arrestins.
The phototransduction cascade in the vertebrate ciliary photoreceptor contains cGMP phosphodiesterase, membrane guanylyl cyclase and cyclic nucleotide-gated channels as major components. Mammals have multiple isoforms of these phototransduction proteins, while only one orthologue is encoded in the genome of C. intestinalis for each of these phototransduction protein subfamilies (T. Kusakabe et al., unpublished data). Ascidian ciliary photoreceptors presumably use these phototrunsduction proteins. Comparative analysis between ascidians and vertebrates suggests that cone- and rod-specific isoforms of phototransduction proteins (and also closely related isoforms used in olfactory cells and other neurons) arose by gene duplications after the divergence between tunicates and vertebrates (Fig. 3).
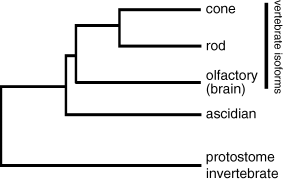
A possible evolutionary history of phototransduction proteins deduced from molecular phylogenetic trees of the arrestin, phosphodiesterase, guanylyl cyclase and cyclic nucleotide-gated channel families.
Visual cycle
The absorption of light by rhodopsin leads to the cis-to-trans isomerization of the chromophore to generate all-trans-retinal. To regenerate rhodopsin and maintain visual sensitivity, the all-trans-retinal must be converted back into the 11-cis-retinal. This process is called “the visual cycle.” In the mammalian eye, the retinal pigment epithelium (RPE) plays an essential role in the visual cycle. RPE65, the highly expressed protein in RPE, plays a key role in the visual cycle by acting as the isomerohydrolase that converts all-trans-retinyl ester to 11-cis retinol in the light-independent manner (27,28). RPE65 and β-carotene 15,15′-monooxygenase (BCO) are similar in amino acid sequences, evolved from a common ancestor, and are both expressed in mammalian RPE (29). Cellular retinaldehyde-binding protein (CRALBP) is of importance as an acceptor of 11-cis retinoid after the isomerization reaction in the visual cycle (30). Another candidate visual cycle protein in mammalian RPE is the retinal G protein-coupled receptor (RGR), which can bind and photoisomerize all-trans-retinal to 11-cis-retinal (31,32).
In C. intestinalis, four genes encoding putative visual cycle proteins, orthologues of RGR (Ci-opsin3 [Fig. 2]), CRALBP (Ci-CRALBP), RPE65 (Ci-RPE65) and BCO (Ci-BCO) have been identified (26,33,34). Ci-opsin3 and Ci-CRALBP are localized in both ocellus photoreceptor cells and surrounding nonphotoreceptor cells in the brain vesicle of the larva (26). In contrast to Ci-BCO, which is predominantly localized in the ocellus photoreceptor cells of the larva, Ci-RPE65 is not significantly expressed in the ocellus and brain vesicle of the larva (34). Ci-RPE65 is expressed in the neural complex, a photoreceptor organ of the adult ascidian, at a level comparable to that of Ci-opsin3 and Ci-CRALBP. These results suggest that the larval visual cycle uses Ci-opsin3 as a photo-isomerase while the visual cycle of the adult photoreceptors is RPE65-dependent. Ci-RPE65 is also expressed in various adult tissues, including the gill, body wall, and intestine, suggesting that Ci-RPE65 plays an unrecognized role in addition to that in the visual cycle (34).
Other photoreceptor organs in ascidians
Three types of light-responsive behaviors have been described in the adult ascidians: siphon contraction (35), phototropism (36) and gamete release (spawning) (37,38). The pigmented spots around the siphon openers (39), the epithelial cells of the sperm duct (40,41) and the cerebral ganglion (42–45) have been suggested to be candidate photoreceptor organs underlying these behaviors.
The C. intestinalis genome contains another vertebrate visual pigment-like opsin gene, Ci-opsin2, which is the paralogue of Ci-opsin1 (Fig. 2) (T. Kusakabe et al., unpublished data). Ci-opsin2 was not found in the photoreceptor cells of the ocellus. Instead, Ci-opsin2 is localized in different parts of the brain vesicle as well as in the oral siphon rudiment (stomodæum) of the larva. Localization patterns of Ci-opsin2 suggest that photoreceptive cells are distributed at multiple extra-ocellar sites in the larva. In adult ascidians, Ci-opsin1- or Ci-opsin2-immunoreactive cells are also found in several different tissues, including the cerebral ganglion and some other tissues that have not been previously identified as photoreceptors.
The origin of vertebrate eyes
Vertebrates have evolved two types of eyes; the lateral eyes (paired eyes) and the median eyes (pineal or parietal eyes). We have proposed a hypothesis that the larval ocellus of ascidians is homologous to the median eye of vertebrates (11,14). This hypothesis is based on their similarities in several aspects.
Larvae of various ascidian species commonly exhibit the shadow response, in which an abrupt decrease in light intensity elicits active swimming (6,11). It is photoreception by the ocellus that triggers this behavior (7,15). The shadow response is also seen in larvae of teleosts and amphibians (46–51). The shadow response in young Xenopus larvae is mediated by the pineal eye (48,52,53). Thus the pineal eye and the ascidian larval ocellus have a similar function at the larval stage.
Both the ascidian ocellus and the pineal eye are the first photoreceptor organ that becomes functional during ontogeny. In lampreys, for example, opsin immunoreactivity appears in the pineal eye of early prolarvae and 3 or 4 days later in the retina (54). Similar observations were reported for teleost embryos (55,56). At the stage when the pineal eye triggers the shadow response, the developing lateral eyes are not competent to respond to the light stimulus in Xenopus tadpole (52).
The other features shared between the median eye of vertebrates and the ocellus of ascidian larvae are their location and developmental origin. Both organs are located in a dorsal part of the forebrain. They are both derived from cells located in lateral parts of the embryonic neural plate (57,58).
If the larval ocellus of ascidians is homologous to the median eye of vertebrates, where do the vertebrate lateral eyes come from? Are there the lateral eye homologues in ascidians? The answer might be “Yes”. We have found that a number of genes related to eye function and development are expressed in the stomodæum (oral siphon rudiment) of the ascidian larva (T. Kusakabe et al., unpublished). The stomodæum develops from cells located at the central part of the neural plate as the vertebrate lateral eyes do (57,58). Thus, the last common ancestor of tunicates and vertebrates may have possessed distinct precursors of the lateral eyes and the median eye of vertebrates.
Acknowledgments
Acknowledgements— Our studies presented in this paper have been supported in part by Grants-in-Aid for Scientific Research from the MEXT (16370075, 17018018, 18370089) and by grants from Japan Space Forum (h160179), the Sumitomo Foundation (041045) and Narishige Zoological Science Award.




