Pressure-induced Isomerization of Retinal on Bacteriorhodopsin as Disclosed by Fast Magic Angle Spinning NMR†
This paper is part of the Proceedings of the 12th International Conference on Retinal Proteins held at Awaji Island, Hyogo, Japan on 4–8 June 2006.
Abstract
Bacteriorhodopsin (bR) is a retinal protein in purple membrane of Halobacterium salinarum, which functions as a light-driven proton pump. We have detected pressure-induced isomerization of retinal in bR by analyzing 15N cross polarization-magic angle spinning (CP-MAS) NMR spectra of [ζ-15N]Lys-labeled bR. In the 15N-NMR spectra, both all-trans and 13-cis retinal configurations have been observed in the Lys Nζ in protonated Schiff base at 148.0 and 155.0 ppm, respectively, at the MAS frequency of 4 kHz in the dark. When the MAS frequency was increased up to 12 kHz corresponding to the sample pressure of 63 bar, the 15N-NMR signals of [ζ-15N]Lys in Schiff base of retinal were broadened. On the other hand, other [ζ-15N]Lys did not show broadening. Subsequently, the increased signal intensity of [ζ-15N]Lys in Schiff base of 13-cis retinal at 155.0 ppm was observed when the MAS frequency was decreased from 12 to 4 kHz. These results showed that the equilibrium constant of [all-trans-bR]/[13-cis-bR] in retinal decreased by the pressure of 63 bar. It was also revealed that the structural changes induced by the pressure occurred in the vicinity of retinal. Therefore, microscopically, hydrogen-bond network around retinal would be disrupted or distorted by a constantly applied pressure. It is, therefore, clearly demonstrated that increased pressure induced by fast MAS frequencies generated isomerization of retinal from all-trans to 13-cis state in the membrane protein bR.
Introduction
Bacteriorhodopsin (bR), from Halobacterium salinarum, is a 26 kDa seven transmembrane helix protein with a retinal as a chromophore via Lys216 in helix G and shows proton pump activity starting from proton release in Schiff base initiated by retinal photoisomerization from all-trans to 13-cis, 15-anti state (1). The retinal configurations of all-trans and 13-cis, 15-syn states in the dark-adapted bR coexist in the isomeric ratio close to 1 (2–4). Primary amino acid residues in the vicinity of Schiff base region of retinal are Arg82, Asp85, Tyr185 and Asp212 forming hydrogen-bond network with strongly hydrogen-bonded water molecules (5). bR assembles into naturally occurring 2D crystalline patches known as a purple membrane (PM) in which its trimeric unit is hexagonally packed under the physiological condition (6). 3D structure of bR with 1.55 Å resolutions has been revealed by X-ray diffraction study (7). To gain insight into the mechanism of proton pump activity of bR, it is important to understand how retinal configurations influence conformation and dynamics of retinal protein in a molecular level.
The above-mentioned isomeric ratio of retinal ([all-trans-bR]/[13-cis-bR]) in the dark-adapted state has been shown to decrease from unity when the pressure was increased because of the negative molar volume changes (8,9). Namely, pressure for bR in the dark causes the shift of isomer equilibrium from all-trans toward 13-cis state with two processes accompanied by the molar volume changes of −21 and −7 mL/mol (10). Because the molar volume change of the first step is quite large, it is expected that even in the small pressure change would give a large change of the population between 13-cis and all-trans state. The isomeric ratio in the dark, however, is insensitive to the temperature change (11,12) and bR is thermally very stable (13), although it is reported that high temperature intermediate in bR exists at a temperature higher than 60°C (14).
We have observed that local conformation and dynamics by 13C-NMR study of site specifically isotopic-labeled bR (15–17). In particular, it turned out that 13C-NMR peaks were well resolved for fully hydrated [3-13C]Ala-, [1-13C]Val-labeled bR, depending upon their local dynamics and conformations of 29 Ala or 21 Val residues, respectively (18,19). In this study, pressure effects to isomeric ratio of retinal were observed in the 15N-NMR spectra in [ζ-15N]Lys-labeled bR in the dark under the fast magic angle spinning (MAS) conditions. MAS can eliminate the chemical shift anisotropy when the rotation axis of sample is inclined to 54.7° toward the static magnetic field and the rotor frequency is faster than the chemical shift anisotropy (20,21). We realized that MAS frequency generates a pressure on the inner wall of the rotor because of the centrifugal forces. Nevertheless, solid-state NMR experiments on this pressure effects have been reported in a few cases. First, interlamellar water of dispersions in multilamellar vesicles (1,2-dioleoyl-sn-glycero-3-phosphocholine [DOPC] and l-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine [POPC]) decreases with spinning frequency in 1H-MAS NMR (22). Second, dynorphin (opioid peptide; 17 residues) has partially dissociated from lipid bilayers on 13C-MAS NMR condition. In the static condition, the peptide binds to membrane and consequently forms magnetically oriented vesicle systems (23,24).
In this study, we will focus attention on pressure effects for bR in PM by solid-state MAS NMR measurements. Retinal configurations in bR were revealed by 15N-NMR experiments of [ζ-15N]Lys-labeled bR under the fast MAS conditions. We consider that this experimental system applies constantly low pressure for fully hydrated sample and the pressure of 63 bar was induced at the MAS speed of 12 kHz.
Materials and methods
H. salinarum S9 was grown in a synthetic medium including [ζ-15N]-l-Lys to yield [ζ-15N]Lys-labeled bR in PM. PM was isolated by the standard method as described (25) and suspended in 5 mM 2-[4-(2-Hydroxyethyl)-1-piperizinyl] ethanesulfonic acid (HEPES) buffer containing 0.02% NaN3 and 10 mM NaCl at pH 7. The sample was concentrated by centrifugation (40 000 × g, 60 min, 40°C) and placed in 4.0 mm outer diameter (o.d.) zirconia pencil-type rotor for the fast MAS experiments. Sample rotor was tightly shielded by a piece of optical-fiber cap and glued to the top part of rotor by rapid Alardyte® to prevent dehydration of pelleted samples. In situ light illumination to sample was made through optical fiber. Namely, light was delivered from outside to inside of the magnet and illuminated to the top of rotor without any contact, so that the fast MAS condition can be achieved.
15N high-resolution solid-state NMR spectra were recorded on a Chemagnetics CMX-400 infinity Fourier transform (FT)-NMR spectrometer operating at 100.16 MHz for carbon and 40.3 MHz for nitrogen nuclei. Cross polarization (CP)-MAS with a variable amplitude contact pulse of observed nucleus (RAMP-CP) were used (26). A double resonance MAS probe equipped with a stator assembly for 4.0 mm o.d. rotor was used throughout the measurements. The spinning frequencies were set to 4–12 kHz and 90° pulse was 5.1 μs. The MAS NMR measurements were performed again at 4 kHz after the measurements at 12 kHz. Ambient conditions of MAS were set at 4 kHz and probe setting temperature at 20°C. Two-pulse phase modulation decoupling (27) was employed during the acquisition time. 15N contact time and repetition time were 2 ms and 4 s, respectively. 15N chemical shifts were externally referenced to 11.59 ppm for the amino nitrogen of glycine from NH4NO3.
Pressure on the samples was naturally applied by the centrifugal force induced by MAS frequency. As the centrifugal force on the inner wall of the cylindrical rotor is proportional to the square of rotor radius and the square of rotor frequency, pressure of inner wall of the rotor with 4 mm o.d. can be estimated to be 7 bar and 63 bar for the rotor frequency of 4 and 12 kHz, respectively. Even though pressure distribution will be presented depending on the location of samples, it is expected that most of the samples are located near the inner wall of rotor to show relatively uniform pressure.
Results and discussion
Figure 1a–d shows 15N CP-MAS NMR spectra of [ζ-15N]Lys-labeled bR at the spinning frequencies of 4, 8, 10 and 12 kHz in the dark. After the NMR spectrum was measured for the spinning sample at the MAS frequency of 12 kHz, CP-MAS spectrum was recorded also at 4 kHz to examine the pressure effects on retinal configurations (Fig. 1e). The 15N signals of free [ζ-15N]Lys, backbone amides of naturally abundant amino acid residues and [ζ-15N]Lys of Schiff base were observed at 11.0 ppm, 90–110 ppm and 140–160 ppm, respectively. The signal intensities of the free lysine was largely decreased at the MAS frequencies of 10 and 12 kHz as compared with those of backbone components, because the Hartmann–Hahn matching condition H1HγH = H1NγN was disturbed by the fast MAS. When the MAS frequency was returned to 4 kHz, the line broadening, intensities and chemical shifts of these signals were recovered.
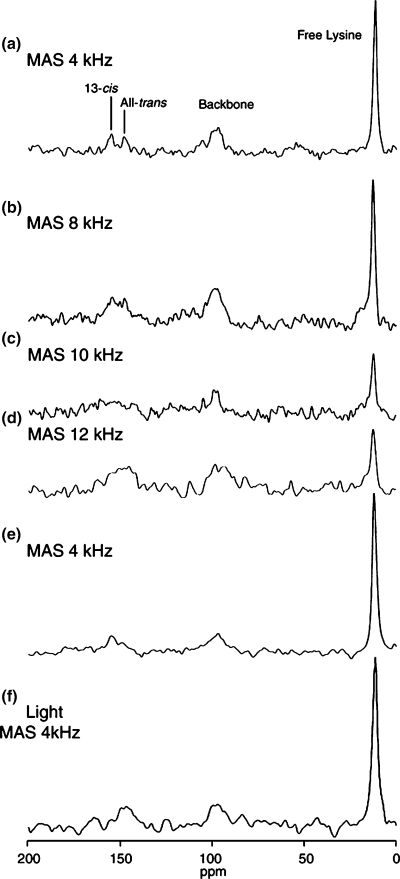
15N cross polarization-magic angle spinning (CP-MAS) NMR spectra of [ζ-15N]Lys-labeled bR at various magic angle spinning frequencies up to 12 kHz (a–d). After spinning sample at 12 kHz, 15N CP-MAS NMR spectrum was recorded at 4 kHz (e). In situ light-irradiation spectrum of bR (f).
Figure 2 shows that the 15N CP-MAS spectra of [ζ-15N]Lys-bR in the protonated Schiff base were observed in the spectral range between 140 and 160 ppm. The signals of the protonated Schiff base are shown to be a sensitive probe on the changes of retinal isomerization and environment (Fig. 2a–d) (10,28,29). Both 15N-NMR signals due to all-trans and 13-cis retinal configurations were observed in the same intensities at 148.0 and 155.0 ppm, respectively, under the MAS frequency of 4 kHz (Fig. 2a). When the MAS frequency was increased up to 12 kHz, the 15N-NMR signals of Schiff base of retinal showed marked broadening (Fig. 2b). This finding indicates that the hydrogen-bond network around the protonated Schiff base is locally distorted with the induced pressure under the fast MAS condition (Fig. 3). The increased signal intensity of the 13-cis retinal at 155.0 ppm appeared increased as the MAS frequency was decreased from 12 to 4 kHz (1, 2). This observation was made possible when the line narrowing in the MAS frequency of 4 kHz recovered to be able to distinguish all-trans from 13-cis signals. It was also noticed that the retinal configurations showed hysteresis by the fast MAS experiments. Namely, the exchange rate from 13-cis to all-trans retinal is very slow. To assign the 15N-NMR signals to either 13-cis or all-trans configuration, white light was irradiated continuously to bR and significant increase of the signal of all-trans was observed (Fig. 2d). It was clearly shown that the all-trans retinal configuration appeared at 148 ppm in the 15N peak-position by illumination of the white light. Therefore, it turned out that retinal configurations of bR was changed from the all-trans to 13-cis retinal of [ζ-15N]Lys-labeled bR by the fast MAS experiments. Obviously, the present observation suggests that the pressure-induced isomerization of retinal also causes distortion of such hydrogen-bond networks in the vicinity of retinal (Fig. 3) (7), together with a possible dehydration effect of surrounding water molecules by centrifuging effect of the fast MAS. It is noticed that the isomerization from all-trans to 13-cis state occurred at the pressure of 63 bar in the solid-state NMR experiment. This pressure is much lower than the reported case where the populations of isomers were estimated from the absorption spectra (8,9). 15N-NMR spectra have been observed previously under the condition of the MAS frequency of 3 kHz and −75°C to show two signals due to all-trans and 13-cis states of bR. However, the increase of 13-cis state was not observed because of the experimental conditions where the pressure was very low (4).
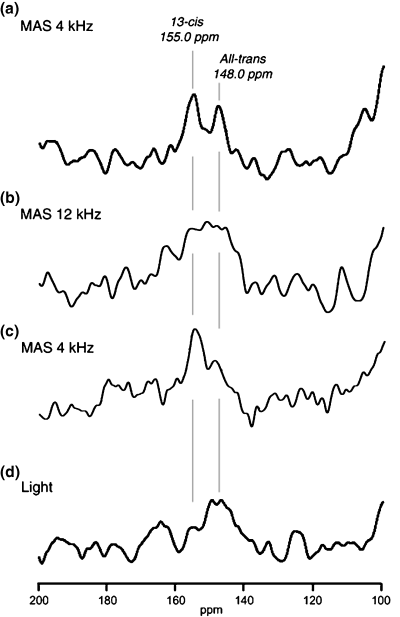
15N cross polarization-magic angle spinning (CP-MAS) NMR spectra of [ζ-15N]Lys of Schiff base in bR at MAS frequency of 4 and 12 kHz, respectively (a and b). After spinning sample at 12 kHz, CP-MAS NMR spectrum was observed at 4 kHz (C). In situ light-irradiation spectrum of bR (d).
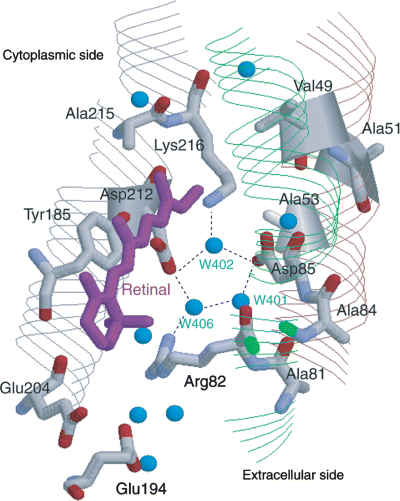
Schematic representation of primary amino acid residues and water in the vicinity of retinal (7) (PDB Code: 1C3W).
It is important to point out that the fast MAS also generate the increased sample temperature because of the heating by friction. Therefore, we have measured the sample temperature within the rotor under the fast MAS condition by monitoring 207Pb chemical shifts of lead nitrate (30). It turned out that the temperature of the sample was increased by 20°C at the MAS frequency of 12 kHz. We, therefore, raised the temperature to 40°C by keeping the MAS frequency at 4 kHz. The increased intensity of 13-cis peak was not observed although some line broadening was noted. These experiments indicate that isomerization from all-trans to 13-cis is mainly caused by the pressure increase, although the line broadening in the fast MAS condition can be caused by both temperature and pressure effects.
Isomerization from all-trans to 13-cis has been studied by extraction techniques and absorption spectroscopy of the range from low to high pressure (1–3000 bar) in which the isomer equilibrium shifts from all-trans to 13-cis state at two processes with the molar volume changes in the dark (10). Thermodynamically, this change of equilibrium constant of [all-trans-bR]/[13-cis-bR] can be attributed to the large molar volume change from all-trans to 13-cis state. Microscopically, locations of water molecules in the vicinity of retinal (7) and hydrogen-bond networks via water molecules that correlate with proton pump activity may be distorted by the increased pressure. In solution high-pressure 15N/1H NMR techniques utilizing pressure cell, fluctuations of hen lysozyme are found to be localized near the cavities containing water molecules (31,32). In general, hydrogen bonding H···O or H···N distances in protein are changed by pressure accompanied by a molar volume change (32). Therefore, the pressure-induced isomerization of retinal and modulation of the hydrogen-bond network can cause the change in structure and motion of residues in proteins located in the vicinity of retinal.
Noticeable pressure effects on bR in PM in the molecular level were observed at the first time in these experiments. Increased pressure by the fast MAS frequencies induces isomerization from the all-trans to 13-cis retinal. It is possible to increase 13-cis state by applying pressure and the all-trans state by irradiation of light. It is stressed that the MAS experiment is a good source of pressure as the induced pressure will be proportional to the square of MAS frequency. This kind of pressure effect in membrane protein has not yet been studied because it was thought that the application of pressure in the solid-state NMR spectroscopy was difficult. This experiment, therefore, clearly showed that pressure-induced experiment can be successfully applied by the fast MAS experiment. It is also important to point out that it is possible to change the protein equilibrium by applying the pressure because a large molar volume change in a large heterogeneous system such as membrane protein can alter the equilibrium constant by relatively small change of pressure.
Acknowledgements— The present work was supported by a Grant-in-Aid for Scientific Research on Priority Area (17048010) from MEXT, Japan and for Scientific Research (17370054) from JSPS and by Research Grant-in-Aid from Magnetic Health Science Foundation. I.K. thanks Yokohama National University for “Research Aid Program for Ph.D. Candidates.”




