Tryptophan 171 in Pharaonis Phoborhodopsin (Sensory Rhodopsin II) Interacts with the Chromophore Retinal and its Substitution with Alanine or Threonine Slowed Down the Decay of M- and O-intermediate†
This paper is part of the Proceedings of the 12th International Conference on Retinal Proteins held at Awaji Island, Hyogo, Japan on 4–8 June 2006.
Abstract
Pharaonis phoborhodopsin (ppR), also called pharaonis sensory rhodopsin II, NpSRII, is a photoreceptor for the photophobic response of Natronomonas pharaonis. Tryptophan 182 (W182) of bacteriorhodopsin (bR) is near the chromophore retinal and has been suggested to interact with retinal during the photoreaction and also to be involved in the hydrogen-bonding network around the retinal. W182 of bR is conserved in ppR as tryptophan 171 (W171). To elucidate whether W171 of ppR interacts with retinal during the photoreaction and/or is involved in the hydrogen-bonding network as in bR, we formed W171-substituted mutants of ppR, W171A and W171T. Our low-temperature spectroscopic study has revealed that the substitution of W171 to Ala or Thr resulted in the stabilization of M- and O-intermediates. The stability of M and absorption spectral changes during the M-decay were different depending on the substituted residue. These findings suggest that W171 in ppR interacts with retinal and the degree of the interaction depends on the substituted residues, which might be rate determining in the M-decay. In addition, the involvement of W171 in the hydrogen-bonding network is suggested by the O-decay. We also found that glycerol slowed the decay of M and not of O.
Introduction
There are four kinds of rhodopsins (retinal proteins) in Halobacterium salinarium; bacteriorhodopsin (bR) and halorhodopsin are light-driven ion pumps, while sensory rhodopsin (sR) and phoborhodopsin (pR; also called as sensory rhodopsin II, SRII) are photoreceptive pigments for the phototaxis. A pR-like pigment was found in a haloalkaliphilic bacterium, Natronomonas (Natronobacterium) pharaonis (1) and was revealed to be a photoreceptor of the photophobic response of this bacterium (2) like pR in H. sarinarium. Thus, the pigment has been named pharaonis phoborhodopsin (ppR) or pharaonis sensory rhodopsin II (NpSRII). Recent development in an expression system of ppR in Escherichia coli cells (3) made it possible to obtain an ample amount of pigment and also various mutants (4). Thus, ppR has been studied extensively instead of pR (5–9).
The absorption maximum of ppR is located at ∼500 nm. Upon photoexcitation of ppR by green-blue light, the chromophore retinal undergoes a cis-trans isomerization from all-trans to 13-cis form, followed by the thermal relaxation via several linearly located intermediates to the original ppR. Thus, the linear and cyclic photochemical reaction is called a photocycle. By low-temperature spectroscopy and time-resolved absorption spectroscopy, K-, L-, M- and O-like intermediates have been identified (7,8,10,11), named after intermediates in the bR photocycle with regard to absorption maxima. Thus, the photocycles of bR and ppR are quite comparable in spite of their amino acid sequence homology being about 53% (12,13). There is a controversy about the N-intermediate: A multiexponential global analysis of the ppR photocycle under several different conditions suggested the presence of an N-intermediate (6), while Fourier transform infrared (FTIR) studies revealed that the N-like species was not found (14,15).
The crystal structure of ppR was reported by two groups (16,17). The X-ray crystallographic studies revealed that the molecular structure of environments of the retinylidene Schiff base of bR and ppR are similar to each other, although the physiological role of both pigments is different. The photocycle of bR and ppR involves not only the reversal of the isomerization of chromophore retinal but also proton transfer steps within the protein (18–20). However, the times required for the completion of the bR and ppR photocycle are different; in the case of bR it is <100 ms, while that of ppR is >1 s. M- and O-intermediates of bR decay within 10 ms, whereas in the ppR photocycle the decay of those intermediates takes several 100 ms to several seconds. Although the molecular origin of this difference has not been well elucidated, several mutant pigments of ppR having accelerated M- and O-decay comparable with those of bR were formed by the amino acid replacement of ppR with the corresponding residue of bR (21–23).
Tryptophan 182 in the F-helix of bR (W182) is near the chromophore retinal with which it has been suggested to interact during the photochemical reaction. The interaction results in large structural changes in the F-helix found in the M- and/or N-states of the bR photocycle, which would have an important role in proton pumping activities (24–26). The 9-methyl group of the chromophore retinal is involved in the interaction with W182 (27,28). W182 is also reported to be involved in the hydrogen-bonding network around the retinal and its substitution with phenylalanine resulted in the formation of L at a much lower temperature than in the wild bR (29). Tryptophan 182 of bR is conserved in ppR as tryptophan 171 (W171).
In this study, we investigated the effects of the mutation of W171 on the photochemical reaction of ppR. In order to examine whether a similar molecular interaction between W182 of bR and chromophore retinal occurs during the photocycle of ppR, we created W171A and W171T mutants of ppR using E. coli expression system to study their photochemical reaction. We especially focused on the formation and decay of the M-intermediate. Low-temperature spectroscopic studies revealed that the substitution of W171 to Ala or Thr resulted in the stabilization of the M- and O-intermediates. The stability of the M-intermediate and the absorption spectral changes during the decay process from the M-intermediate were different depending on the substituted residue, Ala or Thr, suggesting that W171 in ppR interacts with chromophore retinal and the change in this interaction during the photocycle might be rate determining for the decay process from the M-intermediate as in the bR photocycle. We also found that glycerol stabilized the M-intermediate of ppR, not the O-intermediate.
Materials and methods
Preparation of mutant proteins. The plasmids for expression of wild ppR and its mutant proteins were prepared as described previously (30) based on the methods by Kunkel et al. (31). Oligonucleotide primers were designed according to the nucleotide sequence in GenBank (Accession No. Z35086). pGEM-T Easy including a psopII full-length sequence was used as a template for PCR. The fragment obtained was ligated to pET21c (Novagen, Madison, WI). The nucleotide sequences of the constructs were analyzed by an automated sequencer (377 DNA Sequencer; Applied Biosystems). BL21 (DE3) was used for expressing the construct. The expression of the wild type and mutated gene was induced with 1 mM isopropyl-1-thio-β-galactoside. At the same time, 10 μM all-trans retinal was added. The cells expressing the wild ppR, W171A and W171T with C-terminal 6-His tag were spun down and treated with a French Press to obtain the membrane fraction, as described previously (3). The crude membranes were solubilized with 1%n-dodecyl-β-d-maltoside (DDM) and then the proteins were purified with a nickel column.
Absorption measurements. Absorption spectra were measured by MPS 2000 recording spectrophotometer (Shimadzu, Kyoto) or U-3210 spectrophotometer (Hitachi, Tokyo). The formation of M and its decay at 0°C and −30°C were measured by MPS 2000 equipped with a glass Dewar as described previously (32). For spectral measurements below 0°C, the sample was suspended with 66% glycerol in 10 mM Tris–HCl, pH 7.6, 200 mM NaCl, 0.1–0.3% DDM. In order to investigate the effect of glycerol on the decay of M-intermediate of wild or mutant ppR, samples with and without glycerol were prepared to be measured in the same way as mentioned above. The actinic light was provided from a 150 W halogen slide-projector through a combination of an IR-cut filter and a cut-off filter (>500 nm).
Results
Absorption spectra of the samples at 0°C
Figure 1 shows the absorption spectra of wild ppR (curve 1), W171A (curve 2) and W171T (curve 3) at 0°C, where their values of the absorbance at 495 nm are normalized. The medium is described in the legend. As shown in Fig. 1a, substitution of W171 to Ala or Thr did not cause a wavelength shift of the absorption maxima (λmax). For W171A and W171T mutants, the absorbance peak appears around 400 nm probably due to contamination with heme protein(s) in the sample. The amount of mutant proteins expressed was less than that of wild ppR, and the purification was difficult. As shown in Fig. 1b, irradiation of the fully dark-adapted ppR (curve 1 in Fig. 1b) in the presence of 66% glycerol at 0°C caused a small absorbance increase in the wavelength region shorter than 425 nm (curve 6 in Fig. 1b), suggesting the formation of M.
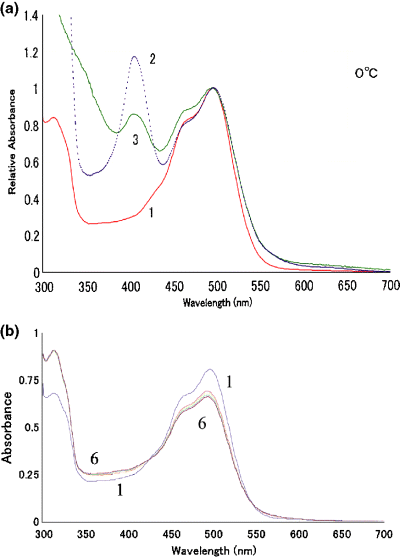
Absorption spectra of the samples used (wild ppR, W171A and W171T) and their photoreaction at 0°C. The absorption spectra of wild pharaonis phoborhodopsin (ppR) (curve 1), W171A (curve 2) and W171T (curve 3) at 0°C are shown in a. After incubation in the dark overnight (curve 1 in b) the wild ppR protein was irradiated with the yellow light (>500 nm) successively for 5, 5, 10, 20 and 40 min (curves 2–6 in b, respectively). The medium was 200 mM NaCl containing 66% glycerol, 10 mM Tris–HCl (pH 7.6) and 0.1%n-dodecyl-β-d-maltoside.
It is well known that bR exhibits light–dark adaptation. An illumination of bR incubated in the dark causes light–dark adaptation (photoisomerization of chromophore retinal from 13-cis to all-trans form) and the formation of M (photocycle of bR with all-trans retinal as its chromophore) (32,33). As the formation of M from bR cannot be detected with a conventional spectrophotometer at 0°C even with 66% glycerol, the difference between absorption spectra of bR before and after the irradiation at 0°C represents a characteristic form for the light–dark adaptation (32). The light–dark absorbance change of ppR shown in Fig. 1b (see also curve 2 in Fig. 5a) did not resemble that of the light–dark adaptation of bR, suggesting no light–dark adaptation in ppR. This is consistent with the previous work reporting that wild ppR lacks this phenomenon (34). Nevertheless, the mutants of W171 were checked for the light–dark adaptation and no adaptation was observed. Thus, we started the following experiments after sufficient dark-incubation to avoid the contamination of any photointermediates in the sample.
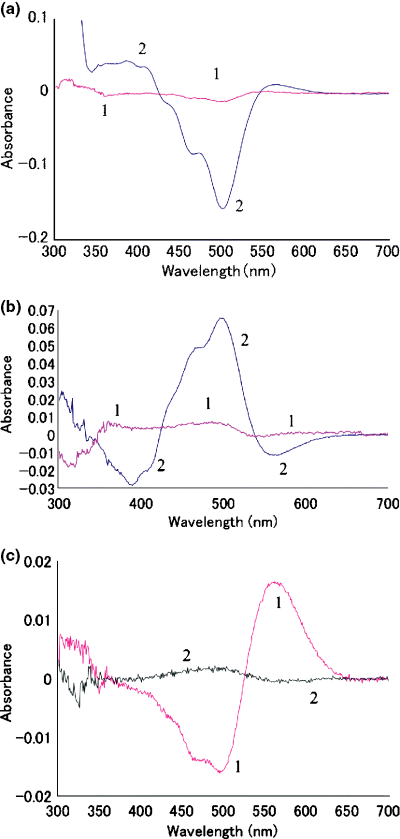
Photoreaction (a) and thermal reaction (b) of pharaonis phoborhodopsin (ppR) in the presence and absence of 66% glycerol at 0°C. Wild ppR without glycerol (curve 1 in a and b) and with 66% glycerol solution (curve 2 in a and b) were irradiated with yellow light for 5 min at 0°C and the difference absorbance was measured (a). After dark incubation for 5 min at 0°C, the absorption spectrum was measured (b). W171T without glycerol (c) was irradiated with yellow light for 5 min at 0°C (curve 1 in c) and then incubated in the dark for 5 min at 0°C (curve 2 in c). The difference absorbance spectra between before and after the treatment were measured at 0°C.
M formation and decay at −30°C
Irradiation of ppR at −30°C with yellow light caused the formation of M-intermediate (Fig. 2a), but successive prolonged irradiation did not fully convert ppR into M-intermediate. It may be that the M-intermediate from wild ppR is not stable at −30°C (Fig. 2b); M-intermediate slowly reverted to ppR even at −30°C in the dark. It was reported that M-intermediate with 66% glycerol was stable at −60°C in the dark (35). By warming the sample to higher temperatures, the reversion from M-intermediate to the original ppR was accelerated (Fig. 2c). As shown in Fig. 3a, difference absorption spectra during the warming had an isosbestic point and the shapes of these spectra are similar, implying that accumulation of warming products other than the original ppR did not occur. However, closer inspection of Fig. 3a shows a small negative band in the longer wavelength region (550–600 nm) due to the O-intermediate. This indicates that irradiation of this sample at −30°C yielded M-intermediate and a small amount of O-intermediate resulting from decay of M-intermediate. There was also an isosbestic point between the original ppR and O-intermediate. The curve number j in Fig. 3d was calculated by the subtraction of curve j in Fig. 3a from the curve j + 1 in Fig. 3a (j = 1–8). In other words, the curves in Fig. 3d show the difference in the difference spectra of Fig. 3a as the temperature is increased by 5°C increments. It is natural that the magnitude of the spectrum j increases and then decreases with increase in j. Interestingly, these “temperature-difference spectra of the difference spectra” (shown in Fig. 3d) are similar to each other in their shape. These findings mean that M-intermediate and a small amount of O-intermediate formed by irradiation at −30°C decay to the original pigment keeping approximately the same ratio when warmed.
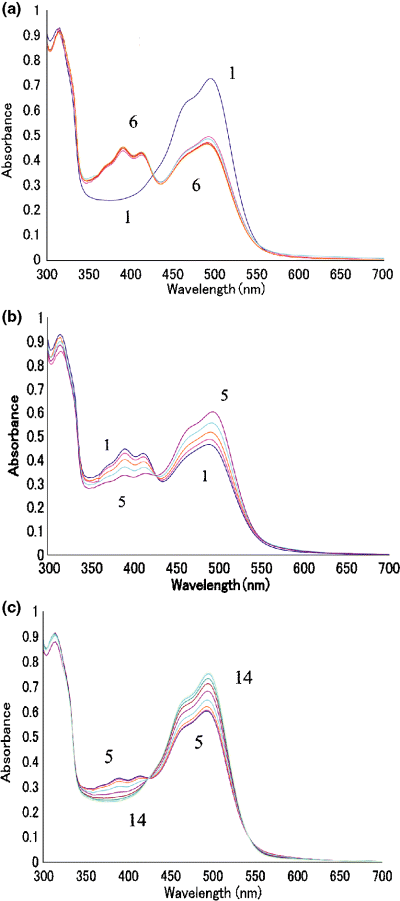
Formation and decay of M-intermediate from wild pharaonis phoborhodopsin (ppR). The wild ppR (curve 1) was successively irradiated with the yellow light for 5, 5, 10, 20 and 40 min (curves 2–6 in a). The product by the irradiation of ppR for total 80 min (curve 1, same as curve 6 in a) was incubated in the dark at −30°C for 5, 10, 20 and 40 min (curves 2–5 in b, respectively). The dark product (curve 5, same as curve 5 in b) was warmed to predetermined temperatures (which are mentioned below), followed by rapid cooling to −30°C to measure the absorption spectrum. Curves 6–14 in (c) are spectra at −25, −20, −15, −10, −5, 0, 10, 15 and 20°C, respectively. The medium is the same as that in Fig. 1.
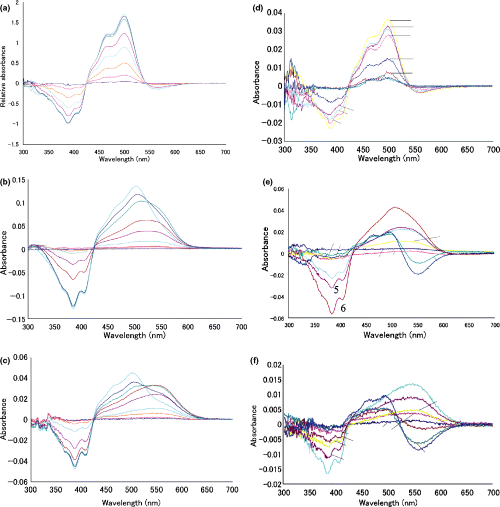
The spectral change in the decay from M-intermediate of wild or mutant pharaonis phoborhodopsin (ppR). The M-intermediate of wild ppR (a), W171A (b) or W171T (c) was warmed up to −25, −20, −15, −10, −5, 0, 10, 15 and 20°C, followed by the rapid cooling to −30°C and absorption measurements at this temperature. Curves 1–9 represent the spectra of the products by the warming to the temperature mentioned, respectively. The right three panels (d, e and f) reveal the “temperature-difference spectrum” calculated from the difference spectra shown in a, b and c. Refer to text for details. This calculation shows clearly the shape change in the difference spectra for the warming. Thus, these panels reveal the transition of absorption bands during the thermal decay.
In the case of W171A or W171T, irradiation with yellow light at −30°C caused a conversion of almost all pigments to their M-intermediate (data not shown), indicating that M-intermediate of these mutant pigments was stable at −30°C in the dark. This was confirmed by the fact that no spectral change was observed by warming to −25°C (3, 4), while apparent changes were noticed for wild ppR (3, 4). Note that this stability is in sharp contrast to the wild ppR. During the warming of M-intermediate from W171A or W171T to higher temperatures, absorbance in the longer wavelengths (greater than 550 nm) reached a maximum at 0°C and decreased at higher temperatures (Fig. 3b,c). The results suggest that the M-intermediate of W171A or W171T was first converted to an intermediate with λmax in the longer wavelength region and then was converted to the original pigment. This decay pathway of the M-intermediate is more clearly elucidated in the “temperature-difference spectra of the difference spectra” as shown in Fig. 3e,f. In the case of warming of M-intermediate from wild ppR, all the difference spectra had difference minima at about 390 nm and maxima at 495 nm (Fig. 3d). On the other hand, in the warming of mutant M-intermediate, the “temperature-difference absorption spectra” between −10°C and −15°C (curve 4 in Fig. 3e,f) represented an absorbance decrease at 390 nm and an increase around 525 nm in W171A (Fig. 3e) or 550 nm in W171T (Fig. 3f). The “temperature-difference spectra” between 0°C and 10°C (curve 7 in Fig. 3e,f, respectively) represent the absorbance decrease in the longer wavelength region (greater than 520 nm) and increase at about 490 nm, which is the λmax of the original pigments.
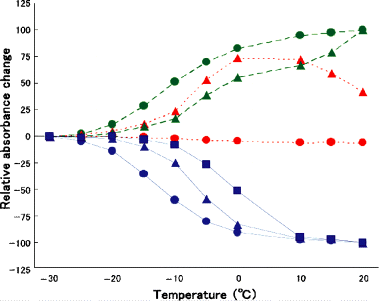
The absorbance changes at 550 nm (dotted line), 500 nm (broken line) and 390 nm (solid line) were plotted against the temperature to which M-intermediate of pharaonis phoborhodopsin (ppR) (circle), W171T (triangle) and W171A (square) were warmed. The absorbance changes at 390, 550 and 500 nm monitored M-, O-intermediate and the original ground state, respectively. All the spectra were measured at −30°C as described in the legend to 2, 3. This figure shows the thermal stability of each intermediate of the wild ppR and mutants.
Figure 4 shows the relative absorbance change at 550 nm (dotted line), 500 nm (broken line) and 390 nm (solid line) of wild ppR, W171T and W171A (only at 390 nm) from those at −30°C when the temperature is increased to that shown in the abscissa. For wild ppR, the small absorbance at 550 nm did not change during the warming, while it began to increase at lower temperatures and then decreased in W171T, implying clearly the difference in the decay process of M-intermediate of both pigments. Therefore, Fig. 4 shows the difference in the stability of M-intermediate of these pigments. From the absorbance decrease at 390 nm (solid lines in Fig. 4), we may conclude that M-intermediate of wild ppR was most unstable and that of W171A was most stable.
Effect of glycerol on the photocycling rate of ppR
During the course of experiments, we found that in the medium containing glycerol the decay of M-intermediate became so slow that the accumulation of M-intermediate by irradiation was observed even at 0°C with a conventional spectrophotometer. Thus, we compared the photoreaction of wild ppR and mutant pigments at 0°C in the absence of glycerol to circumvent the effect of glycerol. Figure 5a,b showed a comparison of photoreaction (Fig. 5a) or dark reaction (Fig. 5b) of wild ppR between the presence and the absence of glycerol. As shown in Fig. 5a, little absorbance change was observed in wild ppR without glycerol by yellow light irradiation for 5 min, although in the presence of 66% glycerol, the amount of absorbance change caused by the same irradiation was drastically increased and accumulation of M was clearly observed. However, the photoproduct, mainly M-intermediate and small amount of O-intermediate, was not stable at 0°C and converted to the original pigment in the dark (Fig. 5b). These results show that glycerol stabilizes the M-intermediate of wild ppR but not the O-intermediate because the accumulation of O-intermediate was not observed during the decay of M-intermediate. In the case of W171T without glycerol, the irradiation with yellow light at 0°C caused a formation of longer wavelength absorption than the original pigment, probably due to O-intermediate (curve 1 in Fig. 5c). Moreover, the photoproduct was almost stable at 0°C for 5 min in the dark (curve 2 in Fig. 5c). The formation of stable O-intermediate was also observed by irradiation of W171A at 0°C (data not shown). These results clearly showed that O-intermediate was stabilized by the substitution of W171 to Ala or Thr.
The flash photolysis measurements revealed that decay of M-intermediate of W171A in the solution without glycerol was five times slowed down by the substitution of W171 with alanine; the decay half-time of M-intermediate of wild ppR was 0.375 s and that of W171A was 1.87 s (H. Yoshida, unpublished results), supporting the results of the low-temperature spectroscopic studies.
Discussion
These low-temperature spectroscopic studies clearly show that in ppR the substitution of W171 to Ala or Thr results in the stabilization of M- and O-intermediate. Although M-intermediate from wild ppR is stable in 66% glycerol solution, M-intermediate from mutant pigments W171A and W171T is more stable under the same conditions as clearly shown in Fig. 4. The M-intermediate from mutant pigments was converted to O-intermediate thermally (Fig. 3b,c,e,f), but the M-intermediate from wild ppR was converted to original ppR without accumulation of the O-intermediate (Fig. 3a,d). In the case of mutant pigments, the formation of the O-intermediate was observed by light irradiation at 0°C even in the absence of glycerol and the O-intermediate formed by the irradiation was stable in the dark at 0°C (Fig. 5c). Thus, we conclude that the substitution of W171 to Ala or Thr results in stabilization of the M- and O-intermediate from ppR, while glycerol stabilizes the M-intermediate but not the O-intermediate.
Hashimoto et al. (26) showed an unusually strong steric repulsion between the indole ring of Trp182 in bR and the 9- and 13-methyl group of the chromophore retinal, suggesting that this steric conflict destabilizes the 13-cis isomeric state of retinal based on their resonance Raman measurements. If such a steric interaction is present in ppR, the substitution of W171, corresponding to W182 in bR, to amino acid residues with a smaller side chain, such as Ala or Thr, would result in the stabilization of the 13-cis state such as the M-intermediate. Thus, the present results are consistent with the presence of a steric interaction between W171 and the chromophore retinal in ppR as is suggested in bR. Such a molecular interaction between retinal and W171 has been proposed and discussed by others (5,25). Yamazaki et al. (27) and Weidlich et al. (28) clearly showed that in bR the 9-methyl residue of the chromophore retinal interacts with W182 from the experiment with a 9-desmethyl analog pigment. In the case of ppR, our present results suggest an interaction between the chromophore retinal and W171 but the position of the methyl in retinal involved in the interaction is not clear at present.
Maeda et al. (29) found that substitution of W182 to Phe resulted in the formation of L at much lower temperatures than in the wild type bR and suggested that the substitution causes a change in hydrogen bonding between retinal, W182 and L93, which occurs upon formation of L in the wild type bR. The mutation of L93 in bR resulted in the slow decay of M- and O-intermediate (36,37). The chromophore retinal of the O-intermediate from this mutant has 13-cis configuration (36), although the O-intermediate from wild bR usually has all-trans retinal as its chromophore. These results indicate that the perturbation in the hydrogen-bonding network near the retinal affects the pK value of the Schiff base and/or D96 and also affects the rate of the reprotonation step of the Schiff base as well as the decay of the M-intermediate, as reported by Iwamoto et al. (23) and Klare et al. (22). The apparent pK value of the Schiff base of W171A and W171T were smaller than that of wild ppR; pKa of the Schiff base of W171A was 7.7 and that of W171T was 8.8, however, that of wild ppR was more than 12 (H. Yoshida, unpublished results). The results suggest that the substitution of W171 results in a perturbation in the hydrogen-bonding network of ppR in the ground state.
The importance of the hydrogen-bonding network in bacterial rhodopsins has become more and more evident recently (38), and the involvement of W182 in the hydrogen bonding in bR has been reported (24,25,29,39,40). We observed the difference in the stability of the M-intermediate depending on the substituted amino acid residues (Fig. 4). The M-intermediate of wild type ppR was the most unstable that from W171T was moderately stable and that from W171A was the most stable. One possible reason for this is the difference in steric interaction depending on the size of the side chain of substituted amino acid. Note that the side chain of Ala is the smallest among the three and that the M-intermediate of this mutant is the most stable. Another possible cause for the difference among them is the fact that the side chain of Ala cannot participate in hydrogen bonding. If this is the case, it may be that W171 also participates in the hydrogen-bonding network, which includes retinal in the M-state of ppR, as reported in the ground state of ppR (16,17). FTIR studies of the M- and O-intermediates of ppR have been performed recently, but the exact involvement of W171 in the hydrogen-bonding network was not reported (15,41–43). In such studies, it was difficult to obtain information on the pure O-intermediate because the O-intermediate was usually observed as a mixture with other intermediates such as the M-intermediate. Thus, the mutants used in the present study would be valuable to investigate the molecular properties of the O-intermediate of ppR.
The recent NMR study by Petkova et al. (38) in bR reported that W182 was not involved significantly in the change of the hydrogen-bonding perturbation in the M-state as was reported earlier and also that the distance between C20 of retinal and the indole nitrogen of W182 in bR changes only slightly from the light-adapted state to the early M-state (the change is about 0.2 Å), suggesting that large structural change does not occur at the early M stage in bR. However, our present results clearly show the difference in the decay process of the M-intermediate of ppR and mutants depending on the amino acid substituted for W171 and this would suggest that the structural and/or the hydrogen-bonding change involving W171 occurs at the M-state and/or in the decay process of M-intermediate (formation of O-intermediate) in ppR system. This agrees with the model presented by Pebay-Peyroula et al. (5).
The absorption maximum did not shift by substitution of W171 to Ala or Thr in ppR. Shimono et al. (18) systematically replaced amino acid residues proximal to the chromophore retinal in ppR with the corresponding amino acids in bR and found that replacement of amino acid residues alone could not explain the difference in λmax between bR and ppR. They also formed chimeric proteins from bR and ppR (44), which have a different combination of the membrane spanning helices from A to G of bR or ppR. They concluded that the interaction between helices D and E and the effect of a hydroxyl group near the protonated Schiff base on helix G (Thr204 in ppR and Ala215 in bR) are important for the ground-state spectral tuning, which distinguish bR and ppR. Our results shown here demonstrate that the maximum wavelength of the decay product from the M-intermediate is different between W171A and W171T; the difference maximum in curve 4 in Fig. 3e (W171A) is about 520 nm, but that in Fig. 3f (W171T) is about 550 nm, although the difference minimum, corresponding to the M-intermediate, was the same in both pigments at about 390 nm and with a shoulder at 405 nm. There are two possible explanations for these results. One is that the chromophore retinal of the decay product from the M-intermediate was affected differently depending on the amino acid residue substituted for W171. Thus, the color of the decay product was different. This suggests that the color tuning mechanism (the interaction between the chromophore retinal and the protein moiety) is different for the ground state and photochemical intermediates. Another possible explanation is that the decay pathway from the M-intermediate is different for W171A and W171T; M from W171T changed into O-intermediate, while M-intermediate from W171A changed into N-like intermediate. If this is the case, however, N-like intermediate should convert into original pigment directly without passing the O-state because the conversion to O-intermediate was not observed in W171A (Fig. 3e). However, the possibility that in the mutant pigment, N- and O-intermediates are in equilibrium and that both pigments revert to the original pigment directly was not excluded.
In conclusion, our results show that a structural and/or hydrogen-bonding change occurs during the M-state and the formation of the O-intermediate and that low-temperature spectroscopy with amino acid substitution and/or with retinal analogs are powerful and sensitive tools for investigating the molecular properties of bacterial rhodopsins.




