Osteoclast Differentiation by RANKL Requires NF-κB-Mediated Downregulation of Cyclin-Dependent Kinase 6 (Cdk6)†
The authors have no conflict of interest
Abstract
This study investigated the involvement of cell cycle factors in RANKL-induced osteoclast differentiation. Among the G1 cell cycle factors, Cdk6 was found to be a key molecule in determining the differentiation rate of osteoclasts as a downstream effector of the NF-κB signaling.
Introduction: A temporal arrest in the G1 phase of the cell cycle is a prerequisite for cell differentiation, making it possible that cell cycle factors regulate not only the proliferation but also the differentiation of cells. This study investigated cell cycle factors that critically influence differentiation of the murine monocytic RAW264.7 cells to osteoclasts induced by RANKL.
Materials and Methods: Growth-arrested RAW cells were stimulated with serum in the presence or absence of soluble RANKL (100 ng/ml). Expressions of the G1 cell cycle factors cyclin D1, D2, D3, E, cyclin-dependent kinase (Cdk) 2, 4, 6, and Cdk inhibitors (p18 and p27) were determined by Western blot analysis. Involvement of NF-κB and c-jun N-terminal kinase (JNK) pathways was examined by overexpressing dominant negative mutants of the IκB kinase 2 (IKKDN) gene and mitogen-activated protein kinase kinase 7 (MKK7DN) gene, respectively, using the adenovirus vectors. To determine the direct effect of Cdk6 on osteoclast differentiation, stable clones of RAW cells transfected with Cdk6 cDNA were established. Osteoclast differentiation was determined by TRACP staining, and cell cycle regulation was determined by BrdU uptake and flow cytometric analysis.
Results and Conclusion: Among the cell cycle factors examined, the Cdk6 level was downregulated by RANKL synchronously with the appearance of multinucleated osteoclasts. Inhibition of the NF-κB pathway by IKKDN overexpression, but not that of the JNK pathway by MKK7DN overexpression, caused the decreases in both Cdk6 downregulation and osteoclastogenesis by RANKL. RAW cells overexpressing Cdk6 resist RANKL-induced osteoclastogenesis; however, cell cycle regulation was not affected by the levels of Cdk6 overexpression, suggesting that the inhibitory effect of Cdk6 on osteoclast differentiation was not exerted through cell cycle regulation. These results indicate that Cdk6 is a critical regulator of RANKL-induced osteoclast differentiation and that its NF-κB-mediated downregulation is essential for efficient osteoclast differentiation.
INTRODUCTION
OSTEOCLASTS ARE DERIVED from hematopoietic myeloid precursors of monocyte/macrophage lineage under the control of systemic and local factors produced by supporting cells such as osteoblasts and bone marrow stromal cells. Among these factors, RANKL is a TNF-related cytokine that stimulates osteoclast differentiation from hematopoietic precursor cells both in vitro and in vivo.1-3 Mice lacking in either RANKL or its receptor RANK have defects in osteoclast differentiation that lead to severe osteopetrosis.4-6 RANK is expressed on the surface of osteoclast progenitor cells and induces intracellular signals, leading to osteoclastogenesis on ligand binding or agonistic anti-RANK antibody stimulation.6, 7 Like other TNF receptor superfamily members, RANK stimulation can induce NF-κB, probably through association with several TNF receptor-associated factors (TRAFs): TRAF2, TRAF5, and TRAF6.6,8-11 Mice deficient in both p50 and p52 subunits of NF-κB have been found to be osteopetrotic because of the failure in osteoclast differentiation, indicating a crucial role of NF-κB in osteoclastogenesis.12, 13 NF-κB activation requires sequential phosphorylation, ubiquitination, and degradation of the inhibitory subunit IκB as well as consequent exposure of a nuclear localization signal on NF-κB.14-16 IκB kinase (IKK) signalsome is the protein complex that contains the inducible IκB kinase activity and consists of IKK1 (IKKα), IKK2 (IKKβ), and the NF-κB essential modulator (NEMO or IKKγ). Among these three components of IKK signalsome, both IKK1 and IKK2 seem to play a critical role in IκB phosphorylation. However, the studies of IKK1 and IKK2 knockout mice indicate that IKK2 is more potent for NF-κB activation by proinflammatory stimuli than IKK1.17-20 This evidence suggests that IKK2 may have a vital function in RANKL-induced NF-κB activation, and in fact, we previously reported that the dominant negative IKK2 (IKK2DN) overexpression suppressed both NF-κB activity and osteoclast formation induced by RANKL using the murine monocytic RAW264.7 cell culture.21
Proliferation of eukaryotic cells depends on their progression through the cell cycle, and at least a temporal cell cycle arrest at the G1 phase is thought to be a prerequisite for cell differentiation.22 Cell cycle control is achieved through the actions of a family of cyclins and cyclin-dependent protein kinases (Cdk's), which phosphorylate and thereby activate cell cycle factors essential for the onset of the next cell cycle phase. In mammalian cells, traverse through G1 and subsequent S phase entry require the activities of the cyclin D-dependent kinases Cdk4 and/or Cdk6 and the cyclin E-dependent kinase Cdk2. These Cdk's are negatively regulated by inhibitory proteins (CKIs) through direct binding to themselves.23, 24 CKIs have been classified into two families: INK4 and Cip/Kip. INK4 (p16, p15, p18, and p19) inhibits only Cdk4 and Cdk6, whereas Cip/Kip (p21, p27, and p57) inhibits all the Cdk's except for the Cdk6-cyclin D3 complex.25 Because the control of cell cycle factors driving S phase onset greatly influences the commitment to cell differentiation in lower eukaryotes, this study investigated the possibility of crucial participation of some cell cycle start factors in RANKL-induced osteoclast differentiation and found that on RANKL treatment Cdk6 was downregulated primarily by RANKL/NF-κB signal-invoked transcriptional repression and that its downregulation was essential for efficient osteoclast differentiation.
MATERIALS AND METHODS
Reagents and antibodies
Human soluble recombinant RANKL was purchased from Wako Pure Chemicals (Osaka, Japan). Recombinant human macrophage-colony stimulating factor (M-CSF) was purchased from R&D Systems (Minneapolis, MN, USA). Antibodies against Cdk2 (H-298), Cdk4 (C-22), Cdk6 (C-21), cyclin D1 (C-20), cyclin D2 (M-20), cyclin D3 (C-16), cyclin E (M-20), p18 (M-20), p27 (F-8), mitogen-activated protein kinase kinase 7 (MKK7; MEK7), and IKK2 (H-470) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against β-actin (AC-15) were purchased from Sigma Chemical (St Louis, MO, USA). DMEM and FBS were also purchased from Sigma Chemical. αMEM was purchased from Life Technologies (Rockville, MD, USA).
Cell culture and osteoclast differentiation assay
The RAW264.7 cell line was purchased from the Riken Cell Bank (Tsukuba, Japan). The cells were inoculated at 5 × 104 cells in a 6-well plate or 5 × 105 cells in a 10-cm plate and were cultured with DMEM containing 10% FBS at 37°C in 5% CO2 in air. For osteoclast differentiation assay, RAW cells were grown in DMEM containing 10% FBS for 16-24 h. The culture medium was changed to DMEM containing 0.5% FBS, and the cells were cultured under serum starvation for 24-48 h. The growth-arrested RAW cells were stimulated with 10% FBS in the presence or absence of RANKL (100 ng/ml) for 1-7 days, fixed with 3.7% (vol/vol) formaldehyde in PBS), and stained at pH 5.0 in the presence of L(+)-tartaric acid using 3-hydroxy-2-naphthoic acid 2,4-dimethylanilide phosphate (Sigma) in N,N-dimethyl formamide (Sigma) as the substrate. TRACP+ cells containing more than three nuclei were counted as osteoclasts.
For studies on primary osteoclast precursors, we used the M-CSF-dependent bone marrow macrophage (M-BMMφ) culture system as described previously.26 Briefly, bone marrow cells from 8-week-old male ddY mice (Sankyo Laboratories Animal Center, Tokyo, Japan) were seeded at 2 × 106 cells in a 6-multiwell plate and cultured in αMEM containing 10% FBS with M-CSF (10 ng/ml). After 2 days, adherent cells were used as M-BMMφ after washing out the nonadherent cells including lymphocytes. The cells were then cultured in αMEM containing 0.5% FBS for 24 h and were further cultured in the presence of M-CSF (10 ng/ml) and RANKL (100 ng/ml) for 4 days. This experiment was performed according to the protocol approved by the Animal Care and Use Committee of the University of Tokyo.
Western blot analysis
Cells were rinsed with ice-cold PBS and lysed with RIPA buffer (100 μl for a well in 6-multiwell plate or 500 μl for a 10-cm plate) containing 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Nonident-P40 (NP-40), 0.1% SDS, 10 μg/ml aprotinin, 0.1 M NaF, 2 mM Na3VO4, and 10 mM β-glycerophosphate. The cell lysates were sonicated briefly and clarified by centrifugation at 15,000g for 20 minutes at 4°C. The protein concentration in the cell lysate was measured using a Protein Assay Kit II (Bio-Rad). Equivalent amounts (10 μg) of cell lysate were electrophoresed by 7.5%, 10%, or 12.5% SDS-PAGE according to the molecular size of the proteins to be detected and were electrotransferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA, USA). After blocking nonspecific binding with 5% skim milk, proteins were immunoblotted with respective antibodies and visualized using the ECL Plus Western Blotting Detection System (Amersham Pharmacia Biotech, Buckinghamshire, UK), following the manufacturer's instructions. Signals were quantified by densitometry (Bio-Rad). Experiments were performed at least three times, and a representative blotting was presented.
Transduction of IKK2DN and MKK7DN using adenovirus
The recombinant adenovirus vectors carrying the IKK2DN (Ser177 and Ser181 to Ala; AxIKK2DN) and the β-galactosidase gene (AxLacZ) were kindly provided by Inder Verma (Salk Institute, La Jolla, CA, USA) and Izumu Saito (Tokyo University), respectively. The recombinant adenovirus vector carrying kinase negative MKK7 (AxMKK7DN, replaced ATP-binding lysine with glutamate residue) was constructed as described.21 Preparation and infection of AxIKK2DN, AxMKK7DN, and AxLacZ were performed as previously reported.21 Titers of the viral stock were determined by modified endpoint cytopathic effect assay with the following modifications. Fifty microliters of DMEM containing 10% FBS was dispensed into each well of a 96-well tissue culture plate, and eight rows of 3-fold serial dilutions of the virus starting from 10−4 dilutions were prepared. HEK293 cells (3 × 105) in 50 μl of DMEM containing 10% FBS were added to each well. The plate was incubated at 37°C in 5% CO2 in air, and 50 μl of DMEM containing 10% FBS was added to each well every 3 days. Twelve days later, the endpoint of the cytopathic effect was determined by microscopy, and the 50% tissue culture infectious dose (TCID50) was calculated. One TCID50 per milliliter approximately corresponds to one plaque-forming unit (PFU) per milliliter. The multiplicity of infection (MOI) is expressed as a measure of titer of how many PFUs are added to every cell. Infection of adenovirus vectors to RAW cells was carried out as follows. The cells were inoculated at the density of 5 × 104 cells per 6-well plate and incubated for 20 h with DMEM containing 10% FBS at 37°C. After further incubation with a small amount of DMEM containing the recombinant adenovirus for 2 h at 37°C at 100 MOI, the cells were washed twice with PBS and again incubated in DMEM containing 10% FBS. Experiments were performed 2 days after the infection.
Establishment of RAW cells stably transfected with Cdk6
RAW cells were inoculated at the density of 5 × 105 cells per 6-cm plate, incubated for 24 h, and transfected with pEF/neoI that carries human Cdk6 cDNA25 using LipofectAMINE reagent (Life Technologies) following the manufacturer's instructions. Twenty-four hours after transfection, the cells were passaged 1:10-1:100 into DMEM containing 10% FBS and 400 μg/ml G418 (Geneticin; Life Technologies) for stable expression. After colony formation, each colony was isolated and passaged. We picked up more than 100 drug-resistant colonies, each of which was derived from a single clone. The expression levels of Cdk6 were quantified by Western blotting, and 12 high-expressing clones and 10 low-expressing clones were established.
RT-PCR
Total RNA (1 μg) was extracted from RAW cells using ISOGEN (Wako Pure Chemicals, Osaka, Japan) following the manufacturer's instructions, reverse transcribed using SUPERSCRIPT First-Strand Synthesis System for RT-PCR (Life Technologies), and amplified within an exponential phase of the amplification with a Perkin Elmer PCR Thermal Cycler (PE-2400). Gene-specific primer pairs were as follows: 5′-GCAACCTCCAGTCAGCA-3′ and 5′-GAAGTCACAGCCCTCAGAATC-3′ for RANK and 5′-CATGTAGGCCATGAGGTCCACCAC-3′ and 5′-TGAAGGTCGGTGTGAACGGATTTGGC-3′ for GAPDH. The cycling parameters were 30 s at 94°C, 30 s at 49°C, and 90 s at 72°C for RANK and 30 s at 94°C, 30s at 55°C, and 90 s at 72°C for GAPDH. Each band intensity was quantified by densitometry (Bio-Rad).
Flow cytometric analysis
About 1 × 105 cells were suspended in 0.02 ml citrate buffer, to which was added 0.18 ml Soln. A (0.03 mg/ml trypsin, 3.4 mM trisodium citrate, 0.1% NP40, 1.5 mM Spermine 4 HCl, and 0.5 mM Tris-HCl [pH 7.6]) and incubated for 10 minutes, and then added with 0.15 ml Soln. B (3.4 mM trisodium citrate, 0.1% NP40, 1.5 mM Spermine 4 HCl, 0.5 mM Tris-HCl [pH 7.6], 0.5 mg/ml trypsin inhibitor, 0.1 mg/ml Ribonuclease A) and incubated for 10 minutes; 0.15 ml Soln. C (4.16 mg/ml propidium iodite, 3.4 mM trisodium citrate, 0.1% NP40, 4.8 mM Spermine 4 HCl, 0.5 mM Tris-HCl [pH 7.6]) was finally added, and the mixture was again incubated for 10 minutes. All procedures were performed at room temperature. The DNA content was analyzed by EPICS XL (Beckman), and the data were analyzed by XL EXPO32 (Beckman).
BrdU incorporation assay
RAW cells were inoculated at a density of 1 × 103 cells per well in a 96-well plate and cultured in DMEM containing 10% FBS with or without hRANKL (100 ng/ml). At 3 days of culture, cells were labeled with BrdU for 2 h, and cell proliferation was determined by BrdU incorporation using a kit (Cell Proliferation ELISA; Roche Molecular Biochemical, Mannheim, Germany) following the manufacturer's instructions.
Statistical analysis
Means of groups were compared by ANOVA, and significance of differences was determined by posthoc testing using the Bonferroni method.
RESULTS
Cdk6 is downregulated by RANKL in RAW cells
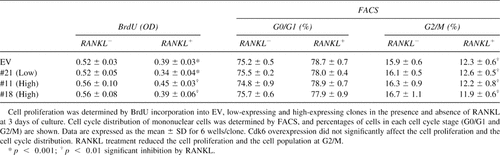
We initially confirmed that RAW cells differentiated into TRACP+ multinucleated osteoclasts after 5 days of treatment with soluble RANKL (100 ng/ml; Fig. 1A). We analyzed the regulation by RANKL of cell cycle factors that critically regulate the onset of S phase: Cdk2, Cdk4, Cdk6, cyclins (D1, D2, D3, and E), and CKIs (p18, p21, and p27) in RAW cells (Figs. 1B and 1C). Western blot analysis revealed that the Cdk6 protein level was decreased by RANKL after 5 days of culture and thereafter, whereas those of cyclins were hardly affected throughout the culture period up to 7 days. Although Cdk4 and Cdk6 have about 70% homology of amino acid sequence27 and share D cyclins as their catalytic partners, only Cdk6 was regulated by RANKL. Levels of Cdk2, p18, and p27 were somewhat decreased by RANKL treatment, and neither p21 nor p57 was detected throughout the experiments in this cell line (data not shown).
(A) Time course of osteoclastogenesis from murine monocytic RAW264.7 cells, (B and C) expression of cell cycle factors by RANKL in the RAW264.7 cell culture, and (D) the murine primary osteoclast precursor M-BMMφ culture. Cells were stimulated with serum in the presence or absence of RANKL (100 ng/ml) for the indicated days. (A) Osteoclastogenesis formed from cultured RAW cells was determined by TRACP staining, and the number of positively stained cells containing more than three nuclei was counted. Bar, 400 μm. The graph is expressed as means (bars) ± SE (error bars) for 8 wells/group. (B-D) The protein levels of cell cycle factors controlling the G1-S transition were determined at the indicated days of culture by Western blot analysis. β-actin was used as a loading control. B and C are from different cultures of RAW cells to confirm the regulation of Cdk's. D is from the M-BMMφ culture for 4 days. The number under each band is the treated/control ratio of the intensity of each band normalized to that of β-actin measured by densitometry. In each figure a representative blotting was shown among at least three independent experiments that showed similar results.
To investigate whether the Cdk6 downregulation is specific to this cell line or a more general phenomenon, we performed the same Western blot analysis with a culture of primary osteoclast precursor M-BMMφ. Treatment with RANKL inhibited induction of Cdk6 at 4 days, whereas Cdk4 was uninfluenced (Fig. 1D). Cdk2 was not decreased but was rather increased in this culture system. These results show that Cdk6 was specifically downregulated during the commitment to RANKL-induced osteoclast differentiation.
NF-κB mediates RANKL-induced downregulation of Cdk6
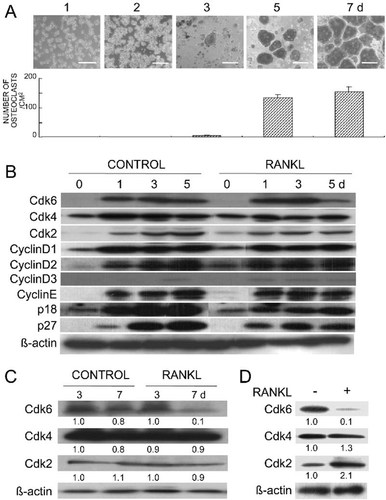
To examine the mediation of the NF-κB pathway, a major signaling pathway of RANKL, in the downregulation of Cdk6, we overexpressed IKK2DN in RAW cells using an adenovirus vector and examined its effect on RANKL-led Cdk6 downregulation. In our previous report, we confirmed that the adenovirus vector could efficiently transduce the IKK2DN gene into RAW cells and specifically suppressed the NF-κB activation in response to RANKL.21 Both the inhibition of Cdk6 and the induction of osteoclastogenesis by RANKL were markedly reversed by the IKK2DN overexpression in RAW cells, whereas the control LacZ adenovirus infection did not affect the RANKL actions (Fig. 2A). These results indicate that the NF-κB signaling mediates not only osteoclast differentiation, but also the Cdk6 downregulation by RANKL.
Effects of overexpression of (A) IKKDN and (B) MKK7DN using adenovirus vectors on the Cdk6 inhibition and osteoclastogenesis by RANKL in the RAW cell culture. RAW cells were infected with a recombinant adenovirus carrying the IKKDN, MKK7DN, or LacZ gene at 100 MOI, and subsequently, growth-arrested by incubation in a low serum medium for 2 days. The cells were stimulated with serum in the presence or absence of RANKL (100 ng/ml) for 5 days. Bar, 100 μm. Expressions of Cdk6, IKK2, and MKK7 were determined by Western blotting with β-actin as loading controls. The number under each band is the treated/control ratio of the intensity of the Cdk6 band normalized to that of β-actin measured by densitometry. Similar results were obtained in independent Western blottings of three separate experiments. The graph indicates means (bars) ± SE (error bars) for 8 wells/group. *Significant difference from the RANKL-treated and AxLacZ-infected culture; p < 0.01.
We further investigated the possible involvement of the c-jun N-terminal kinase (JNK), another major signaling pathway of RANKL, in the downregulation of Cdk6 by RANKL. We previously reported that MKK7 is a vital function in JNK activation and osteoclast formation induced by RANKL, because MKK7DN overexpression using an adenovirus vector suppressed both of them.21 Thus, we examined the effect of the adenovirus-mediated MKK7DN overexpression on RANKL-led Cdk6 downregulation in RAW cells. The MKK7DN overexpression suppressed the induction of osteoclastogenesis by RANKL as previously reported21; however, it did not restore the inhibition of Cdk6 in response to RANKL, unlike IKK2DN overexpression (Fig. 2B), indicating that the RANKL-led Cdk6 downregulation was not mediated by the JNK signaling.
RAW cells overexpressing Cdk6 resist RANKL-induced differentiation
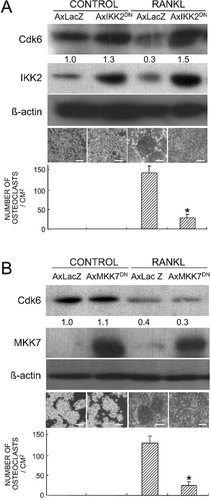
As shown above, Cdk6 downregulation occurred during RANKL-induced osteoclast differentiation. Consequently, a critical question is whether or not this downregulation is essential for osteoclast differentiation. To address this, we generated RAW cell clones stably expressing various levels of Cdk6 by transfecting with an expressing vector harboring Cdk6 cDNA and tested their ability to respond to RANKL and differentiate to osteoclasts. The ability of RANKL to induce TRACP+ multinucleated osteoclast formation was compared among the empty vector-transfected cells and low- (∼1.5-fold) and high- (>5-fold) expressing cells. RANKL induced osteoclastogenesis in the empty vector-transfected cells and low-expressing clones; however, it was markedly decreased in high-expressing clones (Fig. 3A). Cdk6-led resistance to osteoclast differentiation is unlikely to be caused by an unexpected interference of the RANKL/NF-κB signaling by the overexpressed Cdk6, because RANK expression was not significantly affected by Cdk6 expression (Fig. 3B).
(A) RANKL-induced osteoclastogenesis from RAW cells overexpressing Cdk6. Stable clones of RAW cells transfected with Cdk6 cDNA were established as described in the Materials and Methods section, and clones with low expression (21) and high expression (11 and 18) were selected on Western blotting (top panel). These clones and empty vector-transfected RAW (EV) cells were induced for osteoclast differentiation by treating with RANKL (100 ng/ml) for 7 days, and osteoclast formation was determined by TRACP staining. The macroscopic images of culture wells and the microscopic images inside the wells are shown in the middle panels. Bar, 100 μm. The graph in the bottom panel is expressed as means (bars) ± SE (error bars) for 8 wells/group. *Significant difference from the EV cell culture; p < 0.01. (B) RANK mRNA levels by RT-PCR in each clone stimulated for 3 and 6 days with RANKL. The number under each band is the intensity of the RANK band normalized to that of GAPDH measured by densitometry. Other clones with low and high expressions showed similar results.
Overexpression of Cdk6 does not influence cell cycle regulation of osteoclasts
Because Cdk6 promotes the G1-S transition, suppression of osteoclast differentiation by overexpressed Cdk6 could be a mere consequence of its execution of this role. We therefore examined the effects of both RANKL and Cdk6 overexpression on G1-S transition and proliferation of RAW cells. The three kinds of transfected cells were similarly arrested in quiescence, stimulated with serum in the presence and absence of RANKL, and analyzed for cell proliferation and populations in G0/G1 and G2/M by BrdU incorporation and flow cytometric analysis (FACS; Table 1). At 3 days of culture, when osteoclastogenesis just started, RANKL slightly reduced cell proliferation and the G2/M population; however, Cdk6 overexpression caused no significant changes in either of them. These results indicate that the inhibitory effect of Cdk6 on osteoclast differentiation was not exerted through cell cycle regulation.
DISCUSSION
This study showed that Cdk6 is a key molecule in determining the differentiation rate of osteoclasts as a downstream effector of RANKL/NF-κB signaling. Figure 4 shows the mechanism of effect of RANKL on osteoclast differentiation based on present and previous studies. The RANK activation by binding of RANKL stimulates the NF-κB signaling, probably through association with TRAFs.6,8-11 NF-κB moves from the cytoplasm into the nucleus, associates with various transcription factors in the nucleus, and downregulates Cdk6, which is a negative regulator of the transition from the G1 to the differentiation stage.
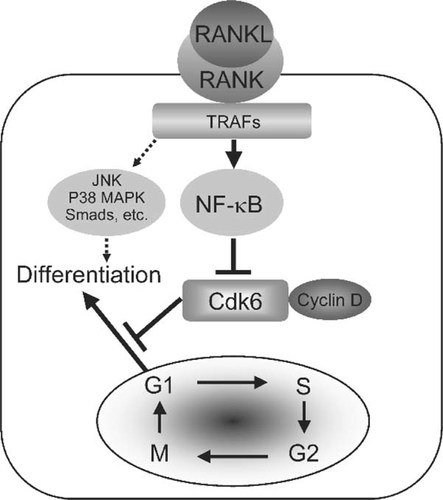
Scheme of Cdk6-dependent RANKL effect on osteoclast differentiation obtained from this study. RANKL stimulates NF-κB signaling by binding to RANK through association with TRAFs. NF-κB downregulates Cdk6, which is a negative regulator of the transition from the G1 phase to the differentiation stage. Other signaling pathways such as JNK, p38 MAPK, and Smads might be involved in osteoclast differentiation through mechanisms independent of Cdk6.
This study failed to show the contribution of JNK, another major pathway lying downstream of RANKL, to the RANKL-led Cdk6 downregulation. Our results, however, do not rule out the possibility that pathways other than NF-κB-mediated Cdk6 inhibition are required for osteoclast differentiation. JNK is known to activate activator protein 1 (AP-1), which is composed of various combinations of Fos and Jun family members. Mice deficient in c-Fos are reported to be osteopetrotic because of the failure in osteoclast differentiation, which can be rescued by Fra-1 as well as c-Fos overexpression,28-31 indicating a crucial role of the JNK/AP-1 signaling in osteoclastogenesis.12, 13 JNK is activated by the phosphorylation of Thr and Tyr residues through MKK4 and/or MKK7. MKK7 seems to be more specific in JNK activation than MKK4 because MKK4 is known to activate p38 MAPK as well. We previously reported that overexpression of MKK7DN in RAW cells by the adenovirus vector inhibited both RANKL-induced JNK activation and osteoclast formation.21 JNK may possibly contribute to osteoclast differentiation through an independent pathway of Cdk6. Other possible pathways for osteoclast differentiation are the p38 MAPK and the Smad signaling. Recently, Matsumoto et al.32 reported that RANKL activates p38 pathways in osteoclast precursors, which is essential for osteoclast differentiation, and Fuller et al.33 and Kaneda et al.34 showed the critical role of transforming growth factor (TGF)-β in osteoclast differentiation and survival. The involvement of cell cycle factors in osteoclast differentiation mediated by these pathways should be clarified in the future.
Cdk6 overexpression did not affect cell cycle regulation of RAW cells. D cyclins and partner kinases Cdk4 and Cdk6 are all known to be critical factors that control the cell growth potential,35, 36 but several specific roles have been reported for Cdk6. The Cdk6-cyclin D3 complex is unique among cyclin D and cognate kinase combinations and evades inhibition by CKIs.25 Therefore, it can greatly enhance the proliferative potential of fibroblasts under growth-suppressive conditions,25 and consequently, sensitizes cells to physical and chemical transformation.37 Cdk6 combined with cyclin K, encoded by human herpes virus 8, which is the causative agent of Kaposi sarcoma, is also reported to be immune to inhibition by CKIs.38 These functions were not seen in Cdk4 complexes. This study discovered a novel function of Cdk6 as an inhibitor of the transition to the differentiation stage without affecting cell cycle regulation. Although Cdk4 has 70% homology of amino acid sequence with Cdk6,27 the Cdk4 protein was not affected by RANKL in this study. We hereby propose that Cdk4 and Cdk6 play different roles and that Cdk4 cannot substitute for Cdk6 in osteoclastic cells.
How could Cdk6 control differentiation without influencing cell cycling? One possibility is that Cdk6 directly controls a factor critically involved in differentiation. This possibility may not be as remote as generally thought. In fission yeast, Pas1 cyclin and its partner kinase Pef1 activate a transcription factor complex functionally equivalent to E2F-DP of mammals, thereby promoting S phase entry, just like Cdk6, yet they independently inhibit the mating pheromone signaling whose activation is essential for differentiation of this yeast cell.39 Thus, this might be a good model for the situation of Cdk6 in RANKL-induced osteoclast differentiation, highlighting a potential functional similarity between Cdk6 and Pef1.
Among cell cycle factors, Cdk's have vital roles in controlling cell cycle progression. Therefore, much attention has been devoted to the view that CKI-led inhibition of G1-specific Cdk's is critical for cell differentiation. Accordingly, potential roles for CKIs in differentiation have been studied extensively, but with mixed results.40, 41 Several studies revealed a certain correlation between induction of p21CIP1 and differentiation in hematopoietic cells including monocytes/macrophages.42-46 For osteoclast differentiation as well, upregulation of p21CIP1 and P27KIP1 is reported to be associated with osteoclast differentiation in the M-BMMφ culture.47 This was inconsistent with the present results in RAW cells that P27KIP1 was not increased, but rather decreased, by RANKL and that p21CIP1 could not be detected. Mice completely ablated for p21CIP1 and/or P27KIP1 or other major CKIs still develop and grow normally without significant bone abnormality such as osteopetrosis, strongly dismissing the current view.41, 48 Although there is evidence for p57KIP2 being involved in differentiation of some cells,49, 50 no one has identified cell cycle factors that are controlled by differentiation signals and critically influence the differentiation commitment process. Because the Cdk6 downregulation was reproducible in RAW cell and M-BMMφ cultures, we believe that Cdk6 generally plays a role in the RANKL-induced osteoclast differentiation. Although we tried to perform the signaling and overexpression experiments in M-BMMφ, adenovirus infection and plasmid transfection were much less efficient in transducing these cells. Matsuo et al.31 reported an efficient gene transfer into osteoclast precursors using a retrovirus vector system. However, the level of gene expression by retrovirus vectors is not enough for experiments using dominant negative molecules like this study. Developing more efficient gene transduction systems to primary osteoclast precursors will be helpful for further analysis of the signaling pathways of osteoclast differentiation.
This study for the first time showed that Cdk6, a G1 cell cycle factor, plays a critical role in controlling RANKL-induced osteoclast differentiation. Numerous signaling molecules, including transcription factors such as a nuclear factor of activated T-cells (NFATs),51, 52 have recently been reported to be involved in osteoclast differentiation. Consequently, one of these factors may be responsible for the RANKL-triggered repression of Cdk6 transcription. Identification of the transcriptional repressor as well as key targets for Cdk6 will definitely be required for deeper understanding of the molecular basis of bone resorption.
Acknowledgements
We thank Reiko Yamaguchi for providing expert technical assistance and Drs Inder Verma and Izumu Saito for kindly providing adenovirus vectors carrying IKK2DN and LacZ, respectively. This study was funded by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports, Culture and Technology (14657358).