Growth Factor Regulation of Fracture Repair
Fracture repair can be considered as a biologically optimal process resulting in the restoration of injured skeletal tissue to a state of normal structure and function. Although the process leads to healing in the vast majority of cases, a small but significant proportion of fractures result in delayed union or persistent nonunion. Surgical interventions have been directed toward enhancing the fracture repair process, normalizing the rate of healing, and decreasing the likelihood of nonunion. During fracture repair, a number of growth factors, cytokines, and their cognate receptors are present at elevated levels in and around the fracture site. Many of these proteins are normally expressed in skeletal tissue, and others are released from associated inflammatory cells at the site of injury. The induction of these proteins is regulated both spatially and temporally, suggesting that they play an active role in promoting fracture repair. The following review will summarize the current literature on the roles of the major cytokines and growth factors involved in fracture repair. In addition, the signaling cascades induced by these molecules will be discussed. While many cytokine and growth factor signaling events have not been specifically examined in the context of fracture repair, a large body of literature on signal transduction has emanated from studies on these molecules in embryonic bone development. Given the conserved nature of these molecules and their signaling cascades from Drosophila to humans, and the similarities between the fracture repair process and embryonic bone development, it seems highly probable that these downstream signaling events are conserved in fracture repair.
Fracture repair can be envisioned as involving five distinguishable processes, including the immediate response to injury, intramembranous bone formation, chondrogenesis, endochondral bone formation leading to the reestablishment of load bearing function, and bone remodeling. While these processes may be discussed individually, it should be recognized that the first four occur simultaneously during fracture repair and are likely to influence one another. Extensive remodeling of the newly formed bone follows these four concurrent processes and facilitates the reestablishment of the full biomechanical integrity of the bone. A number of investigators have commented on the similarities between the repair process and embryonic bone formation and indeed a number of specific events characteristic of embryonic bone formation are reiterated in fracture repair. This is especially evident as one begins to examine the specifics of local factors regulating fracture repair, specifically those involved in the events of endochondral bone formation.
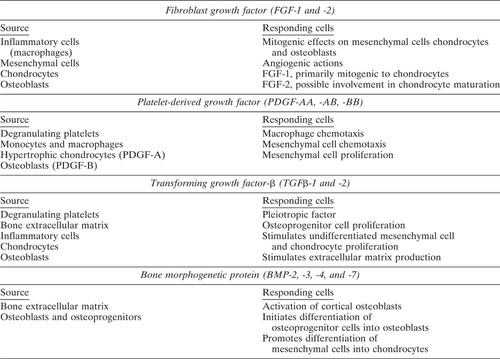
The immediate response to injury from the fracture trauma is the initiating event of the fracture repair process. Fracture trauma involves not only an interruption of skeletal integrity but also a disruption of the normal vascular structures and nutrient flow at the fracture site. This leads to reduce oxygen tension, disruption of the marrow architecture, and results in the infiltration of inflammatory cells, macrophages, and degranulating platelets during formation of a hematoma.1, 2 While it is likely that the mechanical stresses, changes in oxygen tension, and loss of vascular nutrients at the fracture site may signal some aspects of the healing process, the dominant initiators of fracture repair are most likely the numerous cytokines and growth factors released into the fracture site.3 To date the majority of research on fracture repair has focused on the actions of a relatively limited number of cytokines including interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor alpha, and macrophage colony-stimulating factor as well as the local growth factors fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), and the bone morphogenetic proteins (BMPs)-2, -3, -4, and -7.
It is interesting to note that the majority of the factors mentioned have well described roles in the formation and patterning of the embryonic skeleton or in the homeostatic maintenance of the mature skeleton. During the immediate injury response, the first detectable factors include PDGF and TGF-β released by the degranulating platelets.3 This is closely followed by infiltrating macrophages and other inflammatory cells that secrete FGF and additional PDGF and TGF-β. Presumably, these inflammatory cells also release a variety of cytokines including IL-1 and IL-6; however, this has only been demonstrated indirectly and no comprehensive data are available on the spatiotemporal profile of the proinflammatory and immune cytokine expression during the fracture repair process.4 Thus within the first hours post fracture, a complex milieu of factors involved in bone repair can be detected in the fracture site. Some or all of these factors are presumably the initiators of the active repair process acting on the cells of the bone marrow, periosteum, and external soft tissues adjacent to the fracture site.
One of the first tissues to visibly respond to the milieu of factors released by the immediate response to injury is the bone marrow, and in fact preliminary evidence suggests the bone marrow may be a major contributor of some of these factors.2 It has been demonstrated that within hours following the fracture event, the bone marrow undergoes a loss of normal architecture, disappearance of the blood vessels adjacent to the fracture callus clot, and a reorganization of the cellular complement into regions of high and low cellular density (Fig. 1A). Within 24 h post fracture, cells in the high cellular density regions undergo differentiation and take on an osteoblastic phenotype.2 In addition to changes in the bone marrow, osteoblasts lining the cortical bone surface are activated, and adjacent periosteal cambium derived preosteoblasts divide and begin differentiating.1 These resident and differentiating osteoblasts lay down new bone via an intramembranous pathway, thus forming the woven bone or so-called “hard” callus adjacent to the fracture site. These events can be detected as early as 3 days post fracture and continue with proliferation peaking between 7–10 days and disappearing in this region by 14 days post fracture (Figs. 1B, and 2A, 2B).5 Osteocalcin expression in this region is maximal around day 15, indicating continued osteoblastic activity post cessation of proliferation. Under specific conditions in which the fracture is rigidly fixed by a surgical plate and screws, this newly formed bone can bridge the fracture gap and produce fracture repair without the production of a cartilaginous callus.6 This, however, is a relatively rare situation. In the majority of fractures, healing involves an additional chemotactic response by periosteally derived undifferentiated mesenchymal cells and fibroblasts from the external soft tissues that infiltrate and divide replacing the hematoma.
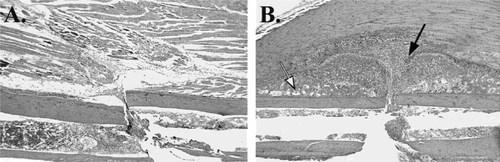
Photomicrographs of early fracture callus formation in the mouse. (A) Fracture site 24 h after fracture showing no detectable fracture callus. (B) Fracture site at day 7 post fracture showing early callus formation and organization. Intramembranous bone formation is detectable under the periosteum (open arrow) and the early chondroid callus representing the initial stages of the endochondral repair process is visible adjacent to the fracture site (closed arrow) (hematoxylin and eosin, ×40).
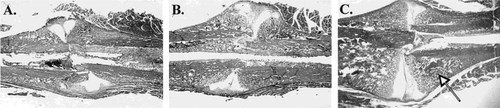
Low power photomicrographs of fracture callus in the rat. (A) Fracture callus at day 9 post fracture showing increased intramembranous bone formation under the periosteum and increased chondroid tissue overlying the fracture site. (B) Fracture callus at 14 days post fracture showing the continued intramembranous bone formation under the periosteum referred to as the hard callus and increased volume of chondroid tissue referred to as the soft callus. (C) Fracture callus 21 days after fracture showing newly formed woven bone adjacent to the fracture site formed as a result of endochondral bone formation (closed arrow) and the residual chondroid tissue still to be replaced with new bone. (hematoxylin and eosin, ×4).
Mesenchymal cell proliferation can be detected as early as day 3 post fracture and remains high for several days.5 The subsequent chondrogenesis by these mesenchymal cells and proliferation of these new chondrocytes continues from day 7 to day 21, leading to the formation of a cartilaginous callus that bridges the fracture site. This cartilaginous callus is referred to as the “soft” callus and provides the initial stabilization of the fracture site (Figs. 1B and 2A–2C). This cascade of events is the initiation of an endochondral bone formation process and is remarkably reminiscent of the proliferative zone of the growth plate. By 9 days post fracture, the chondrocytes of the soft callus adjacent to the woven bone of the hard callus begin to elongate, form elaborate vesicular structures, and turn off the type II collagen and aggrecan expression characteristic of maturing chondrocytes. These chondrocytes undergo hypertrophy and display the characteristic expression of type X collagen typical of hypertrophic chondrocytes of the growth plate.7 In addition, these cells produce and release neutral proteases that begin breaking down the cartilaginous matrix, including proteoglycans, in preparation for calcification.8, 9 Neutral protease expression peaks by day 14 and is followed 3–4 days later by a peak in alkaline phosphatase, indicating an increase in cell-mediated mineralization. The mineralization of the soft callus proceeds in a spatially organized manner with hypertrophy of chondrocytes and calcification beginning at the interface between the maturing cartilage and newly formed woven bone (Figs. 2B and 2C). Following closely after hypertrophic chondrocyte mineralization of the matrix is angiogenesis and the formation of new vascular structures which infiltrate along with accompanying osteoblasts. The resident hypertrophic chondrocytes begin to undergo programmed cell death or apoptosis, and the mineralized matrix is replaced by woven bone laid down by the incoming osteoblasts (Fig. 2C). Thus, the fracture site is repaired with new bone which will subsequently be extensively remodeled by cooperative osteoblast/osteoclast activity, eventually producing bone which is indistinguishable from its original intact state.
While the process by which fracture repair occurs is well described, relatively little is understood about the coordinate regulation of the events leading to successful repair. Furthermore, even less is understood about how the process can fail, leading to cases of delayed union, nonunion, and incomplete repair. It is clear that fracture repair involves the coordinate regulation of cellular chemotaxis, proliferation, and differentiation and that these events are likely to be regulated through signaling via growth factors (Table 1.). The major growth factors characterized as being present in the fracture site are TGF-β1, TGF-β2, BMP-2, BMP-3, BMP-4, and BMP-7 (OP-1), PDGF, and acidic and basic FGF (FGF-1 and FGF-2).1, 3, 10, 11 Given the well described roles of these factors in embryonic bone development and in vitro effects on bone cells, these molecules are likely to be important regulators of fracture repair. It is interesting to note that the signaling pathways utilized by these molecules fall into two distinct groups: those signaling through receptor tyrosine kinases (FGFs and PDGF) and those through serine-threonine kinase receptors (TGF-βs and BMPs). The common theme is signal transduction through a receptor in which kinase activity is induced by receptor occupancy by ligand. The subtle beauty of such a system is the cross talk that this facilitates between distinct signaling pathways and the ability to further modulate the signal cascade inside the cell after the initial receptor binding (Fig. 3).
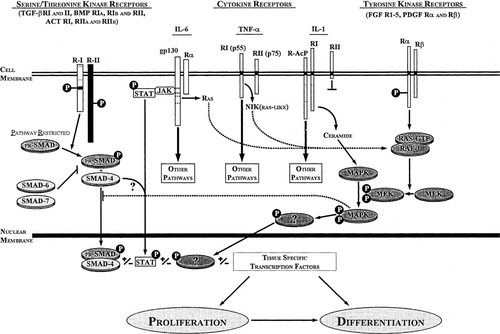
Diagram depicting the cross-talk between the signaling pathways of the major growth factors and cytokines involved in fracture repair. Events involved in receptor-ligand interaction and receptor dimerization are not indicated.
REGULATION VIA THE RECEPTOR TYROSINE KINASE PATHWAYS
Fibroblast growth factors
The FGFs are a family of structurally related polypeptides characterized by a high affinity to heparin. The FGF gene family currently includes nine members expressed in mammals.12 The most abundant in normal adult tissues are acidic FGF and basic FGF, also named FGF-1 and FGF-2. FGF-1 and FGF-2 are expressed in a wide variety of tissues and are abundant in the bone extracellular matrix, presumably due to their affinity for heparin-like glycosaminoglycans.13-15 The FGF family of molecules transduces signals to the cytoplasm via a family of transmembrane receptors with tyrosine kinase activity. There have been five genes encoding FGF receptors identified to date.16, 17 The various FGF receptors dispaly varying affinities for each of the members of the FGF family and are expressed in a wide variety of tissues including bone, although no reports have detailed the FGF receptor profile during the fracture repair process. As with many of the tyrosine kinase receptors, activation of the intrinsic tyrosine kinase activity occurs through receptor dimerization in response to ligand binding. An additional complexity may be added to the receptor–ligand association through the binding of FGF ligand by either secreted or membrane-bound proteoglycans, heparin-like structured proteoglycans in particular.17 These molecules may function by presenting the ligand to the appropriate receptor, thus subtly altering the receptor–ligand complex and modifying the downstream signals. The major downstream signaling cascades include signals generated through the RAS/RAF (retrovirus associated DNA sequences/factors), MEK (MAP kinase kinase), and mitogen-activated protein kinase (MAPK) pathway (Fig. 3).
Both FGF-1 and FGF-2 can be detected in the early stages of fracture repair in the granulation tissue at the fracture site.3, 18 Macrophages and other inflammatory cells express FGFs and this is the likely source of FGF in the granulation tissue. Subsequently, FGFs are expressed by mesenchymal cells, maturing chondrocytes and osteoblasts and have been demonstrated to enhance TGF-β expression in osteoblastic cells.3 FGFs are also abundantly expressed by nerve cells. The FGFs primarily function as mitogens on a variety of mesenchymal cells including fibroblasts, chondrocytes and osteoblasts (Table 1).19, 20 In the case of FGF-1, the mitogenic effects of this molecule appear to be primarily on chondrocytes. This is suggested by the expression profile of FGF-1 that peaks during chondrogenesis. In addition, FGF-1 was tested in rats using a standard closed bilateral femoral fracture model. Injection of FGF-1 into fracture gaps during the first 9 days post fracture was associated with an increased callus size, much of which was attributed to increased proliferation of chondrocytes.18 No biomechanical testing of fractures was reported, but preliminary data indicated a possible weakening of fracture calluses with FGF-1 treatment. This result underscores the recurring theme that fracture callus size is a poor predictor of functional fracture repair and supports the major role of FGF-1 in chondrocyte proliferation.
FGF-2 (basic FGF) is generally more potent than FGF-121 and has been accorded much more attention in fracture repair studies. FGF-2 is expressed by osteoblasts and has also been detected in the upper hypertrophic zones of the growth plate during development, an observation that suggests a role in chondrocyte maturation and endochondral bone formation (Table 1). In addition to its mitogenic and angiogenic properties, FGF-2 also stimulates bone resorption. These properties suggest that FGF-2 has the potential to influence many phases of fracture repair, from early post-traumatic events to late remodeling of the callus. This hypothesis is supported by the in vivo data.
In a canine tibial osteotomy model, a single injection of FGF-2 was associated with an early increase in callus size.22 This stimulation was attributed to the stimulation of periosteal progenitor cell proliferation and was associated with increased mechanical strength at 16 weeks post fracture. FGF-2 treatment was also associated with a more rapid resolution of the soft callus, such that at 32 weeks post fracture there was no difference in mechanical strength between fractures treated with FGF-2 or with vehicle. In a fibular fracture model, FGF-2 was injected at the time of fibular fracture to the fracture site of normal rats and rats were rendered experimentally diabetic via streptozotocin treatment. In these studies, FGF-2 dose-dependently increased the volume and the mineral content of the callus and improved the mechanical properties of the healing fractures.23 Similar results have been obtained by using FGF-2 suspended in a viscous gel formation of hyaluronan, an extracellular matrix component, and given as a single administration to osteotomies generated in the fibulae of baboons.24 Unlike most other growth factors, there appears to be a positive correlation between FGF-2–induced fracture callus size and mechanical strength. It is tempting to speculate that the stimulation of fibroblast and osteoblast proliferation by FGF-2 would result in enhanced collagen synthesis within the callus and concomitantly increase its mechanical stability. This possibility requires further direct study.
Other studies have concluded that neither FGF-1 nor FGF-2 accelerates fracture repair in rabbits. A single injection of either compound into calluses 4 days after tibial fracture had no effect on callus size or its relative amounts of bone and cartilage on day 10.25 Mechanical testing was not performed in this study, and it remains possible that the short period of follow-up was not optimal for demonstrating positive effects. It is also of interest that a single treatment with FGFs 4 days after fracture was ineffective in stimulating callus formation, whereas in studies where FGFs are administerer within 24 h after fracture, an increase in callus size is typically observed.22, 23, 26 This anecdote suggests that the window for anabolic effects with exogenous FGF treatment may be limited to the very early post fracture phase of healing. Consistent with this notion, in normal unfractured rabbit femurs a single intraosseous injection of FGF-2 induces the proliferation or recruitment of mesenchymal cells around existing trabeculae within 3 days.27 This response was associated with a significant and dose-dependent increase in bone mineral density, but only after 4 weeks post injection.27 In this model, and perhaps in fractured bone, FGF-2 administration may be causing a rapid induction of minimally differentiated mesenchymal cells, which is followed by a significant period of cell maturation before increased bone formation or repair are evident.
Platelet-derived growth factor
PDGF is a dimeric molecule consisting of disulfide-bonded A- and B-polypeptide chains. PDGF can exist either as a homodimeric (PDGF-AA, PDGF-BB) or a heterodimeric form (PDGF-AB) according to the relative levels of each subunit generating a level of ligand complexity in cells in which both polypeptides are expressed.28 The different PDGF isoforms (AA, AB, BB) exert their effect on target cells by binding with different specificity to two structurally related protein tyrosine kinase receptors, denoted as the α-receptors and β-receptors.28 The PDGF receptors are activated by ligand binding and subsequent receptor dimerization. Because each subunit of the dimeric PDGF molecule contains a receptor-binding site, one complete PDGE molecule binds two receptor molecules simultaneously. The receptors also display polypeptide preference in that the α-receptor will bind either an A-chain or B-chain but the β-receptor only binds the B-chain. Thus, PDGF-AA induces αα receptor dimers, PDGF-AB induces αα or αβ receptor dimers, and PDGF-BB induces all three possible combinations. Receptor dimerization leads to autophosphorylation, which regulates the intrinsic tyrosine kinase activity. Downstream signal transduction molecules associate with activated PDGF receptor complexes through their Src homology 2 domains (SH2). Molecules demonstrated as able to bind with PDGF α-receptors and β-receptors include phosphatidylinositol 3′ kinase, phospholipase C gamma, the Src family of tyrosine kinases, RAS, and signal transducer and activation of transcription (STAT) of the Jak/stat pathway (Fig. 3).28
PDGF is initially released by degranulating platelets in the fracture hematoma and may be important in promoting chemotaxis.29, 30 In this early stage PDGF-A is more abundant than PDGF-B and most cells appear to be making the homodimeric PDGF-AA. PDGF is also expressed by macrophages that migrate into the fracture site in response to the fracture trauma and initial release of PDGF by platelets. Later in fracture repair, PDGF protein is detectable in both early and mature hypertrophic chondrocytes, although this is primarily PDGF-A.3 Osteoblasts express only PDGF-B, suggesting the PDGF-BB form is the primary isotype in these cells.3
Studies using PDGF in vivo include a unilateral tibial osteotomy model in rabbits. PDGF or vehicle was suspended in a collagen carrier and injected into osteotomies. PDGF treatment was associated with a subjective increase in callus density.31 After 28 days post surgery, PDGF-treated osteotomies were as strong as non-operated control tibiae, whereas vehicle-treated osteotomies were still weaker than controls. These PDGF effects were also associated with an earlier return to normal weight bearing. This pilot study used a small number of animals, and these promising results need further support through larger studies. The mechanisms by which exogenous PDGF might influence fracture repair have yet to be defined. One study demonstrated that PDGF actually inhibits the bone regeneration induced by osteogenin (BMP-3), in a rat cranial defect model.32 The mechanism for this inhibition was attributed to a significant stimulation of soft tissue repair with PDGF, which may have mitigated the effects of BMP-3 on osteogenesis. These studies shed light on the potential for PDGF to influence bone formation and on its limitations in effecting the full cascade of events required for fracture repair.
REGULATION VIA THE SERINE-THREONINE RECEPTOR KINASE PATHWAYS
Transforming growth factor beta
The TGF-β molecules are members of a large family of secreted factors collectively referred to as the TGF-β superfamily. This superfamily contains not only the TGF-β isoforms but also the activins, BMPs, the growth and differentiation factors (GDFs or CDMPs), and several other secreted factors.33, 34 TGF-β–related proteins are found in all vertebrate species, the fruitfly Drosophila, and the nematode Caenorhabditis elegans. All members of the TGF-β superfamily are synthesized as large precursors which are proteolytically cleaved to yield carboxy-terminal mature protein dimers.34, 35 These evolutionarily conserved molecules play important roles in cell differentiation and proliferation during development and appear to play a variety of regulatory roles in the adult organism.
Five isoforms of TGF-β have been identified to date.33 Two isoforms, TGF-β1 and TGF-β2, have received the most attention regarding fracture repair, and for discussion purposes may be referred to collectively as TGF-β. TGF-β signaling involves two receptor types, TGF-β receptor type I and type II.34-36 Most cells within the fracture site as well as elsewhere in the body express TGF-β receptors on their surface. The specificity of downstream signals is generated according to which of the various type I receptors is associated with the receptor ligand complex. TGF-β ligand initially binds an oligomeric form of type II receptor followed by the recruitment of a type I receptor into the complex, possibly also in an oligomeric form, resulting in a heterotetrameric receptor complex associated with the ligand. The type II receptor has a constituitively active serine-threonine kinase activity, which phosphorylates the type I receptor in the GS domain, thus activating the type I receptor serine-threonine kinase activity. The activated type I receptor kinase is responsible for the downstream transmission of the signal through the superfamily signal effector (SMAD) family of molecules.37 Signaling through the downstream SMAD family of molecules is characteristic of the TGF-β superfamily member receptors (Fig. 3). Given the complexity of the signaling pathway and the potential for cross-talk between individual members of the TGF-β superfamily of molecules, several of which will be discussed in the context of the fracture repair process, a separate section on SMAD signaling molecules is included below.
TGF-β is a pleiotropic growth factor initially released by the degranulating platelets in the hematoma and by the bone extracellular matrix at the fracture site.3, 38 In the initial stages of fracture repair, TGF-β can be immunolocalized to the region of the hard callus where it defines the region of periosteal proliferation and intramembranous bone formation.38 Evidence suggests that TGF-β is likely to be primarily involved in the stimulation of proliferation by the preosteoblasts in this region. In addition, the expression of TGF-β is elevated during chondrogenesis and endochondrial bone formation with an initial peak in mRNA levels detected around day 6 post fracture followed by a nadir at day 10. TGF-β expression peaks again by day 14 and remains elevated until week 4. The nadir of TGF-β expression correlates with the peak in type II collagen expression, and the subsequent peak temporally coincides with chondrocyte hypertrophy.38 TGF-β is primarily thought to be a stimulator of undifferentiated mesenchymal cell and chondrocyte proliferation and extracellular matrix production during chondrogenesis and endochondral bone formation (Table 1). TGF-β may also be involved in the normal coupling of bone formation with resorption.
The role of endogenous TGF-β, or of any growth factor, in normal fracture repair is inherently difficult to resolve. However, the importance of TGF-β to this process is implied by the ability of exogenous TGF-β to stimulate fracture repair in several models. Early evidence for the bone-healing effects of TGF-β came from studies on critical size defect repair in the rabbit skull.39 A single application of TGF-β induced complete bony bridging of skull defects that would otherwise heal with fibrous connective tissue. While healing in this model was via intramembranous bone formation, this was nonetheless the first demonstration of a purified growth factor inducing bone repair in the absence of bone morphogenetic proteins.
The ability of TGF-β to stimulate long bone healing was first demonstrated in midtibial osteotomies in rabbits treated with a compression plate.40 Continuous infusion of the osteotomy site with high doses of TGF-β (1–10 μg/day) for 6 weeks resulted in a dose-dependent increase in callus volume and increased mechanical strength compared with untreated osteotomies. In a rat tibial fracture study, TGF-β (4–40 ng) was injected around the fracture site every 2 days during a 40-day healing period.41 TGF-β dose dependently increased the cross-sectional area of the callus, and mechanical testing demonstrated a higher ultimate load in fractures treated with the high dose of TGF-β. The positive results of these two studies might be attributable to the rigorous dosing schedules applied. Continuous infusion or frequent injections into the fracture site would have limited clinical utility, and studies using more convenient dosing regimens have yielded less impressive results. For example, the healing of rabbit tibial fractures was studied after a single injection of TGF-β2 into the callus 4 days after fracture.42 Fractures were either stabilized by plating or remained mechanically unstable, and healing was assessed just 14 days post fracture. This time interval permits abundant cartilaginous callus to form but is not sufficient to achieve mechanically stable cortical union. Mechanical testing was not performed in this study. Injection of TGF-β2 was associated with significant edema around the injection site. In mechanically stable fractures, the high dose of TGF-β2 caused an increase in total callus volume but did not increase the bone content of the callus. In mechanically unstable fractures, TGF-β2 did not increase total callus volume or its bone content, but did increase the fibrous component of the callus. The latter result is consistent with impaired callus development with TGF-β2 treatment and emphasizes the requirement for proper surgical management of fractures if growth factors are to be successfully used to enhance healing.
Other studies involving a single treatment at the time of osteotomy have also failed to show significant bone healing effects. Large calvarial defects in adult baboons were treated with a collagenous matrix containing various doses of human TGF-β1.43 After 30 days, the amount of bone and osteoid in defects treated with TGF-β1 was not significantly different than in defects treated without TGF-β1. In a rabbit model, TGF-β1 or vehicle was suspended in a paste of tricalcium phosphate and amylopectin and applied to a 1 cm radial osteotomy.44 Radiographically, callus appeared to be larger and to persist longer in defects treated with TGF-β1. However, this callus response was not associated with improved union or bridging of the defects, which at 8 weeks were not different than defects left untreated. Mechanical testing also demonstrated that TGF-β1–treated defects were not different than those left untreated. The results of these studies suggest that the ability of TGF-β to stimulate fracture repair may require persistent dosing or very high concentrations.
Bone morphogenetic proteins
The BMPs are a subfamily of the TGF-β superfamily of polypeptides. BMPs are distinguished from other members of the family by having, in general, seven, rather than nine, conserved cysteines in the mature region.45, 46 The BMPs play critical roles in regulating cell growth, differentiation, and apoptosis in a variety of cells during development, including osteoblasts and chondrocytes. Compared with TGF-β, BMPs have more selective effects on bone and also have shown more promising results in animal models of fracture healing. The identification of BMPs was facilitated by the seminal discovery by Marshall R. Urist that demineralized bone powder had the capacity to induce de novo bone formation in an intramuscular pouch.47 There are currently 16 known members of this family that can be subdivided into several classes based on structure.45
BMP signal transduction occurs by a mechanism similar to the other members of the TGF-β superfamily (Fig. 3). BMP ligand can associate with several serine-threonine kinase receptors, including BMP receptor type II, receptor type IA, and receptor type IB (BMPR-II, BMPR-IA, and BMPR-IB, respectively) as well as the related activin receptors (ActR-II, ActR-IIB, and ActR-I).48 As with TGF-β, the BMP ligand binds to the type II receptor, and this receptor occupancy leads to association of the complex with an appropriate type I receptor forming an active receptor–ligand complex. This interaction can be blocked by the antagonists of BMPs, noggin and chordin, which can bind and block BMP activity by preventing receptor binding.49, 50 This antagonist function of noggin and chordin has been specifically demonstrated in osteoblastic cells. The expression of the BMP receptors BMPR-IA and BMPR-IB is dramatically increased in osteogenic cells of the periosteum near the ends of the fracture in the early post fracture period.51 BMPR-IA and BMPR-IB levels are also elevated in proliferating and mature chondrocytes in day 7 cartilage but are not highly expressed in hypertrophic chondrocytes. BMPR-II is coexpressed with BMPR-IA and BMPR-IB but at significantly lower levels.52 The activin receptor ActR-I is also expressed in proliferating and mature chondrocytes, while activin A is only minimally expressed around the cartilage island.51, 52 Therefore, BMP signaling involves a complex receptor pattern in addition to the multitude of BMPs expressed during fracture repair. BMP receptor signaling, as with the TGF-βs, is transmitted through the SMAD family of signal effectors, again providing for a high degree of cross-talk between signals generated by multiple members of the TGF-β superfamily of polypeptides (Fig. 3).
During fracture repair, the BMPs reported to be expressed include BMP-2, BMP-3 (osteogenin), BMP-4, and BMP-7 (osteogenetic protein, OP-1). BMP-2 and BMP-4 are closely related to the Drosophila molecule decapentaplegic (dpp) and signal through a common receptor. Several reports have demonstrated that BMPs are expressed in the early stages of fracture repair where it is likely that small amounts are released from the extracellular matrix of the fractured bone (Table 1). During intramembranous bone formation, osteoprogenitor cells in the cambium layer of the periosteum may respond to this initial low level of release from the extracellular matrix and begin differentiating. BMP-4 mRNA levels do transiently increase in osteoprogenitor cells in this region, and immunolocalization demonstrates an increase in detectable BMP-2/4 near the fracture ends in the cambium region of the periosteum.52, 53 In this region, it appears that BMP-2/4 are driving the osteoprogenitor cells to complete their differentiation into mature osteoblasts. Staining for BMP-2, BMP-4, and BMP-7 is minimal around the hematoma but is up-regulated in primitive mesenchymal cells as they infiltrate into the fracture site and proliferate.52 By days 7–14 post fracture, the expression of BMP-2/4 is maximal in chondroid precursors, while hypertrophic chondrocytes and osteoblasts only show moderate levels of expression. BMP-7 expression was not detectable in day 14 chondrocytes. The current view of the role of BMPs in fracture repair is that these molecules are primarily activators of differentiation in osteoprogenitor and mesenchymal cells destined to become osteoblasts and chrondrocytes (Table 1). This activation by BMPs, specifically BMP-2, is inhibited by the molecules noggin and chordin which have been demonstrated to block BMP-2 interaction with its receptor.50 As these primitive cells mature, BMP expression is dramatically reduced. BMP expression emerges transiently in chondrocytes and osteoblasts during their respective periods of matrix formation, and returns to low levels during callus remodeling.54 It is interesting to note that while mature osteoblasts and chondrocytes do not express significant levels of BMPs in normal bone, they both have greatly increased BMP expression later in fracture repair.
The earliest studies on fracture repair utilized partially purified preparations of BMPs obtained from fractionated demineralized bone matrix. These preparations typically contain multiple BMP isoforms and include noncollagenous bone matrix proteins. Even without BMP purification steps, demineralized bone matrix is an effective graft material in promoting the union of large segmental defects in the rat femur.55 Partially purified human BMPs have also been used clinically in combination with autogenic cancellous bone grafts to successfully repair large segmental tibial defects.56 Early clinical trials for BMPs involved attempts to achieve union in patients with “intractable” fractures. In one study, femoral union was achieved in all 12 patients treated with a combination of internal fixation and implants of partially purified human BMP.57 Prior to enrollment, these patients had failed to respond to an average of 4.3 surgical attempts at union. In a similar study, human BMP was implanted into resistant nonunions in combination with internal fixation, and union was achieved in 24 out of 25 patients.58
While these early data are encouraging, the lack of a no-BMP control group limits the interpretation of BMP effects. Animal studies with appropriate control groups have provided significant evidence that BMPs are capable of inducing the union of large segmental defects that would fail to heal in the absence of exogenous BMPs. In dogs, the addition of partially purified canine BMP to carrier implants caused a significant increase in new bone formation within radial segmental defects.59 Mechanical testing was not reported. In this study, carrier implants without BMP or with bovine BMP did induce significant bone formation in the defects, indicating possible species specificity for BMPs at least in dogs. Another dog study tested the efficacy of recombinant human BMP-7 (rhBMP-7), also known as OP-1, in an ulnar segmental defect model.60 Defects treated with a collagen carrier failed to achieve union or show evidence of new bone formation, whereas the addition of BMP-7 to the carrier was associated with complete radiographic bridging of the defect by 8 weeks. Mechanical testing of the BMP-7–treated ulnae showed that the strength of these repaired bones was similar to that of the contralateral intact ulna. Similar results have been obtained with rhBMP-7 using a rabbit ulnar nonunion model.61 Segmental defects treated with a collagen carrier alone or with no implant showed no significant bone formation or bridging during an 8-week follow-up, whereas implants containing rhBMP-7 induced complete radiographic bridging within 8 weeks. The mechanical strength of the unions achieved with rhBMP-7 was comparable to that of intact bones.
rhBMP-2 has proven to be effective in a number of segmental defect models. The first report demonstrated that BMP-2 dose-dependently stimulated new bone formation in the femoral defects of rats.62 The highest does of BMP-2 was associated with union in 8 of 10 rats. All six control defects treated with a collagenous carrier alone failed to achieve union. Mechanical testing demonstrated that the stiffness of femurs treated with BMP-2 was comparable to that of the contralateral intact femur. rhBMP-2 has also been demonstrated to promote union in sheep studies. In one study, a 2.5 cm segmental defect was created in the right femur of sheep.63 All defects that were filled with either an inactive bone matrix carrier or with nothing resulted in nonunion. Defects filled with the inactive bone matrix carrier plus rhBMP-2 all attained union within 3 months. Interestingly, autogeneic bone grafts that did not include exogenously added BMPs also achieved union. This result might indicate that the endogenous levels of BMPs in bone graft material are sufficient to induce adequate bone formation and union. Bones that achieved union through autogeneic bone grafting or through BMP-2/carrier treatment had mechanical strength that was comparable to the contralateral intact bone.63 rhBMP-2 was also demonstrated to dose-dependently stimulate bone formation in a rabbit ulnar defect model.64 In this study, union was achieved in all ulnar defects treated with a high dose of BMP-2, while a low dose resulted in union in half the defects. Carrier without BMP-2 failed to result in union in all animals. Mechanical testing revealed that those ulnae that achieved union after BMP-2 treatment had strength and stiffness that were at least as great as intact control ulnae.
Partially purified bovine BMP-3 has been tested in a rat femoral defect model, using a hydroxyapatite/ceramic composite carrier.65 Defects treated with BMP-3 demonstrated a greater induction of bone both within the carrier implant and within the defect. However, biomechanical testing did not reveal any effect of BMP-3 on parameters of mechanical strength. Partially purified BMP-3 has also been reported to induce the healing of critical size defects in the cranium of adult baboons.66 More animal studies must be conducted, with biomechanical testing as one endpoint, to adequately resolve the question of whether purified and/or recombinant BMP-3 will have similar efficacy to that attained with BMP-2 or BMP-7.
TGF-β superfamily signal effectors SMADs
SMADs are a class of proteins that function as intracellular signaling effectors for the TGF-β superfamily of secreted polypeptides.37, 48, 67 Superfamily signal effectors (SMADs) received their name as a contraction of the names of the C. elegans gene Sma and Drosophila gene Mad (mothers against decapentaplegic), the first identified members of this evolutionarily conserved class of proteins. SMADS have a structure that contains a conserved N-terminal MH1 domain and C-terminal MH2 domain separated by a less conserved linker (L) domain. There are currently seven members of the SMAD family divided into three subclasses including the pathway-restricted SMADs (SMAD1, 2, 3, and 5), common-mediator SMADs (SMAD4) and the inhibitory SMADs (SMAD6 and SMAD7). TGF-β signaling occurs through the pathway restricted SMAD2 and SMAD3, while BMP signals are transmitted through SMAD1 and SMAD5. The activated type I receptor activates the appropriate pathway restricted SMAD by phosphorylating the C-terminal SSXS domain, thus initiating the interaction of the pathway-restricted SMAD with the common-mediator SMAD (SMAD 4). This complex is than translocated to the nucleus where it acts by asssociating with other factors and directly binding DNA (Fig. 3). Given SMAD4 interacts with each of the pathway-restricted SMADs and is required for proper signaling it is likely that this molecule plays an important role in the integration of signals generated simultaneously by multiple members of the TGF-β superfamily.
CROSS-TALK BETWEEN SIGNALING PATHWAYS
While the mechanisms for signal transduction through individual growth factors and their associated receptors are well described, there still exists no clear understanding of how these multiple factors when expressed in a restricted spatial context can cooperatively regulate a complex process such as fracture repair. Nor is it clear how these growth factors cooperatively interact with the signaling by cytokines and their associated receptors. As previously stated, there is a growing consensus that the initiation of fracture repair may involve signals generated by the cytokines expressed as part of the initial trauma response and inflammation. Preliminary reports demonstrate that IL-1, IL-6, and TNF are expressed early in the fracture repair process but no comprehensive examination has been made of the spatiotemporal profile of the cytokines expressed in the fracture site during the repair process.4, 68 Signaling through cytokine receptors is a rapidly growing area of research that has generated a large body of literature, primarily because of the complicated nature of these signaling pathways. Cytokine receptors, for the most part, do not contain intrinsic catalytic domains in their cytoplasmic portions and therefore these molecules do not fall under one of the aforementioned general categories. These receptors utilize cytosolic molecules that associate with the cytoplasmic domains of the receptors for signal transduction. In the case of IL-6 these include the Janus family of tyrosine kinase (JAK) molecules.69, 70 These molecules are activated indirectly by receptor occupancy and signal by activating the downstream STAT molecules. These molecules directly translocate to the nucleus and participate in DNA binding. As previously mentioned, this pathway may be also involved in downstream signals generated by the PDGF receptors. Interestingly, it has recently been demonstrated that there can be exist a synergy between STAT3 and SMAD4 activity suggesting cross-talk between signals generated by the JAK/STAT pathway and those from TGF-β superfamily members (Fig. 3).71, 72 Another area of potential cross talk between signaling systems involved in fracture repair is the MAPK cascade. Both FGF and PDGF receptor signals may, in part, signal through this kinase cascade via activation of the upstream molecule RAS.28 Another member of the MAPK family, TGF-β associated kinase (TAK-1), has been shown to be stimulated by TGF-β and BMP-4 and there is evidence that members of the MAPK family when activated through the epidermal growth factor or hepatocyte growth factor receptor tyrosine kinases phosphorylate SMAD1 in the linker region inhibiting its translocation to the nucleus.71, 72 These data further suggest an active mechanism of cross talk between the tyrosine kinase and the serine threonine receptors, through the MAPK signaling cascades (Fig. 3). In addition, there is evidence that IL-1 and TNF receptor signaling also utilizes the MAPK signaling modules through an upstream signaling molecule NF-κB inducing kinase (NIK) with similarities to RAS as well as RAS itself (Fig. 3).73, 74 While the MAPK pathway is only one of several signaling pathways, these reports taken together suggest this point of convergence in the signaling from multiple growth factor and cytokine receptors is an important site for integrating incoming signals from the cell surface (Fig. 3).
Despite the growing volume of data on the biology of fracture repair and the molecules involved in its regulation, a number of complex issues still remain to be elucidated. For example, currently there does not exist a complete understanding of which of the aforementioned molecules are required for successful fracture repair and which are accessories. Nor is there a clear picture of what cells are expressing these factors and which elaborate the appropriate receptors and internal signal transduction machinery to respond to their signals. Additionally, there is very little information on which factors may modify the ability of the effectors discussed to regulate target cells. One area in which this issue is becoming evident is in BMP signaling and its antagonism by noggin and chordin. Similar modifying molecules that antagonize, amplify, or alter growth factor and cytokine activities are likely to exist throughout the fracture repair process, and the correct function of these secondary modifier molecules is as equally relevant as the growth factors themselves. Finally, this review has focused on growth factors and cytokines and their signaling through membrane associated receptors; however, one must also recognize that for many of these molecules there are soluble counterparts and systemic effectors of the process of fracture repair. Therefore, much remains to be discovered before a complete understanding of fracture repair can be achieved.
Acknowledgements
The authors thank Christopher A. George for preparing the photomicrographs and graphics depicted in this manuscript.