No Difference in Intestinal Strontium Absorption After an Oral or an Intravenous 1,25(OH)2D3 bolus in Normal Subjects
Abstract
It has been suggested that 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) stimulates intestinal calcium absorption less via the intravenous (iv) than the oral route, because the first avoids direct contact of the drug with the enterocytes. However, no study has addressed the issue directly. This investigation was designed to measure the effect of a single oral or iv dose of 1,25(OH)2D3 on calcium absorption, using stable strontium (Sr) as a surrogate for calcium, and measuring the Sr fractional absorbed dose (FAD%) over 240 minutes after Sr administration. In 10 healthy volunteers, five tests were performed in a cross-over design, with a wash-out period between two consecutive tests: Sr absorption without 1,25(OH)2D3 (test A); Sr absorption immediately after either oral (test B) or iv (test C) 1,25(OH)2D3 (1.5 μg/m2 of body surface area [BSA]); Sr absorption (24 hr after either oral (test D) or iv (test E) 1,25(OH)2D3 (1.5 μg/m2 BSA). The concurrent administration of 1,25(OH)2D3 and Sr (tests B and C) did not significantly change the area under the Sr FAD%–time curve with respect to test A (test A: 4090 ± 345; test B: 4510 ± 345; test C: 4210 ± 345), whereas Sr absorption was significantly increased (p < 0.001) when Sr was given 24 hr after either oral or iv 1,25(OH)2D3 (test D: 5710 ± 345; test E: 5510 ± 345). It was concluded that 1,25(OH)2D3 is likely to influence calcium absorption significantly only via its genomic effect, independent of its administration route.
INTRODUCTION
The clinical use of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) in chronic renal failure is often limited by hypercalcemia. Calcitriol pulse therapy is considered an important part of the standard treatment of secondary renal hyperparathyroidism, since it has been shown to suppress parathyroid cell proliferation, thus preventing parathyroid gland hyperplasia,1 and to reduce parathyroid hormone (PTH) gene transcription.2 It has been postulated that intravenous (iv) therapy with 1,25(OH)2D3 is more effective than oral treatment in decreasing PTH plasma levels.3, 4 At the same time, iv 1,25(OH)2D3 is believed to decrease the frequency of hypercalcemia by avoiding direct contact with the enterocytes during the intestinal calcium absorption process.3 However, no direct study addressing the question as to whether iv and oral 1,25(OH)2D3 have different effects on intestinal calcium absorption has been published. To evaluate intestinal calcium absorption in response to a single oral or iv dose of 1,25(OH)2D3, we measured it in healthy volunteers, using stable strontium (Sr) as a surrogate tracer for calcium.5 A cross-over design was used, with adequate wash-out periods between tests.
MATERIALS AND METHODS
Healthy volunteers
Ten healthy caucasian volunteers (six men and four women; age range 27–42 years; weight 66.7 ± 14.1 kg) were studied. They had no medical history suggesting any renal, endocrine, cardiovascular, hepatic, or other diseases. They had normal physical examination and biochemical findings, including calcium, phosphorus, albumin, creatinine, blood urea nitrogen (BUN), bicarbonate, alkaline phosphatase (ALP), intact PTH (iPTH), and 1,25(OH)2D3 measured in serum or plasma in the morning, after an overnight fast. None of them was taking any medication on a regular basis, nor did they take any drugs before or during the study. The four female subjects were premenopausal with regular menstruation.
Study protocol
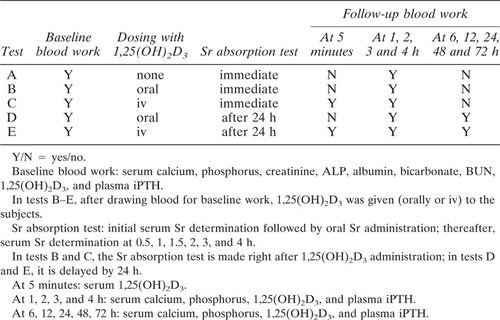
Each subject was submitted to five separate Sr absorption tests with intervals of at least 21 days (wash-out periods), during which the serum Sr concentration was allowed to return to baseline values. The five tests are identified by the letters A–E: test A, Sr absorption without 1,25(OH)2D3; test B, Sr absorption immediately after administration of oral 1,25(OH)2D3; test C, Sr absorption immediately after administration of iv 1,25(OH)2D3; test D, Sr absorption, 24 h after oral 1,25(OH)2D3; and test E, Sr absorption, 24 h after iv 1,25(OH)2D3.
A cross-over design was chosen for the study. Test A was always performed as the first test, whereas tests B–E, were performed in a random sequence.
All subjects had been instructed to maintain a self-selected diet containing a normal calcium intake (assessed through an interview with the dietician) throughout the week before and during the study period, but to avoid toothpaste in the 12 h preceding the test, since toothpaste often contains Sr.
For each test, the subject was admitted to the hospital in the early morning after an overnight (12 h) fast. Weight and height were recorded, and an in-dwelling catheter was placed in a forearm vein. Blood samples were drawn for baseline laboratory evaluation: serum calcium, phosphorus, creatinine, ALP, albumin, bicarbonate, BUN, 1,25(OH)2D3, and plasma iPTH (Table 1).
At this point, in test A (Sr absorption without 1,25(OH)2D3, the Sr absorption test, as described below, was started immediately. In tests B and C, 1,25(OH)2D3 was administered per os or iv respectively (see below), then the Sr absorption test was started immedately. In tests D and E, 1,25(OH)2D3 was administered per os or iv, respectively, then, after 24 h, the Sr absorption test was performed. In all tests (A–E), serum calcium, phosphorus, 1,25(OH)2D3, and plasma iPTH concentrations were measured at 1, 2, 3, and 4 h. In tests D and E, serum calcium, phosphorus, 1,25(OH)2D3, and plasma iPTH concentrations were also measured 6, 12, 24, 48, and 72 h after 1,25(OH)2D3 administration. In tests C and E, blood was also drawn at 5 minutes after iv 1,25(OH)2D3 for serum 1,25(OH)2D3 determination. The protocol is summarized in Table 1.
For the serum determinations, blood in tubes was allowed to clot for 30 minutes at room temperature before centrifugation. All serum and plasma samples were frozen immediately after centrifugation and stored at −20°C until analysis.
The study was approved by the Institutional Review Board of the Department of Pediatrics, University of Milan and the Ethical Committee of the University of Heidelberg. Written informed consent was obtained from each volunteer.
Sr absorption test
A blood sample (2 ml of whole blood) was drawn for baseline Sr determination. Then the standard dose of hexahydrated Sr chloride (8.06 mg/kg of body weight, equivalent to 2.65 mg of SrCl2) was administered per os in 200 ml of deionized water to be drunk in 1 minute.5
Thereafter, Sr determination was made at 0.5, 1, 1.5, 2, 3, and 4 h. Between blood samplings, the vascular access was maintained with 0.9% saline solution (heparinized catheter). The subjects were not allowed to eat any food during the 4 h of the Sr absorption test.
1,25(OH)2D3 administration
A dose of 1.5 μg/m2 of body surface area (BSA) rounded to the nearest multiple of 0.25 μg, was administered. Rocaltrol® capsules of 0.25 μg or 0.5 μg (Hoffman-La Roche, Basel, Switzerland) were used for oral administration, and Calcijex® 1 μg vials (Abbott Laboratories, North Chicago, IL, U.S.A.) for iv administration. Intravenous 1,25(OH)2D3 was given through a vascular access separate from that used for blood sampling.
Analytical methods
Serum calcium, phosphorus, albumin, bicarbonate, ALP, creatinine, and BUN were determined using standard laboratory procedures. Serum 1,25(OH)2D3 was determined by radio receptor assay (Nichols Institute Diagnostics, San Juan Capistrano, CA, U.S.A.), with mean intra- and interassay coefficients of variation (CVs) of 7.8% and 13.7%, respectively. The normal range is 18–62 pg/ml. Plasma iPTH was measured in duplicate using a two-site radioimmunoassay (Allegro Intact PTH kit; Nichols Institute Diagnostics). The assay has a sensitivity of 1 pg/ml; the duplicate measurements showed a mean intra- and interassay CV of 4.1% and 5.8% respectively. Sr was measured using an atomic absorption spectrophotometer (4000 Atomic Absorption; Perkin Elmer, Norwalk, CT, U.S.A.), previously calibrated with a Sr solution of known concentration. The procedure involved stabilization of the air-acetylene flame temperature for 10 minutes, calibration of the equipment, analysis of the samples, and reading of the values at a wavelength of 460.7 nm (slit 0.7).5 All samples from each subject were processed and analyzed in duplicate in the same assay. The reproducibility of the method was evaluated on 60 samples reanalyzed on different days. The CV at our laboratory was 4.1%.
Statistical analysis
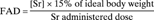
For all variables, the Shapiro–Wilk's test for normality was used6 in order to choose the appropriate statistical analysis. All the studied variables were found to be normally distributed within time and groups. The baseline descriptive variables are given as mean ± SD. The values of the variables measured over time (1,25(OH)2D3, Sr, and iPTH) are presented as least squares means ± SE.8
Analysis of variance was used to evaluate baseline values for Sr, calcium, phosphorus, iPTH, 1,25(OH)2D3, maximum Sr concentration (Cmax), and the area under the Sr FAD%–time curve over the 240 minutes (SrAUC0–240). The AUCs were calculated using the trapezoidal rule (Bezout integral); the computer-calculated areas were rounded.
The general linear model for unbalanced data (Proc. Glm SAS/PC version 6.04; SAS Institute, Cary, NC, U.S.A.) with a split-plot design was used to compare the effects of different administration routes of 1,25(OH)2D3 at different times. The means calculated within this model (population marginal means) are obtained by the least squares method and are the expected values of class or subclass means that would be expected for a balanced design. The standard error in this analysis is referred to the mean value for the general linear model. The linear and quadratic trends between and within groups were evaluated using orthogonal polynomial fitting especially constructed for data unequally spaced in time. According to Scheffé's test, multiple within-group comparisons were performed when the linear or quadratic trends were significant.7, 9 A p value ≤ 0.05 was considered statistically significant. All of the statistical tests were two sided.
RESULTS
All the subjects had normal serum calcium, phosphorus, 1,25(OH)2D3, and plasma iPTH levels at baseline. The mean basline level of 1,25(OH)2D3 was 33.8 ± 6.4 pg/ml (range 18–50 pg/ml), and that of iPTH was 28.1 ± 12 pg/ml (range 13.7–58.0).
The 1,25(OH)2D3 serum concentration versus time after oral and iv 1,25(OH)2D3 administration is shown in Fig. 1. As expected, the kinetics of 1,25(OH)2D3 were substantially different between the two administration modalities. The peak 1,25(OH)2D3 concentration (oral: 101 ± 15 pg/ml; iv: 297 ± 24 pg/ml; p < 0.001) but also the area under the 1,25(OH)2D3 concentration–time curve (oral: 3180 ± 291 pg/ml · h; iv: 4260 ± 429 pg/ml · h; p < 0.01) were significantly higher after iv administration. Six hours after the administration of the 1,25(OH)2D3, the serum concentration of 1,25(OH)2D3 was no longer significantly different in the two groups.
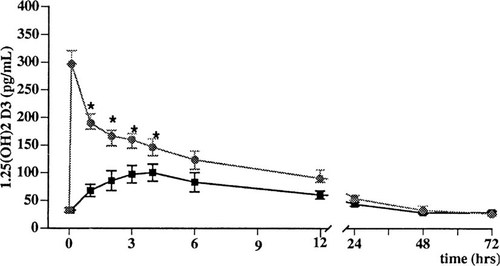
Serum concentrations of 1,25-dihydroxyvitamin D after 1,25(OH)2D3 (1.5 μg/m2 of BSA) given orally (▪) or iv (hex grid) in normal subjects. Values are expressed as least squares means ± SE. *p < 0.05.
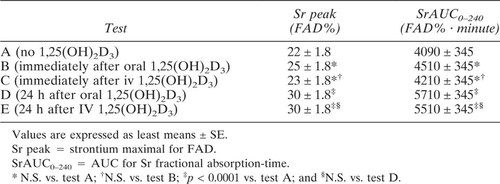
Figure 2 shows Sr fractional absorption over time in the five tests. When oral or iv 1,25(OH)2D3 was administered at the start of the Sr absorption test (tests B and C), Sr fractional absorption was not changed with respect to test A, where no 1,25(OH)2D3 was given. On the contrary, Sr absorption measured 24 h after 1,25(OH)2D3 administration was significantly increased with both the oral (test D) and the iv (test E) route. Similar findings were observed when the results were analyzed as the area under the Sr fractional absorption–time curve and in terms of peak Sr concentration (Table 2). Again, there was no difference between the oral or iv 1,25(OH)2D3 administration routes.
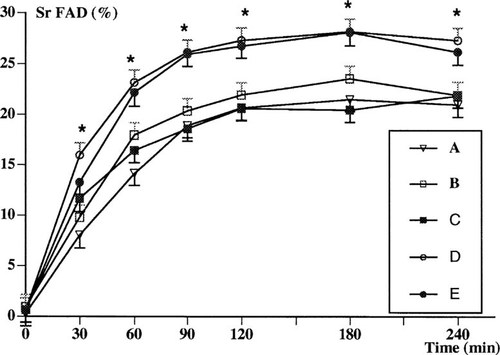
Time course of Sr FAD in normal subjects under different conditions with regard to the route and timing of administration of 1,25(OH)2D3. Sr absorption test: without 1,25(OH)2D3 (A); immediately after oral 1,25(OH)2D3 (B); immediately after iv 1,25(OH)2D3 (C); 24 h after oral 1,25(OH)2D3 (D); and 24 h after iv 1,25(OH)2D3 (E). Values are expressed as least squares means ± SE; *p < 0.001 (tests D and E vs. tests A, B, and C).
Neither serum calcium and phosphorus levels nor plasma iPTH levels showed any significant changes in relation to the 1,25(OH)2D3 administration route. In particular, the mean of the maximum concentrations of serum calcium and phosphorus levels after oral or iv 1,25(OH)2D3 were, respectively, 9.87 ± 0.37 mg/dl and 9.78 ± 0.31 mg/dl (calcium) and 4.47 ± 0.10 mg/dl and 4.24 ± 0.22 mg/dl (phosphorus); the mean iPTH minimum concentrations were 15.7 ± 4.8 pg/ml and 16.7 ± 3.1 pg/ml.
DISCUSSION
The present study demonstrates that, in normal human subjects, a pharmacological dose of 1,25(OH)2D3 increases Sr absorption (used as a surrogate for measuring calcium absorption) by 35% as measured 24 h after 1,25(OH)2D3 administration, whereas no early increase in Sr absorption could be detected immediately after use of the drug. The study also demonstrates for the first time that the increase in intestinal calcium absorption is not influenced by the route of administration of 1,25(OH)2D3.
The use of stable Sr to investigate intestinal calcium absorption deserves comment. Calcium and Sr actually compete for intestinal absorption,10 bone distribution,11 and renal excretion,12 and their metabolism is controlled by the same hormones.13 According to the periodic table, Sr is the most similar element to calcium.14 Moreover, Sr shares a number of other common characteristics with calcium: it mimics calcium in many biochemical reactions, it can substitute for calcium in transport systems, and it shares with calcium the process for uptake in the mitochondria. The high Sr affinity for calcium-binding proteins, although less than that of calcium in absolute value,15-17 theoretically makes it a reliable means of measuring intestional calcium absorption,18-22 especially in a comparative analysis. Reid et al.5 who first developed the test as presently used, demonstrated a very close correlation (r = 0.93) between Sr and calcium absorption as comeasured by45Ca: the fractional absorption of Sr was lower than that of45Ca, but its time course curve closely resembled that of radioactive calcium. The same conclusion was reached by Milson et al., who performed the test under different clinical conditions, characterized by abnormalities in calcium absorption.23 The stable Sr test is, therefore, currently considered an easy, safe, and reliable method for estimating the intestinal absorption of calcium.24, 25 Especially in the present research setting, in which repeated measurements were made in young subjects—including females of reproductive age—over a short time span, the absence of radioactivity in stable Sr makes it preferable to45Ca for estimating calcium absorption.
1,25(OH)2D3 is reported to influence the intestinal calcium absorption in vertebrates with at least two separate actions: a rapid (nongenomic) action on the cellular membranes and transport systems and a delayed action which involves gene activation and protein synthesis.26, 27 There appear to be several different mechanisms ensuring calcium entry across the brush-border membrane into the intestinal cell, as well as its translocation through the cytoplasm and its release across the basal lateral membrane into the circulation.28-31 In vitamin D–deficient animals, a biphasic response has been observed after a single iv dose of 1,25(OH)2D3: the first phase begins within 2 h and peaks by 6 h; the second phase begins after 12 h and peaks at 24 h 32 In vitamin D–replete animals, the “rapid” (nongenomic) action of 1,25(OH)2D3, not fully explained yet, is called transcaltachia and develops within a few minutes.33 It seems to involve the opening of membrane calcium channels and calcium transport through the cell in lysosome-like vesicles, with subsequent release in the circulation.34
The main action of 1,25(OH)2D3 on intestinal calcium absorption, however, requires several hours to develop, depending on the activation of nuclear vitamin D receptors (VDRs), gene transcription, and protein synthesis; the membrane calcium pumps are potentiated, and the intracellular calcium is taken up by newly synthesized proteins (calbindins) and actively transported toward the capillaries across the epithelial cells.35, 36
In our subjects, we measured intestinal Sr absorption with separate tests, after either oral or iv 1,25(OH)2D3 in order to study both the early and the delayed actions of the drug. However, during the first 4 h after either oral or iv administration of calcitriol, no “early” induction of intestinal Sr absorption was detected. It thus seems possible that transcaltachia is not very relevant in ostensibly healthy young adults on self-selected diets.
On the contrary, substantial stimulation of Sr absorption (> 35%) was observed 24 h after 1,25(OH)2D3 administration. The increase was in the same range as that observed in other studies in which, 8–16 h after calcitriol, calcium transport was increased by ∼25% and the net calcium absorption by 33%.37, 38 The observation of a significant increase in the intestinal absorption of Sr 24 h after 1,25(OH)2D3 administration is consistent with the delayed mechanism of action of calcitriol, i.e., the increase of calcium transport as a result of genomic effects (synthesis of calbindins and other proteins).38
The most interesting finding of our study was the fact that the route of 1,25(OH)2D3 administration did not affect intestinal Sr absorption. In 1984, Slatopolsky et al.3 favored the use of iv 1,25(OH)2D3 in the treatment of renal patients with secondary hyperparthyroidism, since the use of oral 1,25(OH)2D3 was limited by hypercalcemia and hyperphosphatemia. It was postulated that intermittent iv therapy with 1,25(OH)2D3 was superior to oral administration as a result of a number of mechanisms: higher peak concentration leading to greater occupancy of the parathyroid VDR, increased bioavailability through circumvention of the “first-pass” hepatic degradation of 1,25(OH)2D3; and decreased frequency of hypercalcemia and hyperphosphatemia by the avoidance of direct contact between 1,25(OH)2D3 and intestinal epithelial cells.3, 4
No previous study specifically addressed this last point in human subjects; as a result of the present study, Slatopolsky's hypothesis of a significant difference in the stimulation of intestinal calcium absorption between the two routes of administration of 1,25(OH)2D3 is not supported, at least in healthy subjects. Moreover, while both routes of administration of 1,25(OH)2D3 induced a decrease in iPTH levels, no different effects on iPTH were observed between them in our normal subjects.
In conclusion, our study does not support the view that intestinal calcium absorption is influenced by the route of 1,25(OH)2D3 administration. However, the study was conducted in normal subjects, and whether this also holds true for patients with chronic renal failure and secondary hyperparathyroidism remains to be demonstrated.
Acknowledgements
We thank Mrs. R. Greiffenhaghen for her precious assistance. This work was supported by a research grant from the “Associazione per il Bambino Nefropatico.”