Bone Physiology During Pregnancy and Lactation in Young Macaques
Abstract
We used a nonhuman primate model (Macaca nemestrina) of adolescent human pregnancy to characterize bone remodeling at midpregnancy and at weaning and the associated changes in bone mass. In this longitudinal study, 125 nulliparous females were followed through pregnancy, 6 months of lactation, and 3 months postweaning; 13 nonpregnant females served as controls. Between early pregnancy and midpregnancy, the whole body bone mineral increased. There was no significant change between midpregnancy and parturition. Between parturition and 3 months lactation, the animals lost 3.0% of their bone mineral (p < 0.01), which was regained by 3 months after weaning. The vertebral bone mineral apparent density decreased during pregnancy and 6 months of lactation, followed by an increase during the 3 months after weaning. Calcium, phosphate, 25-hydroxyvitamin D, and osteocalcin increased significantly from midpregnancy to weaning whereas 1,25-dihydroxyvitamin D values showed significant decreases. Histomorphometric measurements from bone biopsies showed significant increases in most parameters of bone formation between pregnancy and weaning. These results are consistent with the hypothesis that at midpregnancy bone formation is decreased and cancellous bone resorption may have increased. During lactation, losses occur in both cortical and cancellous bone, partially depleting the maternal reservoir of calcium, but a subsequent increase in bone formation enables restoration of bone mineral after weaning to values similar to those in the control group.
INTRODUCTION
A low peak bone mass is a risk factor for the development of postmenopausal osteoporosis. The increased calcium demands of pregnancy and lactation could adversely affect the attainment and/or maintenance of peak bone mass, especially if imposed during adolescence, when bone apposition is accelerated. Several studies have examined changes in bone mass during pregnancy and lactation, but most of the subjects have been mature women.1 We have used a nonhuman primate model (Macaca nemestrina) of adolescent human pregnancy because tetracycline-labeled bone biopsies are contraindicated in human pregnancy. The aims of this study were to measure changes in bone mass of the whole skeleton and vertebrae, to document biochemical changes in the calcium–parathyroid axis and markers of osteoblastic activity during midpregnancy and at weaning and to compare these measurements with the static and kinetic parameters of bone remodeling.
MATERIALS AND METHODS
Sample
The female macaques were from the breeding colony of the former Primate Field Station of the University of Washington Regional Primate Research Center, located in Medical Lake, Washington. The study was approved by the Animal Care Committee at the University of Washington. Standard breeding and animal care protocols were maintained throughout the study. Animals were fed Purina High Protein monkey chow ad libitum (Ralston Purina Co., St. Louis, MO, U.S.A.), supplemented with apples. The stated composition of the chow was 1% calcium, 0.6% phosphorus, and 6.6 IU/g of vitamin D. Three samples of the chow were measured every month during the study to monitor mineral content. The calcium was 9.6 ± 1.3 mg/g and phosphate was 7.2 ± 0.7 mg/g.
Of the 149 animals in the base population, 17 were feral animals that had been imported as juveniles from Southeast Asia; the others had been born at the Field Station. Age of the feral animals was estimated from body weight at the time of admission to the colony and could be inaccurate by a year. Young (<6 years) nulliparous females were enrolled in the study if they were healthy and sexually mature, as determined by routine screening laboratory tests and daily monitoring. After one normal estrus cycle they were placed in a harem breeding group with one sexually mature male and seven females. They were then examined monthly by ultrasound which was used to determine date of conception. Pregnant and lactating females were moved into housing with eight females of like reproductive status.
Thirteen females were intentionally housed without males; these were the controls, aged 2.4–3.9 years at the time of enrollment into the study. Eleven females never became pregnant and were dropped from the study. The reproductive cohort contained 125 females aged 3.3–6.0 years at midpregnancy; 97 were enrolled at least 3 months before diagnosis of pregnancy (10 of these aborted before 100 days gestation and were treated as nulliparous; their measurements were entered into the database starting at early second pregnancy), 9 during early pregnancy, 9 during midpregnancy, and 10 at parturition. The average gestational duration was 171 days. Four females had midtrimester abortions and did not have parturition data.
Postpartum, 3 females died, 76 completed 6 months of lactation, 12 lactated less than 6 months, and 30 did not lactate. The reasons for nonlactation included third trimester abortion or stillbirth (n = 10), death of the infant (n = 9), removal of the infant for other research (n = 6), and rejection of the infant (n = 5). The postpartum data on the animals that did not lactate for 6 months is not included in this analysis.
Study design
Bone mass was measured in the control group every 3 months at the time of routine tuberculosis testing. Prepregnant females were also measured at 3-month intervals. When pregnancy was diagnosed, at an average gestational age of 38 days, bone mass was measured, and subsequent measurements were done at 100 days of pregnancy, 1–5 days after delivery, 3 and 6 months postpartum, and 3 months postweaning. Missing or inadequate measurements occurred in eight females at 3 months before pregnancy and four or fewer at the other time points. By the 3-month postweaning measurement, 76% of animals had become pregnant again, with an average gestational age of 41 days.
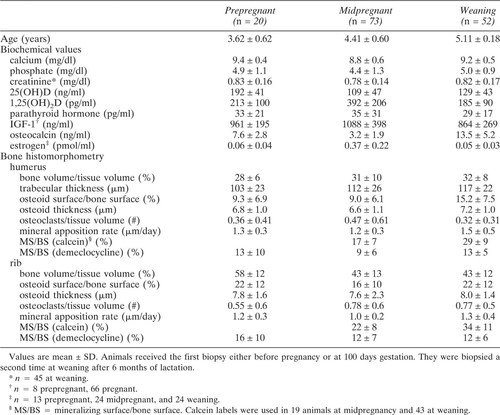
Bone biopsies were performed in 20 females before they became pregnant (data from these measurements are termed “prepregnant”) and in 73 other females at 100 days (range 93–107 days) of pregnancy, which is late in the second trimester. A second biopsy was performed 6 months postpartum, at the time of weaning by separation, in 52 dams that completed full lactation. Twelve of the second biopsies were from dams whose first biopsy had been performed when they were prepregnant and the remaining were from dams whose first biopsy was at midpregnancy. Blood was collected for biochemical tests at the time of the biopsies. The control group did not have bone biopsies or biochemical determinations.
Bone densitometry
The females were anesthetized and scanned in the lateral position with a dual-energy absorptiometer equipped with software for small subjects (Norland Corp., Fort Atkinson, WI, U.S.A.). Bone mineral of the whole body and the spine (2nd, 3rd, and 4th lumbar vertebrae) was measured. Pixel size was 3.0 mm × 3.0 mm for whole body and 1.0 mm × 1.0 mm for the spine; scan speed was 60 mm/s. The entire vertebra was included in the region of interest, including the posterior and transverse processes. The lateral position was used so that the fetus could be excluded from the measurements.

Biochemical measurements
Plasma and serum were obtained from blood by centrifugation and frozen and stored at –70°C for later analysis. 25-hydroxyvitamin D (25(OH)D) was measured by competitive protein binding,2 1,25-dihydroxyvitamin D (1,25(OH)2D) by competitive binding to chick thymus receptor,3 and intact parathyroid hormone (PTH) by a double-site radioimmunoassay,4 or a chemiluminescence intact PTH assay.5 The intra-assay coefficients of variation for the vitamin D assays were <10% and for PTH <6%. Insulin-like growth factor 1 (IGF-1) was measured following acid-ethanol extraction of plasma utilizing a double antibody,6 antibodies were rabbit and the standard human prepared from recombinant DNA. The intra-assay coefficient of variation (CV) was <11%. Reagents from the above assays were obtained from Nichols (San Juan Capistrano, CA, U.S.A.). Osteocalcin was measured on plasma using a two-site immunoradiometric assay with rabbit antibodies and human standards.7 The intra-assay CV was 6%. Reagents were obtained from Immunotopics, Inc. (San Clemente, CA, U.S.A.). Serum calcium, phosphate, and creatinine were determined by autoanalyzer (PARAMAX; American-Dade Chemistry Systems, Costa Mesa, CA, U.S.A.). The intra-assay CV for these tests was <4%.
Estrogen assays were done on a subset of 13 nonpregnant, 24 pregnant, and 24 weaning animals, using a radioimmunoassay double-antibody125I kit from ICN Biomedical Include (Carson, CA, U.S.A.).8 The intra-assay variability was 7.1%.
Bone histomorphometry
A female was given two fluorescent labels, one 10–14 days, and the other 3–5 days before each biopsy. Three different kinds of labels were used: demeclocycline (D), 25–30 mg/kg administered by gastric tube; calcein (C), 12–20 mg/kg intravenously; and tetracycline (T), 20 mg/kg orally or by gastric tube. The calcein was used during the later part of the study because the tetracycline labels were often faint and difficult to measure. The labeling schedule was: nonpregnant, nulliparous females: D-T; midpregnancy: D-T (n = 17), D-D (n = 35); D-C (n = 18), C-D (n = 1), none (n = 1); weaning: D-T (n = 1), D-D (n = 6), C-D (n = 43). All except three females had a different labeling sequence on the second biopsy than on the first.
Bone biopsies were performed under general anesthesia with ketamine. A 1.5-cm piece from the anteriolateral aspect of the 6th rib was removed. Approximately 1.5 cm of cancellous bone was removed from the proximal humerus, along the longitudinal axis, with a 2-mm trephine. The second biopsy was done on the opposite side of the body than used for the first. These anatomic locations were chosen to provide adequate samples of both cancellous and cortical bone; pilot studies had shown that the iliac crest contained very little cancellous bone in these adolescent animals.
Bone was embedded in methyl methacrylate and sectioned with a Jung K microtome to 8 μm. The rib was sectioned transversely at the center of the specimen. Two sets of sections of the humerus were made 100 μm apart and stained with Goldner's stain, alkaline phosphatase stain, or left unstained for fluorescent microscopy.
The Osteometric program (Osteometric, Atlanta, GA, U.S.A.) was used to measure the biopsies. The entire rib section, which is predominantly cortical bone, was measured except the periosteal surface. Only cancellous bone of the humerus was measured. The growth plate and adjacent 0.5 mm were excluded.
Direct measurements included tissue area, bone area, bone perimeter, osteoid perimeter, osteoid width, number of osteoclasts, length of fluorescent labels, and distance between labels. The mineralizing surface was the length of the calcein-labeled surface in the biopsies labeled with calcein and the first demeclocycline-labeled surface in the remaining biopsies. In 24 biopsies (evenly distributed between reproductive stages) the second label was too faint to measure, so the mineral apposition rate (MAR) was calculated by dividing the distance between the first label and mineralization front by the number of days between the first label and the biopsy. The MARs calculated with the single label were significantly shorter; therefore, the rates were adjusted by the mean difference for each reproductive stage. This did not change the outcome of any of the analyses.
Eroded surfaces were not measured because they did not represent resorptive surfaces, which were frequently smooth and shallow. The calculated parameters used the standardized nomenclature and derivations.9 A plate model was used to calculate trabecular thickness.
Statistical analysis
Descriptive statistics show mean and SD. Linear regression analysis, one-way analysis of variance, paired and unpaired t-tests, and nonparametric tests were done using the StatView 4.1 program (Abacus, Berkeley, CA, U.S.A.).
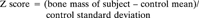
Linear regression was not used to calculate Z scores because the increase in bone mass with age was not linear (Fig. 1).
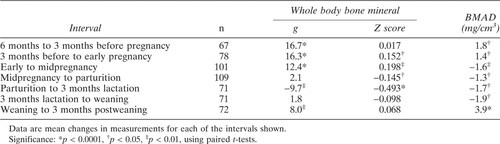
Changes in bone mass measurements in control cohort. Upper graph is whole body bone mineral, lower graph is vertebral BMAD. Each line plots data from an individual animal.
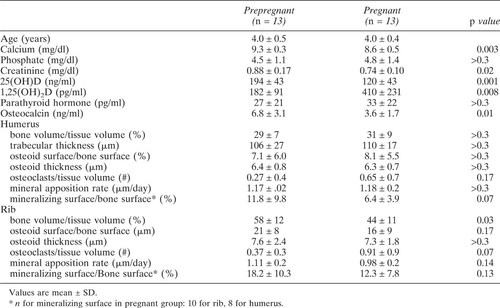
Several of the biochemical and histomorphometric measurements taken at the prepregnant time point correlated with age, which confounded comparison between measurements at prepregnancy and pregnancy. Therefore, to compare data between prepregnant and midpregnant females, each prepregnant animal was age matched (within 0.2 years) to one at midpregnancy. Thirteen were of comparable ages. The other seven were younger than 3.3 years, which was the youngest age at midpregnancy. Differences in mean values between the prepregnant and age-matched pregnant animals were tested by paired t-tests. A p value of < 0.05 was accepted as significant.
RESULTS
Bone densitometry
The bone mass measurements of the control animals are shown in Fig. 1. Between 2.5 and 6 years of age, the whole body bone mineral increased by 93% but the vertebral BMAD showed no consistent change related to age.
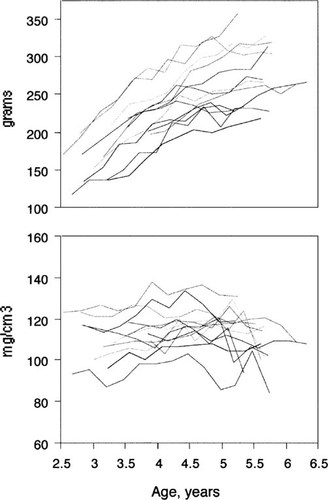
The longitudinal changes in bone densitometry measurements for each interval between reproductive stages are given in Table 1. The mean values for whole body bone mineral measurements throughout reproduction are shown in Fig. 2. Bone mass increased from 6 months prior to diagnosed pregnancy until parturition. The change between midpregnancy and parturition was not significant. Between parturition and 3 months lactation, the animals lost 3.3 ± 8.5% of their bone mineral, which was regained by 3 months postweaning.
Whole body bone mineral measurements. The upper graph shows values in grams, the lower graph shows Z scores (SD from the mean value of controls of the same age). Points show mean value ± SE for each reproductive stage: 6 months and 3 months before pregnancy (–6 month, –3 month); early pregnancy (Early); midpregnancy (Mid); parturition (Part); 3 months of lactation (Lact); weaning (Wean); and 3 months postweaning (Post). Number of animals that had adequate bone density measurements is shown in parenthesis.
The whole body bone mineral Z scores are reported in Table 1 and Fig. 2. Z scores did not change during the interval from 6 months before pregnancy until 3 months before pregnancy; this indicates that the whole body bone mass was increasing at the same rate as seen in controls. The Z scores of females at midpregnancy (when the mean age was 4.4 years, range 3.3–6.0 years) were compared with the Z scores of the control females when they were 4.3–4.6 years old. Z scores were higher in the pregnant group (0.59 ± 1.1 vs. –0.04 ± 0.97, p = 0.04). Similar analysis at the other reproductive stages showed no difference between reproductive and control females. The Z scores in the animals at postweaning were not different from their Z scores 6 months before pregnancy.
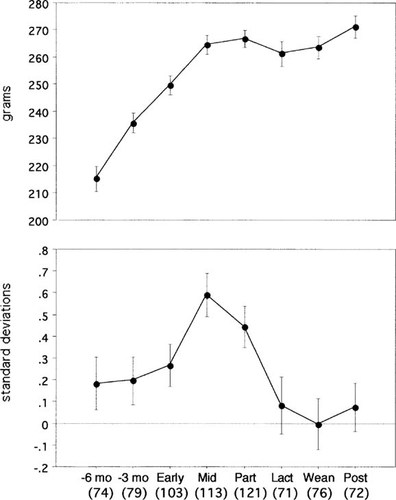
The vertebral BMAD results are shown in Table 1 and Fig. 3. The BMAD at postweaning was not different than at 6 months before pregnancy.
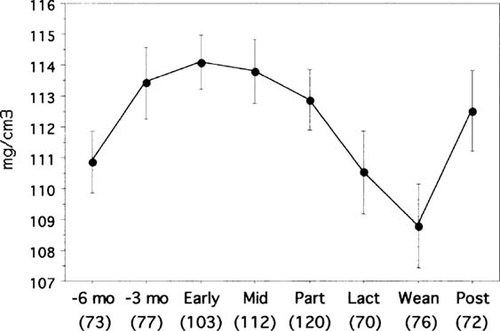
BMAD measurements. Points show mean value ± SE for each reproductive stage: 6 months and 3 months before diagnosis of pregnancy (–6 month, –3 month); early pregnancy (Early); midpregnancy (Mid); parturition (Part); 3 months of lactation (Lact); weaning (Wean); and 3 months postweaning (Post). The number of animals that had adequate bone density examinations is given in parenthesis.
Areal BMD measurements increased during each interval from 6 months before pregnancy until midpregnancy. They also increased between weaning and postweaning. During the other intervals, the changes were not significant.
Biochemistry
Descriptive data are shown in Table 2 for the reproductive cohort. Table 3 shows comparison between age-matched prepregnant females and pregnant females at midpregnancy (100 days gestation).
Table 4 showed paired t-test results from the females which had biochemical measurements at both midpregnancy and weaning. Paired comparisons could not be done with estrogen levels because the samples did not overlap. The estrogen levels were high during pregnancy; levels were similar between weaning and prepregnant females. There was a significant correlation between estrogen and 1,25(OH)2D in the pregnant females (r = 0.47, p = 0.02).
There were significant correlations between osteocalcin and the cancellous osteoid surface in prepregnant females (r = 0.46, p = 0.04) as well as in pregnant females (r = 0.46, p < 0.001) and females at weaning (r = 0.29, p = 0.03).
Histomorphometry
Basic descriptive measurements are shown in Table 2. The calcein-labeled surfaces were approximately twice as long as the demeclocycline-labeled surfaces. Therefore, direct comparison of mineralizing surfaces or bone formation rates (BFRs) could be done only in animals that had received a calcein label at both biopsies or no calcein label at either biopsy. The calcein-labeled surface was also much greater than the osteoid surface; this suggests that either there was osteoid that was still mineralizing but too thin to detect or that some of the calcein-labeling was not specific.
In the biopsies from the prepregnant females younger than 3.3 years, the MAR at the rib was significantly higher than in the older prepregnant females (1.46 ± 0.7 μm/day and 1.10 ± 0.5 μm/day, respectively). Cancellous osteoid surface was also higher in the younger than in the older females (13.4 ± 7% vs. 7.2 ± 6%). The only measurement that differed significantly between age-matched prepregnant and pregnant females was the rib bone volume, which was lower in the pregnant females (Table 3).
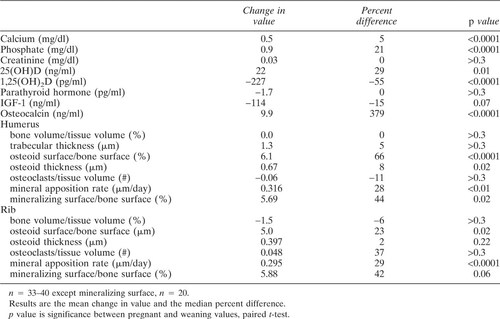
Differences in measurements between midpregnancy and weaning are shown in Table 4 and Fig. 4. There were significant increases in most parameters of bone formation between pregnancy and weaning.
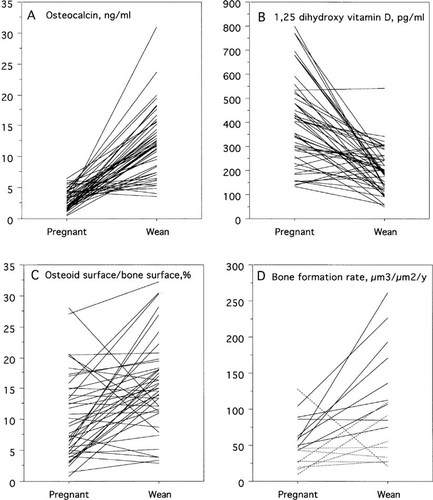
Measurements at midpregnancy and after 6 months of lactation (at weaning). Each line represents data from a different animal. (A) Plasma osteocalcin. (B) 1,25(OH)2D. (C) Osteoid surface/bone surface from bone biopsies of the humerus. (D) BFR per bone surface from bone biopsies of the rib. Dotted lines were from biopsies labeled with demeclocycline and solid lines labeled with calcein.
DISCUSSION
In these young females that had not yet achieved full growth and skeletal consolidation, the complex processes of bone growth, modeling, and remodeling underwent marked changes during reproduction. The fetal and infant calcium requirements require adjustments to the maternal calcium economy, which may be regulated by several hormones and by alterations in intestinal absorption, renal excretion, and bone exchange. It appears that several of these mechanisms are operative during reproduction. Our results, combined with those from the literature, suggest that during pregnancy the vitamin D axis is active and fetal calcium needs are primarily met by enhanced intestinal absorption. The slight vertebral bone loss seen during pregnancy could be due to increased bone resorption and/or decreased bone formation. During lactation the bone calcium reservoir is partially depleted in both cortical and cancellous bone; there is increased bone resorption followed by increased bone formation. After weaning, bone resorption decreases, while bone formation continues at a high rate, so that bone mineral is restored. This discussion will focus on bone physiology in this age group and during the reproductive phases of prepregnancy, early pregnancy, midpregnancy, lactation, and postweaning.
The macaques in this study were aged 2.5–6 years, which corresponds to ages 10–24 in humans. The interpretation of results in this age group is more difficult than in adults due to the confounding factor of growth, which can affect the measurement of bone density when using dual-energy X-ray absorptiometry. Our previous studies in this species had shown that the vertebral areal bone density measurement increased with size even when there was no change in the three-dimensional density (Archemedean density). The prediction of three-dimensional density could be improved by using the BMAD calculation, and it was improved further by a depth measurement from spinal radiographs.10 In this study, the third dimension (which is the width of the vertebra because bone densitometry was done in the lateral position) could not be measured because the fetus would have obscured the vertebrae in an anterior view; therefore, the BMAD was calculated to estimate the bone density. In the control females the BMAD did not increase with age. Therefore, in this age range, the skeleton was growing but the Archimedean bone density was not increasing. The importance of calculating the Archimedean density is demonstrated by the fact that the BMAD decreased during late pregnancy and lactation, whereas the areal BMD did not change.
Early pregnancy
During early to midpregnancy, the whole body bone mineral continued to increase while vertebral BMAD deceased. The ration of bone volume/tissue volume in the ribs also decreased. The contrasting patterns suggested that the increase in whole body bone mineral was predominantly a result of skeletal growth and not of increased density. By midpregnancy the whole body bone mineral was 0.6 SD higher than expected for age.
Midpregnancy to late pregnancy
The fetus is rapidly accumulating bone mineral at this stage.11, 12 If the fetal demands for calcium were met by resorption of bone calcium, then the whole body bone mineral would have decreased. We found, however, that the whole body bone mineral did not change significantly between midpregnancy and parturition. Thus, our data are compatible with the hypothesis that the calcium demands of the fetus are primarily met by increased intestinal calcium absorption (stimulated by vitamin D). Although bone resorption was insufficient to account for the fetal requirements, the cancellous bone resorption could have been increased, as evidenced by the decrease in vertebral BMAD and increase in osteoclast count. Studies in humans of calcium balance13 and urine collagen cross-linking molecules14, 15 suggest that bone resorption increases during pregnancy, but not until the last trimester. The bone density in mature human females does not change during pregnancy.16
Osteocalcin generally is a biochemical marker for bone formation in remodeling bone and at the growth plates. In this study, the osteocalcin levels were significantly lower in females at midpregnancy than in age-matched prepregnant females. Published studies in fully grown human adults have also found diminished osteocalcin levels during pregnancy.17, 18 Interpretation of osteocalcin results is complicated because there may be enhanced placental clearance of osteocalcin.17 The significant correlation between osteocalcin and cancellous osteoid surfaces that was seen in our study suggests that osteocalcin probably could be used as a marker of bone formation during normal pregnancy.
The histomorphometric results at midpregnancy did not show consistent decreases in the BFRs or osteoid surfaces when compared with age-matched prepregnant females, but the sample size was small. The mineralizing surface (which is the major determinant of BFR) was nearly twice as high in prepregnant females as in pregnant ones, but this difference was not statistically significant. At some time between midpregnancy and weaning the BFRs increased, but our data do not allow us to determine whether this occurred during late pregnancy or lactation. A recent report of biopsies in cynomolgus macaques showed no difference in BFRs between controls and females in the third trimester but an increase after 3 months of lactation.19
We found no other reports of bone biopsies during the second trimester of pregnancy in humans, because tetracycline is contraindicated during pregnancy. There has been one study of women who were planning an abortion and were biopsied at 8–10 weeks of pregnancy. They had lower osteoid and lower (but not significantly) mineralization rates compared with nonpregnant controls.20 These results are consistent with the trends observed in this study.
Levels of 25(OH)D were lower in females at midpregnancy than in age-matched nonpregnant females. The explanation for this is not clear; some of the decrease may have been caused by a demand on vitamin D stores.21 Most studies report normal 25(OH)D levels in pregnant women,22 but these levels may be low in populations with marginal baseline vitamin D status.23 There is a direct relationship between dietary vitamin D intake and plasma 25(OH)D during pregnancy,24, 25 and in humans vitamin D shows seasonal fluctuations related to sunlight exposure. The latter was not a factor in our study because the animals were maintained indoors with a constant cycle of artificial lighting, and throughout the study they were given the same Chow diet.
Levels of 1,25(OH)2D were markedly higher in pregnant than in age-matched nonpregnant females. Interpretation of the 1,25(OH)2D levels is complicated because D-binding proteins also increase during pregnancy.26 Several other studies have also shown an increase in 1,25(OH)2D,27, 28 free 1,25(OH)2D,26 as well as enhanced intestinal absorption of calcium and increased urine calcium28, 29 during pregnancy. In our animals, the 1,25(OH)2D correlated with estrogen, which has been shown to stimulate the 1α-hydroxylase.30 When bone from normal nonpregnant animals and humans is exposed to 1,25(OH)2D in vitro, osteocalcin production increases, and preosteoclasts are stimulated to differentiate, enhancing bone resorption.31 These effects were not all seen in our study. Despite increased 1,25(OH)2D levels, the osteocalcin levels were decreased at midpregnancy. There may have been an effect, however, on the preosteoclasts. The number of osteoclasts was higher in pregnant than in nonpregnant animals, but with marginal significance.
Lactation
During the first 3 months of lactation, the whole body bone mineral decreased by 3.3% and remained stable for the last 3 months of lactation. The vertebral BMAD decreased over the 6 months of lactation with a total loss of 3.3%. These values are similar to those reported in mature humans, whose bone density decreases by 4–7% in 6 months.22, 32-34 Longitudinal studies of bone resorption markers have shown high levels during early lactation.15, 35 These changes are associated with decreased estrogen, increased prolactin, and increased PTH-related protein1, 32 but not with PTH35 or intestinal absorption of calcium.36 Dietary calcium supplementation does not prevent the bone resorption associated with lactation.15, 37, 38
In our study, bone biopsies were taken after 6 months of lactation. The measurements reflecting bone formation (osteoid surface, mineralizing surface, MAR) were markedly higher than at midpregnancy, with no change in the number of osteoclasts. The osteocalcin levels also showed dramatic increases, consistent with enhanced bone formation and similar to results from several other studies.14, 15, 22, 32, 35 In normal bone, high resorption rates lead to high formation rates. Our results suggest that the probable increased bone resorption of early lactation had stimulated the bone formation. By the time the biopsies were taken at weaning, the resorption was no longer obvious. This is consistent with reports of decreasing N-telopeptide during later months of lactation.35
The IGF levels in this study showed a trend toward lower levels after 6 months of lactation compared with midpregnancy. Others have reported high39, 40 or normal41 IGF levels during pregnancy. The change in IGF must be interpreted with caution because of possible expansion of plasma volume during pregnancy and changes in IGF binding proteins, but it may reflect the increased caloric and protein intake seen during pregnancy.42 We have monitored food consumption during pregnancy in these animals and it increased during late pregnancy (data not shown).
The 1,25(OH)2D levels were significantly lower after lactation than at midpregnancy, in the opposite direction from the marked increase in osteocalcin. The decrease in 1,25(OH)2D may have partially caused the putative decrease in bone resorption prior to the biopsy taken at weaning.
Postweaning
In our study, the postweaning females showed an increase in vertebral BMAD as well as whole body bone mineral. After the reproductive cycle, at 3 months postweaning, the whole body bone mineral Z score was not significantly different from the Z score 6 months before pregnancy. From an evolutionary perspective, one would hypothesize that a species that produces sequential offspring would be under selective pressure to evolve mechanisms that would maintain the maternal skeleton, while providing the fetus and infant a reliable source of calcium. Several large retrospective studies have found no associations between bone mass in elderly women and a history of lactation or parity.43, 44 Thus, normal reproduction does not appear to have a lasting negative impact on maternal bone mass.
Results from our study raise additional questions for future research. The increased skeletal growth observed during early pregnancy would not be expected in a fully mature animal, and thus the skeletal effects of pregnancy and lactation may be different for older females. The females were all maintained on an adequate calcium intake; the effects of poor calcium intake, especially during the postweaning recovery period, are not well understood. Finally, we were not able to address the question of delayed effects of pregnancy itself on bone mass because in the breeding colony it was not possible to randomly remove infants from mothers to assess the difference between lactating and nonlactating postpartum females.
Acknowledgements
We acknowledge the assistance of Craig Aumann, Barbara Carter, Diana Greenlee, and Janet Long. Sharon West and Mark Murchison made the measurements on the animals and participated in their care. Elmer Feist and Mary Ann Berrie assisted in bone histomorphometric measurements. William Bremner, M.D., and Elizabeth Van Gaver performed estradiol measurements. The work was supported by PHS Grants RO1 AR40813–01 and RR00166, and also from the Clinical Nutrition Research Unit, NIH P30 DK35816.