Intermittent and Continuous Administration of the Bisphosphonate Ibandronate in Ovariohysterectomized Beagle Dogs: Effects on Bone Morphometry and Mineral Properties
Abstract
Bisphosphonates have emerged as a valuable treatment for postmenopausal osteoporosis. Bisphosphonate treatment is usually accompanied by a 3–6% gain in bone mineral density (BMD) during the first year of treatment and by a decrease in bone turnover. Despite low bone turnover, BMD continues to increase slowly beyond the first year of treatment. There is evidence that bisphosphonates not only increase bone volume but also enhance secondary mineralization. The present study was conducted to address this issue and to compare the effects of continuous and intermittent bisphosphonate therapy on static and dynamic parameters of bone structure, formation, and resorption and on mineral properties of bone. Sixty dogs were ovariohysterectomized (OHX) and 10 animals were sham-operated (Sham). Four months after surgery, OHX dogs were divided in six groups (n = 10 each). They received for 1 year ibandronate daily (5 out of 7 days) at a dose of 0, 0.8, 1.2, 4.1, and 14 μg/kg/day or intermittently (65 μg/kg/day, 2 weeks on, 11 weeks off). Sham dogs received vehicle daily. At month 4, there was a significant decrease in bone volume in OHX animals (p < 0.05). Doses of ibandronate ≥ 4.1 μg/kg/day stopped or completely reversed bone loss. Bone turnover (activation frequency) was significantly depressed in OHX dogs given ibandronate at the dose of 14 μg/kg/day. This was accompanied by significantly higher crystal size, a higher mineral-to-matrix ratio, and a more uniformly mineralized bone matrix than in control dogs. This finding lends support to the hypothesis that an increase in secondary mineralization plays a role in gain in BMD associated with bisphosphonate treatment. Moreover, intermittent and continuous therapies had a similar effect on bone volume. However, intermittent therapy was more sparing on bone turnover and bone mineral properties. Intermittent therapy could therefore represent an attractive alternative approach to continuous therapy.
INTRODUCTION
Bisphosphonates have emerged as valuable therapeutic agents for treating metabolic bone diseases with predominant increase in bone resorption, in particular postmenopausal osteoporosis.1, 2 Several animal and clinical studies have demonstrated that second and third generations of bisphosphonates such as clodronate,3-6 pamidronate,7-11 alendronate,12-19 risedronate,20 and tiludronate21-23 are able to correct, totally or partially, bone loss after cessation of ovarian function. Bone mineral density (BMD) increases rapidly during the first year of treatment and 3–6% gain can be observed.14, 15 However, this bone gain is accompanied by a depression in bone turnover assessed by noninvasive methods24-27 or directly by bone histology.28 Despite a state of low bone turnover, a smaller but progressive increase in bone mass occurs beyond the first year of bisphosphonate therapy.5, 7, 15, 18, 29, 30 The reasons for this continuous increase in BMD are not entirely clear. There is some evidence that an increase in secondary mineralization of bone packets occurs because of the extended time lapse before they can undergo resorption.31-33 Moreover, there are uncertainties and concerns about the long-term effects of bisphosphonate on the skeleton, and alternative approaches such as intermittent therapies have been suggested.8, 34-36
The present study was undertaken to determine if the long-term treatment with bisphosphonate induces modifications in hydroxyapatite crystal size and to compare the effects of continuous versus intermittent regimens on bone volume and bone turnover in the ovariohysterectomized beagle dog model of established bone loss. For this purpose, we used a newer third-generation bisphosphonate, ibandronate, 1-hydroxy-3-(methylpentylamino) propylidiende bisphosphonate monosodium salt monohydrate (BM 21.0955).37 This bisphosphonate has been shown to be a more potent inhibitor of bone resorption than the previously studied bisphosphonates. It prevents retinoid-induced hypercalcemia in thyroparathyroidectomized rats at lesser doses than risedronate (2×), alendronate (10×), pamidronate (50×), and clodronate (500×).37 Also, we have previously demonstrated that ibandronate at the low dose of 1 μg/kg was able to prevent completely the bone loss occurring after cessation of ovarian function in beagle dogs without significantly decreasing bone turnover.38
MATERIALS AND METHODS
Animals and diet
Seventy full-grown, colony-raised 5- to 10-year-old female beagle dogs were purchased from a USDA-licensed dealer (Hazleton Research Products, Inc., Kalamazoo, MI, U.S.A.). In accordance with the guidelines of the National Research Council, they were housed in runs and fed a nutritionally complete and balanced diet (Agway Prolab Canine 1600; Agway, Inc., Syracuse, NY, U.S.A.) containing 1.67% calcium, 1.26% phosphate, 21% crude protein, 8% crude fat, 5.5% crude fiber, and 11% moisture. The animals were individually fed 200 g of food/day, an amount appropriate for a normal active dog. Access to water was ad libitum. The dogs were housed under identical conditions, and they were allowed ample exercise and regular exposure to sunlight. Physical well being and activity were monitored at least twice daily, and body weight was recorded monthly.
Design and protocol
This study was designed as a longitudinal controlled experiment. After a 2-week quarantine and an additional adaptation period of 2 weeks, the animals underwent baseline blood drawings and were scheduled to undergo surgical iliac crest bone biopsies immediately followed either by ovariohysterectomy (OHX, n = 60) or sham-operation (Sham, n = 10).
The dogs were then followed for 4 months to allow bone loss to develop in the OHX animals. At month 4, blood drawings and bone biopsies were repeated. Immediately after the second bone biopsies, the 60 OHX dogs were randomly divided into six groups of 10 animals each. Five groups of dogs received ibandronate in a continuous manner at the following doses: 0 (vehicle), 0.8, 1.2, 4.1, and 14 μg/kg of body weight (bw)/day of free acid equivalent. In these dogs, ibandronate or vehicle was subcutaneously administered 5 out of 7 days per week. The sixth OHX group received ibandronate intermittently at a dose of 65 μg/kg of bw/day for 2 weeks (14 consecutive days) followed by 11 weeks without therapy. These cycles were repeated four times. The cumulative dose of ibandronate was the same in dogs treated either intermittently or continuously with the highest dose (total 3640 μg/kg). The 10 Sham dogs received vehicle continuously.
Ibandronate was dissolved in sterile physiologic saline (0.9% NaCl), and the pH was adjusted to 7.4. Vehicle solution consisted of physiologic sterile saline (0.9% NaCl). After 16 months of experimental observation, all dogs underwent a third iliac crest bone biopsy and were sacrificed. Autopsies were performed.
Double labeling of bone was done in all animals before each bone biopsy. To allow differentiation of the labels given before each biopsy, two different bone markers were used: tetracycline hydrochloride at a dose of 20 mg/kg of bw/day and calcein at a dose of 15 mg/kg of bw/day. The respective bone markers were given for 2 days, followed by a labeling-free interval of 6 days before the second 2-day administration of marker. Biopsies were done 3 days after the second label was given.
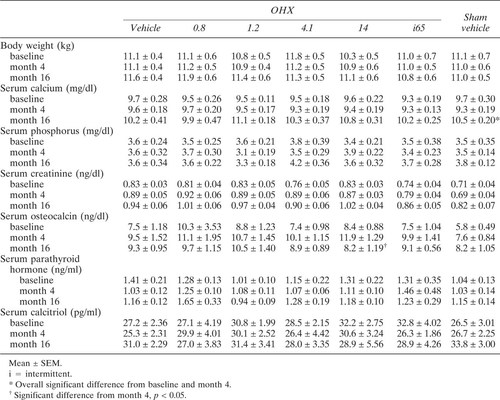
Blood drawings were performed for determinations of serum calcium, phosphorus, creatinine, parathyroid hormone (PTH), calcitriol, and osteocalcin levels.
Surgical methods
All surgical procedures were performed under general anesthesia with Isofluorane (1–1.5%). OHX was done using the standard technique of sterilization of female dogs.39 Sham operation was performed by entering the peritoneum through the same median vertical infraumbilical incision, pulling out the ovaries, and examining them for normality. Ovaries were then put back in situ, and closure of the abdominal wall was performed following standard techniques.
Open iliac crest bone biopsies were taken from the left and right iliac bone in an alternating manner through a lateral flank incision with a low-speed Stryker bone saw equipped with stainless steel blades (Orthopedic Frame Co., Kalamazoo, MI, U.S.A.). The first sample was taken from the anterior two-thirds of the iliac bone. The second sample was obtained in the same manner and included the entire iliac crest. The third bone sample was surgically obtained prior to euthanasia and consisted of the remainder of the iliac bone from the side of the first biopsy. The bone specimens measured ∼0.5 × 3 × 4 cm and included the superior and inferior area of the iliac crest. The dogs tolerated the surgical procedures well and resumed normal activity within 24 h after surgery.
At the end of the experiment, autopsies were done in all dogs. Macroscopic examination of all organs was performed. Absence or presence of ovaries was verified. All experimental procedures were in compliance with the guiding principles in the care and use of research animals.40
Biochemical measurements
All determinations of serum indices were done at the end of the study. Each assay included samples from dogs from various groups and the various time points.
Serum calcium, phosphorus, and creatinine concentrations were determined by the Kodak Ektachem DT 60 Analyzer (Eastman Kodak, Rochester, NY, U.S.A.). Serum osteocalcin concentrations were measured in duplicate employing our polyclonal antiserum-based radioimmunoassay for dog osteocalcin.41 The detection limit of the assay is 0.08 ng/ml; the intra-assay variation in the present study was <3.5%, and the interassay variation was 3.8%. Serum PTH levels were determined in duplicate with the Incstar C-terminal radioimmunoassay (Incstar Corp., Stillwater, MN, U.S.A.).42 The results are expressed in nanograms per milliliter. The intra-assay variation was <7.6%, and the interassay variation was 9.8%. Measurements of serum calcitriol levels were done with the 1,25-dihydroxyvitamin D D assay kit (Nichols Institute, San Juan Capistrano, CA, U.S.A.).43 The intra-assay variation was <6.4%, and the interassay variation was 4.8%.
Mineralized bone histology and histomorphometry
Bone samples were fixed in absolute ethanol, dehydrated, and embedded in methylmethacrylate as previously described.44 Serial sections 3 μm and 7 μm thick were cut with a Microm, model HM360 microtome (C. Zeiss, Thornwood, NY, U.S.A.). Sections 3 μm thick were stained with the modified Goldner Trichrome stain.45 Unstained sections 7 μm thick were prepared for phase contrast and fluorescent light microscopy. Two bone samples were routinely cut from each iliac bone specimen and then processed and embedded, allowing sagittal and medial sectioning. Histomorphometry of bone was performed in cancellous bone at a standardized site below subcortical and cortical bone.
Static and dynamic parameters of bone structure, formation, and resorption were measured with the Osteoplan system II (Kontron, Munich, Germany).46, 47 For measurement of parameters of bone structure, a minimum area of 7 mm2 of bone was evaluated at a magnification of ×31.25. For static and dynamic parameters of bone formation and resorption, a minimum of 50 optical fields was evaluated at a magnification of ×200. In addition, erosion depth was measured by reconstruction of resorption cavities as described by Cohen-Solal et al.48 The histologic sections were analyzed after completion of the study without knowledge of animal number or group. Comparison of histomorphometric parameters from the anterior and half of the posterior part with the posterior part of the iliac bone sample taken during the second biopsy was done and did not reveal any difference. Calculation and presentation of all histomorphometric parameters follow the guidelines of the histomorphometry nomenclature committee of the American Society of Bone and Mineral Research.49
Mineral properties
Mineral properties of the iliac bone specimens were determined by Fourier transform infrared macrospectroscopy as previously described.50 Briefly, bone samples were fixed in ethanol, dehydrated through serial acetones, and embedded in methyl methacrylate. Five-micrometer-thick sections were placed on BaF2 Fourier transform infrared macrospectroscopy windows and covered with a second window. Spectra were recorded in the transmission mode with a UMA 500 microscope with a motorized xy stage, coupled with an FTS-40 spectrometer (Bio-Rad Inc., Cambridge, MA, U.S.A.). Spectral resolution was 4 cm−1, and the sampling area of each spectroscopic analysis was 50 × 50 μm2. Sequential spectra were collected on the periosteal and endosteal sites in cortical bone and at the edge and the center of trabecules in cancellous bone (spectra set I). Spectra at identical positions were also collected after the sections were decalcified with 5% solution of EDTA (spectra set II). Mineral-to-matrix ratios, as indices of the degree of mineralization, were calculated by integrating the area under the peaks at 900–1200 cm−1 (phosphate) and ∼1585–1725 cm−1 (Amide I) in spectra set I. To eliminate the collagen contribution in the 900–1200 cm−1, spectra set II was subtracted from spectra set I and second-derivative spectra were calculated. After curvefitting routines (GRAMS/386; Galactic Industries Corp., Salem, NH, U.S.A.), the ratio of the areas under the peaks 1020 and 1030 was calculated. This ratio is a sensitive indicator of mineral crystallinity/maturity. Crystallinity/maturity is a parameter dependent on crystallite size, hydroxyapatite stoichiometry, and abundance of substituting ions such as CO . The more “crystalline/mature,” the bigger the crystallite size, the more hydroxyapatite-like stochiometry, the less ion substitution.50-52
. The more “crystalline/mature,” the bigger the crystallite size, the more hydroxyapatite-like stochiometry, the less ion substitution.50-52
Statistical analysis
Results are expressed as mean ± SEM. All statistical tests were two-sided. Normality of distribution was assessed by the Lilliefors test, and homogeneity of variance was tested with the Levene test. Adequate transformations of the data were done when results did not meet the characteristics assumed for analysis of variance (ANOVA).53 Comparability of baseline results between groups was done using one-way ANOVA with the Scheffe a posteriori test. Serial changes were analyzed by multiple ANOVA for repeated measurements. All computations were done using the SPSS software package for Windows release 6.0 (SPSS, Chicago, IL, U.S.A.).
RESULTS
Physical well being and body weight of animals
All animals underwent baseline bone biopsy, OHX, and Sham without any complications. Daily injections of ibandronate or vehicle were well tolerated at the site of injection and systemically. During the course of the experiment, two dogs died, one vehicle and one ibandronate treated (14 μg/kg/day). Two other OHX dogs developed mild renal insufficiency, one receiving vehicle and the other ibandronate (0.8 μg/kg of bw). These four dogs were not included in the analysis. In all other dogs, autopsies at the end of the experiment did not reveal any organ abnormality, and there was no remnant ovarian tissue in the OHX dogs. Body weights were not different at baseline among the groups and did not change significantly thereafter (Table 1).
Serum biochemical and hormonal parameters
Serum creatinine, phosphorus, PTH, and calcitriol were not different among the groups at baseline and did not change thereafter (Table 1). Serum calcium showed an overall significant increase during the experiment (Table 1). However, the serum concentrations remained in the normal range and no differences among the various groups were observed (Table 1). Serum osteocalcin levels increased significantly 4 months after OHX (8.3 ± 0.67 vs. 10.5 ± 0.58 ng/ml, p < 0.01). After treatment with ibandronate at the high dose of 14 μg/kg/day, there was a significant decrease in serum osteocalcin levels in OHX dogs to levels not different from baseline values or levels obtained in other groups at the end of the study (Table 1).
Bone histomorphometry
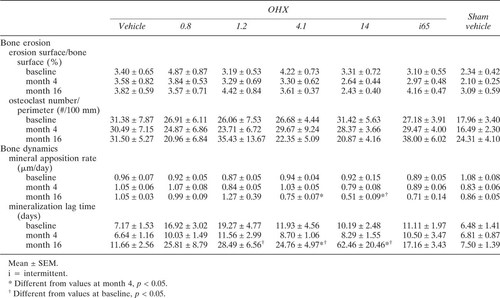
Bone samples from two OHX dogs given 1.2 μg/kg/day and 14 μg/kg/day of ibandronate and from one OHX treated intermittently were inadequate for histomorphometry (cancellous bone area <7 mm2) and were not included in the analysis. Baseline histomorphometric parameters of bone in the remaining 63 dogs were not different among the groups (Table 2).
Four months after surgeries, there was a significant decrease in cancellous bone volume/tissue volume in the OHX dogs with an increase in trabecular separation and no changes in trabecular thickness (Table 2). Thereafter, ibandronate administration induced a dose-dependent increase in bone volume (Fig. 1A). At the highest doses, ibandronate given either continuously (14 μg/kg/day) or intermittently (65 μg/kg/day) completely reversed this bone loss (Fig. 1). At the end of the experiment, bone volume/tissue volume in dogs treated with these high-dose regimens was similar to their baseline values (Fig. 1B). This bone gain was accompanied by an increase in trabecular thickness (Table 2), which, however, reached significance only when ibandronate was administered continuously (14 μg/kg/day).Ibandronate at the dose of 4.1 μg/kg/day did not reverse the bone loss. However, it stabilized bone volumes to values similar to those observed 4 months after OHX and prevented the further decrease in bone volume seen in OHX animals given vehicle or the smallest doses of ibandronate, i.e., 0.8 μg/kg/day and 1.2 μg/kg/day (Fig. 1). There were no changes in parameters of bone structure in Sham animals given vehicle (Fig. 1, Table 2).
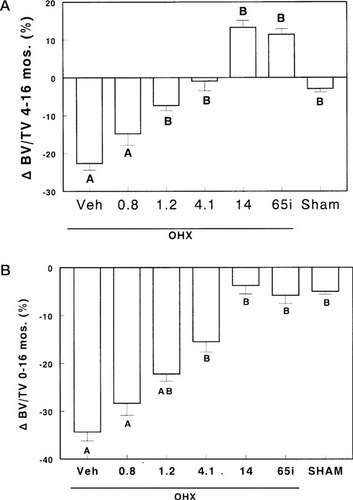
Percent changes in bone volume/tissue volume (ΔBV/TV) from months 4–16 (A) and from months 0–16 (B) in ovariectomized (OHX) or sham-operated (Sham) dogs receiving vehicle (Veh) or ibandronate continuously or intermittently (i). Data with the same letter are not significantly different, one-way ANOVA.
Four months after surgeries, there was a significant increase in bone turnover in OHX dogs, and activation frequency rose from 0.81 ± 0.09–1.15 ± 0.10 years−1 (p < 0.01). Ibandronate therapy at any dose induced a significant decrease in parameters of bone formation (Table 2) and activation frequency, which, however, reached levels significantly below those of Sham animals only for the dose of 14 μg/kg/day (Fig. 2). Moreover, in these animals, there was a reduction in both osteoid thickness and mineral apposition rate (Tables 2 and 3). However, the decrease in mineralization rate and activation frequency was relatively more pronounced than the decrease in the extent of osteoid surface and osteoid thickness resulting in an increased mineralization lag time (Table 3). This was also observed to a lesser degree in OHX animals treated with 4.1 μg/day of ibandronate (Tables 2 and 3). There were no changes in erosion surface/bone surface or number of osteoclasts/bone perimeter. However, at the end of the study, erosion depth were slightly, but significantly, lower in OHX dogs receiving ibandronate in a continuous manner than in OHX and Sham animals receiving vehicle (Fig. 3). OHX dogs treated intermittently with ibandronate did not exhibit a reduction in erosion depth (Fig. 3).
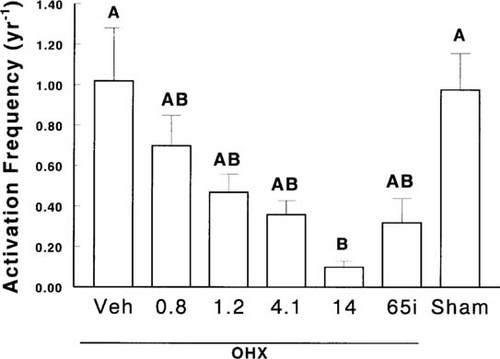
Activation frequency at month 16 in ovariectomized (OHX) or sham-operated (Sham) dogs receiving vehicle (Veh) or ibandronate continuously or intermittently (i). Data with the same letter are not significantly different, one-way ANOVA.
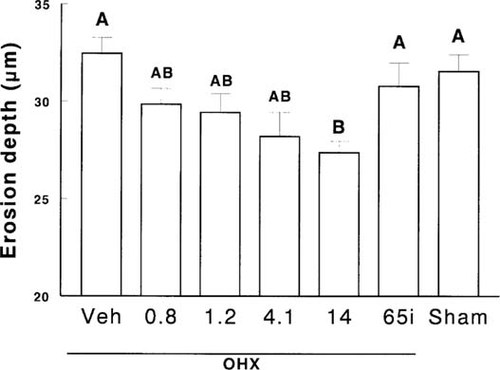
Erosion depth at month 16 in ovariectomized (OHX) or sham-operated (Sham) dogs receiving vehicle (Veh) or ibandronate continuously or intermittently (i). Data with the same letter are not significantly different, one-way ANOVA.
Mineral properties
OHX dogs receiving the continuous doses of 4.1 μg/kg and 14 μg/kg of ibandronate had a higher degree of mineralization (mineral-to-matrix ratios), greater crystal size, and more mature hydroxyapatite crystals than all other groups at any site (Table 4, Fig. 4). Moreover, in those dogs, the mineralization was more uniform, that is, the normal pattern of higher mineralization in trabecular center or cortical endosteum (older bone) and lower mineralization in trabecular edge and cortical periosteum (newer bone) were no longer observed. Degree of mineralization and crystallinity/maturity were found equally distributed between periphery and center of trabeculae and cortices (Table 4). This finding was observed to a lesser degree, and not at all sites, in OHX dogs treated intermittently or dogs given the lower dose (4.1 μg/kg/day) of ibandronate continuously (Table 4).
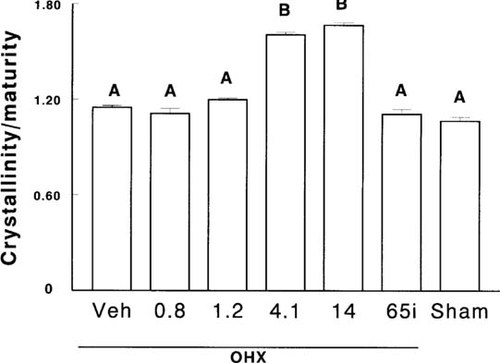
Crystallinity/maturity at month 16 in ovariectomized (OHX) or sham-operated (Sham) dogs receiving vehicle (Veh) or ibandronate continuously or intermittently (i). Data with the same letter are not significantly different, one-way ANOVA.
DISCUSSION
The present study shows that ibandronate administered continuously has a dose-dependent effect on bone volume in OHX dogs. This finding is in keeping with previous studies using ibandronate in ovariectomized rats54 or postmenopausal women36, 55 or in studies testing other bisphosphonates.56-59 However, the dose of ibandronate needed to completely reverse the bone loss after ovariohysterectomy (14 μg/kg/day) was higher than the lowest dose (1 μg/kg/day) able to prevent bone loss 1 month after OHX in the same animal model.38 This discrepancy may be due to the fact that ibandronate was started after OHX during two distinct periods characterized by different osteoclastic activity. Osteoclasts exhibit a high level of activity soon after cessation of ovarian function as evidenced by deep erosion depth38 or decrease in trabecular connectivity,60-63 whereas long after initiation of bone loss, during new steady state, osteoclastic resorption is less pronounced.64 Therefore, it is conceivable that osteoclasts with high metabolic cellular activity are more receptive to direct65, 66 or indirect67, 68 effects of bisphosphonates than osteoclasts observed during steady state.
It is of note, however, that the antiresorptive effect of continuous administration of ibandronate at 4.1 μg/kg/day and 14 μg/kg/day was marked only by a significant decrease in erosion depth without change in the number of osteoclasts and the extent of erosion surface. This was also observed in our previous short-term study when ibandronate was given to prevent bone loss in OHX dogs.38 This discrepancy between osteoclastic activity (erosion depth) and eroded surface was also noted in cortical bone of dogs treated with risedronate,69 in ovariectomized baboons given alendronate,12 and in postmenopausal women treated with etidronate70 or alendronate.28 These in vivo observations are in contrast with in vitro data that point to bisphosphonates as inhibitors of the number of osteoclasts through decrease in osteoclast recruitment71, 72 or shortening of the osteoclast lifespan due to cytotoxicity73 or apoptosis.74 However, other in vitro studies showed that aminobisphosphonates such as alendronate are not cytotoxic75 and do not alter osteoclast number.76 These data, together with the histomorphometric findings, suggest that bisphosphonates act in vivo primarily on osteoclast activity.
In the present study, the dose-dependent increase in bone volume observed with continuous administration of ibandronate was associated with a concomitant dose-dependent decrease in bone turnover. Gain in bone volume at the expense of bone turnover in osteoporotic patients treated with aminobisphosphonate is a well documented phenomenon.28 Moreover, the high dose of 4.1 μg/kg/day and 14 μg/kg/d of ibandronate administered continuously was also associated with a marked decrease in mineralization surfaces resulting in prolonged mineralization lag time without any signs of osteomalacia. The long-term consequences of low bone turnover and delayed mineralization are unclear. It is of note that intermittent administration of ibandronate did not induce such a decline in bone turnover and increase in mineralization lag time.
An interesting finding of the study is that continuous long-term administration of ibandronate at the effective doses of 4.1 μg/kg/day and 14 μg/kg/day induced bigger crystal size and more mature hydroxyapatite crystal. This is in keeping with the observation of a dose-dependent increase in hydroxyapatite crystal size (assessed by X-ray diffraction), following treatment with increasing doses of pamidronate in vitro.77 However, this is in contrast with the results obtained in minipigs treated with 1 mg/kg/day of alendronate for 1 year.78 In that experiment, no changes in thickness of mineral crystals were observed in vertebrae using small-angle scattering and backscattered electron imaging.78 Differences in sensitivity of methods of measurements of crystal size,79 or potency of doses used between the two bisphosphonates or differences between the two bisphosphonate molecules and/or animal model and/or bone turnover may, at least in part, explain this discrepancy. OHX dogs given the two highest continuous doses of ibandronate also had higher mineral-to-matrix ratio, that is, a higher degree of mineralization of cortical and cancellous iliac bone than the other groups. A higher mean degree of mineralization was not observed in vertebrae of minipigs treated with alendronate,78 whereas it was found higher in ribs of the same animals.32 The present findings are also consistent with the observation of higher degree of mineralization in tibia of one baboon given alendronate for 2 years,31 and in cortical iliac bone of osteoporotic women treated with alendronate for 2 and 3 years.33 Moreover, crystal size and degree of mineralization (mineral to matrix ratio) were equal or greater at the edge than at the center of the trabecules in dogs given ibandronate at the highest continuous dose. In untreated bone, crystal size and mineral-to-matrix ratio were greater in the center than in the periphery. These observations are in keeping with data obtained in the above mentioned minipig ribs,32 and osteoporotic women33 in which bone matrix mineralization was more uniform after treatment with alendronate. The increase in hydroxyapatite crystal size and the uniform mineralization in OHX dogs treated continuously with the highest doses of ibandronate lend support to the hypothesis of a progressive increase in secondary mineralization in the presence of low bone turnover.31 This, in turn, may contribute to the slow but ongoing increase in BMD seen 313, 15, 17, 18 and 430, 80, 81 years after initiation of bisphosphonate therapy.
Continuous and intermittent treatments with the same cumulative dose of ibandronate were as efficient in reversing bone loss after cessation of ovarian function. This is in agreement with previous studies in which both treatment modalities were found to impact positively on calcium balance in intact rats,82 mechanical strength in ovariectomized rats54 or intact dogs,83 and BMD in postmenopausal women.84 However, in the present study, erosion depth and bone turnover were not significantly depressed at the end of the study in the dogs treated intermittently. This suggests that during the intermittent treatment periods, the highest daily dose of ibandronate (65 μg/kg/day) was more potent in suppressing bone resorption and thus inducing a stronger transient positive effects on bone balance than the continuous daily dosing and allowing relatively more bone to be formed during the treatment-free periods. Moreover, it is of note that animals intermittently treated did not exhibit changes in bone crystallinity and mineralization lag time. The impact of the higher hydroxyapatite crystal size on bone mechanical strength remains to be elucidated. There is no available direct evidence that increased bone crystals may have a negative effect on bone mechanical properties, other than in fluoride treated bone,78 a condition that is also often associated with mineralization defect and mottled bone,85, 86 cancellous and cortical microfracture, and microcallus formations containing mechanically inferior woven bone.87, 88 It has even been shown that lifelong administration of daily doses of ibandronate far in excess of any therapeutically intended dose increased bone mass and maintained compressive strength of lumbar vertebrae in rats.89 It awaits further study to determine whether similar results can be obtained in humans. In any event, since ibandronate can be administered by bolus intravenous injections without side effects specific to aminobisphosphonates,36, 90 intermittent ibandronate therapy could represent an attractive alternative approach to continuous therapy with increased tolerability and compliance in those osteoporotic patients who cannot tolerate chronic oral administration. However, it awaits further studies to determine whether the less suppressive effect of intermittent therapy on bone turnover and the maintenance of normal bone mineral structure are advantageous in the long term compared with continuous therapy.
Acknowledgements
This work was supported in part by grants from DCI S-679 and S-680. The authors thank Terry Sexton, Richard Wheaton, and Kenloch Westberry for technical assistance and Louise Tipton for editorial help.