Femoral Neck Length and Hip Fracture Risk
Abstract
To determine whether there are differences in femoral skeletal geometry in fracture-prone subjects when size, positioning diagnosis, and age are controlled, we compared femoral measurements made from the uninvolved hip on 119 plane anteroposterior pelvis radiographs of women without fracture to those of the contralateral hip in a group of 43 female patients with hip fractures (neck, 23; intertrochanteric, 20). The hip was imaged in a standardized position of rotation and adduction. Race, age, and musculoskeletal diagnosis were known. Subjects were grouped by diagnosis of the opposite hip condition (rheumatoid arthritis, osteoarthritis, and normal) and compared. Measurements were also analyzed as ratios to head diameter (HD), neck diameter (ND), and pelvic width. Femoral neck length (NL) was measured from skeletal preparations and imaged in controlled positions of abduction and external rotation. No differences were found between the neck and intertrochanteric fracture groups. The differences between the fracture group and the controls were a thinner femoral cortex (measured at a point one head radius below the lesser trochanter) a larger femoral head, and a larger femoral ND in the fracture group (p < 0.025). The difference in cortical thickness was still significant when scaled by size, but the ratio of HD to ND was equivalent in fractures and controls. No difference in femoral NL could be demonstrated. The experimental measurements showed that apparent NL is significantly position sensitive and this may explain previously reported differences in fracture-prone groups.
INTRODUCTION
Several reports have suggested that the dimensions of the femoral neck influence hip fracture risk.1-5 Of these studies, the largest and most frequently cited are those which utilize the “hip axis” length (HAL), an automated measurement of distance from the lateral femur to the pelvic brim in images generated during dual-energy X-ray absorptiometry (DXA).3-6 This technique is of interest because individual hip fracture risk cannot be determined by bone density alone, and HAL appears to be an independent risk factor easily obtained in the course of bone density measurement. Subsequent plane radiographic and DXA investigations have identified racial and generational differences in the length of the hip axis or femoral neck raising the possibility that skeletal anatomy might explain fracture risk differences among these groups.7-12
However, the literature on this topic does not present a clear consensus. Using plane radiographs to compare fracture and control samples, Karlsson et al.13 and Ferris et al.1 found that femoral neck length (NL) was actually smaller in the fracture group. The only computed tomography (CT) comparison reported that the femoral neck was shorter in a fracture group.2
To judge the reliability of these quantitative studies, one must consider factors that affect measurement accuracy. Skeletal measurements based on radiographs, including DXA images, are subject to significant errors. A comparison of radiography to direct measurements of femoral shaft diameter (SD) has reported an error of 2.4 mm in the standard radiographs versus an 0.9-mm error with CT.14 Magnification contributes to error in plane radiographs (average 12%)15 and in DXA imaging (about 7%).6, 16 By comparison, the reported differences in HAL measurements range from 3.5 mm to as little as 2.3 mm, or about 3%. Movement of the hip joint changes the orientation of the femoral neck and thus changes its apparent length on radiographs.
The size of the hip is influenced both by subject height and body mass. Specifically, femoral length is proportional to height, but SD more closely reflects body volume.17 Furthermore, body habitus affects magnification in radiographic images, to the degree that soft tissues raise the pelvis from the film.15, 16 Body height and mass are thought to affect fracture risk and fracture type.18-20 If this is true, some or all of the observed skeletal differences in fracture groups simply reflect these differences in body shape. Scaling and multivariate analysis have been used to correct for body height differences, but measurements of HAL and NL include a segment of the shaft and/or femoral head. For these reasons, scaling by body height alone does not truly normalize these values.
Control groups for fracture-risk studies often contain a disproportionate number of osteoarthritic hips. Joint space narrowing, stiffness, and other anatomic features of osteoarthritis may affect apparent hip shape,21 thus this diagnosis should be identified and considered in any morphometric study.
In the study reported here, we compare the dimensions of femora in patients with fractures to those of a nonfracture group using measurements taken from plane radiographs and in conditions where positioning was rigidly controlled. Our objective was to generate standardized data which could be compared against those of published studies and thus confirm or refute the reported differences between normal and fracture-prone individuals. To correct for the effect of body size and magnification, length measurements were analyzed before and after expression as ratios of femoral head and neck diameter (ND) and pelvic width. The effects of hip positioning on length and angle measurements were assessed by a variety of methods. We knew whether control subjects had osteoarthritis or inflammatory arthritis and were thus able to analyze these groups separately.
MATERIALS AND METHODS
Two groups were compared: 43 women with hip fractures suffered in falls and 119 women scheduled for elective total hip replacement. In each case, the contralateral (unbroken, undiseased) hip was measured. None of the fracture patients had radiographic evidence of arthritis, metabolic disease, or tumor. The surgical diagnoses for the control patients were rheumatoid arthritis (20 subjects), osteoarthritis (85 subjects), and secondary osteoarthritis (14 subjects). In each case, the measured hip was radiographically normal. The subjects were all said to be Caucasian. Subject height and weight were unknown.
Measurements, in millimeter units, were made from anteroposterior 1-m radiographs of the pelvis taken in a standardized fashion with the patient supine, the beam centered on the midline, and the toes touching. The radiographs of fracture patients were made in the period following surgery, when the patient was comfortably able to lie supine in the required position.
Fourteen basic parameters were measured on each radiograph. The most salient of these are identified in Fig. 1. NL was measured in three ways: length of the medial cortex (MCL), true neck length (NL, from shaft axis to head center), and from the lateral cortex to head apex (“femoral axis length”) (Fig. 1). Neck orientation was determined using two methods, one based on the neck axis (NSA) and the other on the orientation of the medial trabecular system (MTA), the dense band of trabeculae which extends from the medial neck cortex to the head center. Measurements of the width of the femoral shaft (SD) and the medullary canal (ID) were arbitrarily made at a level one head radius below the lesser trochanter.
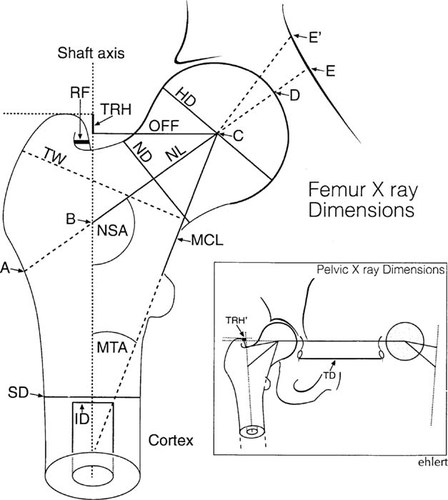
Measurements taken from proximal femur or pelvis and key to abbreviations used in tables. HD, diameter of femoral head; OFF, distance of head center from shaft axis; ND, width of femoral neck at its most narrow point; NL, distance from the shaft axis to the head center measured along the central axis of femoral neck; MCL, distance from lesser trochanter to the head center in a line passing along the medial cortex and medial trabecular system; MTA, angle between medial trabecular system and shaft axis; NSA, conventional neck shaft angle measured as angle between neck and shaft axes; TW, width of the intertrochanteric region in a line perpendicular to the medial trabecular system; TRH, height of the tip of the greater trochanter above a line perpendicular to the shaft axis and passing through the head center; TRH′, height of the tip of the greater trochanter above a line passing through both femoral head centers; SD, diameter of the femoral shaft at a point one HD below lesser trochanter; ID, inside diameter of the medullary canal at same level; RF, distance from posterior border of trochanter to internus fossa. In studies of hip fracture risk, femoral NL is defined as: “hip axis length” (A–E3-5, 7, 12), “femoral axis length” (A–D2, 5, 13), and “neck length” (A–C7 or B–C9) are most common. We measured femoral axis length and NL (B–C) here. With hip adduction, the medial segment (A–E′) of HAL increases due to the inner shape of the pelvis.
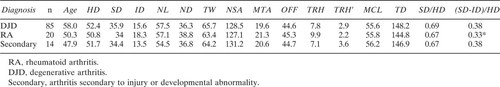
To ascertain the effect of underlying diagnosis, the control group was separated into three groups based on the disease which lead to surgery on the contralateral hip: rheumatoid arthritis, osteoarthritis, and secondary arthritis. The secondary arthritis group was composed of patients with osteonecrosis of the femoral head or developmental dysplasia on the operated side only, not that measured. Two subjects in this group had suffered traumatic acetabular injuries, but none had evidence of proximal femur fractures. In all subjects, the measured hip was concentric with a normal joint space and read as normal by the clinic radiologist as well as the senior author (J.M.C.).
All of the groups were then compared using a standard t-test, with a hypothesis of no difference between fracture and no-fracture groups, p value < 0.025. The relationship of all variables was analyzed by calculation of correlation coefficients. To control for the influence of body size, dimensional measurements were normalized by dividing each value by the femoral head diameter (HD), ND, or by the distance from the midline to the hip center and the analysis was repeated.
The effect of position was evaluated in two ways. First, the hips of two subjects were imaged using a QDR 4500 A DXA unit with a pencil beam (Hologic, Waltham, MA, U.S.A.). Five scans were made of each subject and the length of the “hip axis” (the distance from the lateral cortex of the femur to the inner table of the pelvis passing through the head and neck centers) was measured in our standard position and positions of hip abduction, external rotation and combined abduction, external rotation. Second, the plane radiographs were made of an articulated hemipelvis, with the hip in 25 different positions of rotation and abduction. Abduction was varied from –20° to +35°, and external rotation from –20° to +45° each at 5° increments. All of the femoral measurements used in the clinical study were then repeated for each position. To assess rotation and abduction, the height (TRH′) and medial displacement (RF) of the greater trochanter were assessed on all of the study films.
To provide an accurate estimate of the relationship between body size and femoral HD, the femoral measurements were also taken from a group of 72 cadaver femora which could be measured without magnification and rotational artifact. Height and body weight were known in 49 of these subjects: 30 females and 19 males. Correlation coefficients were also calculated for measurements in this group.
RESULTS
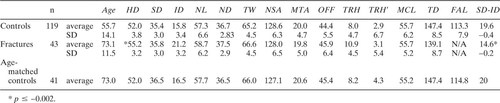
The basic data from the nonfracture and fracture groups are provided in Table 1. All measurements displayed a normal distribution. The strongest correlations among length measurements were to neck and HD and to distance between the head center and the midline (on the pelvis films).
The only significant differences found among the groups were in the total thickness of the femoral cortex (SD-ID), measured at a level one head radius below the lesser trochanter, head, and ND). The cortex was also thin in the subjects with rheumatoid arthritis, but still significantly thicker than in the fracture patients. When scaled to HD or pelvic width, cortical thickness in the fracture patients and controls was even more disparate compared with controls. All other dimensions, including head and ND, were equivalent when scaled this way (Table 2). Diagnosis did not influence hip shape (Table 3).
Because the control group was younger, analysis was repeated with ages matched, and the results were unchanged. A small but significant correlation was found between age and the thickness of the femoral cortex, but the cortical thickness was still significantly thinner in fracture patients when controls were age matched.
When measured on the DXA scans of the two volunteers, both males, 1.73 m in height, abduction of 15° with 10° of external rotation changed the hip axis from 70 mm to 63 mm. Pure abduction of 15° reduced the axis length from 70 mm to 65 mm, and external rotation reduced the apparent length of the femoral neck by 4 mm.
Measurements taken from our skeletal preparations showed that external rotation beyond a neutral position reduced apparent length of the neck, the “femoral axis,” and the “hip axis” (Figs. 2A and 2B). Specifically, HAL decreased from 118 mm at 15° to 105 mm at 45° of external rotation. As previously shown by Cser et al.,22 the apparent NSA increased with external hip rotation beyond 0°. Adduction caused a marked increase in HAL, which measured 156 mm at 5° of abduction (neutral defined as femur vertical), and 180 mm at 15° of adduction. Medial displacement of the greater trochanter, measured from the obturator fossa (RF) increased linearly between 0° and 35° of external rotation. We used this value to compare rotation in our groups and RF was equivalent in all.
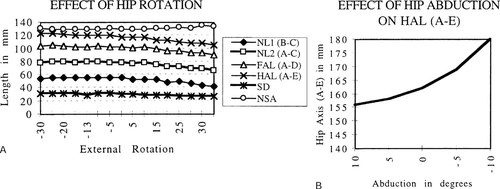
(A and B) Graphs illustrating changes in parameters of femoral NL and HAL created by external rotation (A) or abduction (B) of the hip in an experimental model. Little change occurred between maximum internal rotation and neutral position, but all parameters decreased linearly between neutral and 30° of external rotation.
DISCUSSION
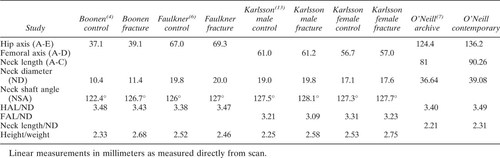
Previous radiograph- and DXA-based surveys have found differences in femoral NL in groups of fracture-prone subjects. In that body of work, NL is defined in a variety of ways (Fig. 1 and Tables 4 and 5). Hypothetically, length of the neck could influence bending forces in the proximal femur, predisposing those individuals with longer necks to fracture9 In this radiographic study, however, we could not demonstrate a significant difference in femoral NL—defined by various methods—when a fracture group was compared with controls. Furthermore, the ratios of NL to width, to femoral HD, and to pelvic width were very similar among groups. Given the discrepancies in the published studies and the recognized limitations of X-ray imaging, the common belief that length of the femoral neck is an independent fracture risk remains unproved. Any definitive study must address and control several interrelated variables which diminish the accuracy of X-ray imaging of the hip.
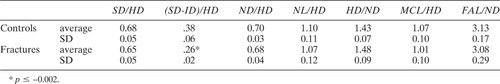
Evaluation of the published dimensional data is complicated by the fact that DXA-derived length measurements are not standardized and cannot be directly compared either with other DXA studies or with radiographic studies. Fortunately, most include a measurement of femoral neck width in similar units, and the ratios of NL to neck width can be compared. In this study, the FAL/neck width ratio was quite similar (about 3.10:1), for all groups. Use of such a ratio should normalize for variations in body size and magnification. In other published studies, the ratios to ND vary widely, for both HAL and FAL (Tables 4 and 5). O'Neill et al.7 and Reid et al.12 showed, on the basis of archival films, that NL/width ratios were smaller in previous generations of women, and suggested that an increasing NL/width ratio may explain increasing fracture rates. Nakamura et al.9 used a similar argument (comparing NL with cross-section moment of inertia) to explain lower fracture rates in Japanese women, when compared with Caucasian Americans. Yet, in several of the studies which compare a fracture-prone group with controls, the NL/width ratio in the control group is equivalent or smaller than in the fracture group, and no difference was found here.
The presence of such marked differences between studies suggests unrecognized technical errors. In addition to the direct error of measuring from the image with a ruler or automated machine, other significant sources of error are magnification and subject positioning. Our experimental measurements indicate that abduction shortens the apparent hip axis length and external rotation shortens apparent femoral NL (Figs. 2A and 2B). Radiographic technicians attempt to standardize hip rotation and abduction in pelvic images by placing the thighs and toes together when a study is performed. This standard is not always achieved because of patient-related factors. For example, one of the published radiographic studies reports that patients were positioned in “15° to 30° of internal rotation” when the pelvis films were performed.23 By our observation and that of others, the hips lie in external rotation on injury films, because the patients are more comfortable in that position. All parameters of femoral NL will be shorter if measurements are made from these films, and this could explain the shorter neck measurements in some radiographic studies.
The published DXA-derived measurements were not made in an acute, postfracture setting, but hip position in those studies will be influenced by body habitus. Especially when lying supine, hip adduction is limited by girth of the thighs. Patients with relatively thick thighs cannot put their knees together, but thin women can, and adduct to a greater degree. Asking stout subjects to place the great toes together actually increases internal rotation of the hip, but does not force the knees together. If thin women are indeed more likely than obese women to break a hip,24 they as a group would be positioned in more adduction and external rotation. Such positioning would explain the seemingly contradictory but consistent observation of greater hip axis length and shorter femoral NL in at-risk patients. This hypothesis is compatible with work which argues that a larger body mass index and specifically increased thigh girth, reduces hip fracture risk in falls.25 The reported variations between fracture groups and controls are quite small, making even minimal positional changes potentially significant. For example, the 3.4% increase in hip axis length observed in the Faulkner fracture group could be created by about 5° of hip adduction.
Neck-shaft angle varies among the published studies on NL and fracture risk. In every comparison study except those of Cody and Nahigian2 (a CT study) and Ferris et al.1 (where the hips were held in maximum internal rotation), the NSA is larger in the fracture-prone group. This is consistent with the hypothesis that generally, these patients were imaged in a relatively externally rotated position, given that external rotation increases apparent NSA.22 The measurements of NSA reported by Boonen et al.4 and Peacock et al.5 are notably smaller than others, including our own. As with the NL/width values, such variation is difficult to explain, and again raises the possibility that methods of measurement as well as positioning are not adequately standardized.
As other studies have reported,2, 23 femoral head and NDs were significantly larger in the fracture group, but the head/ND ratio was equivalent between groups. Other length measurements (SD, NLs, distance to the midline, and trochanteric width) were also slightly (although not significantly) larger in the fracture group, which suggests that these subjects simply had larger bones than did the controls. When Cody et al.2 normalized HD to femoral shaft length, their fracture and control groups were equivalent. Another possibility is that osteoporosis is associated with a general dilatation of the femur, which heretofore was ascribed simply to aging.
People with osteoarthritis of the hip have a low risk of fracture26 and may be over-represented in control groups. Some studies have suggested that proximal femoral anatomy may be different in subjects with primary osteoarthritis.21, 27 Karlsson et al.13 speculated that joint space narrowing in arthritic subjects may artificially shorten the hip axis in controls. In this study, significant differences were not found among controls with osteoarthritis, rheumatoid arthritis, and secondary arthritis. This finding does not necessarily contradict reports which state that osteoarthritic hips are different, because all of our measurements were taken from the uninvolved, radiographically normal hip in subjects admitted for replacement of the opposite side.
The clearest risk for fracture is age-related osteoporosis.28 Thinner cortices in the femoral shaft and neck have been observed by other investigators.5, 23 This phenomenon is somewhat independent of bone mass and thus may help predict risk. We found that the difference is even greater when cortical thickness was scaled to shaft, neck, and HDs. Chronic illness may also affect cortical thickness, which was reduced in the rheumatoid patients. This illustrates the limitations of studies based on chronically ill subjects or cadaver bones.
On the basis of this study, we conclude that femoral NL does not necessarily change hip fracture risk. Thinning of the cortical shell from within may be the anatomic process that places these subjects at increased risk. Small increases in length of the hip axis in fracture studies could be an artifact of positioning, and future studies should adopt strict criteria for a standard position, defining abduction and rotation as well as an error value for linear and angular measurements.