Growth Retardation in Children with Chronic Renal Failure
Abstract
Growth retardation is a major obstacle to full rehabilitation of children with chronic renal failure (CRF). Several factors have been identified as contributors to impaired linear growth and they include protein and calorie malnutrition, metabolic acidosis, growth hormone resistance, anemia, and renal osteodystrophy. Although therapeutic interventions such as the use of recombinant human growth hormone, recombinant human erythropoietin, and calcitriol have made substantial contributions, the optimal therapeutic strategy remains to be defined. Indeed, growth failure persists in a substantial proportion of children with renal failure and those treated with maintenance dialysis. In addition, the increasing prevalence of adynamic lesions of renal osteodystrophy and its effect on growth have raised concern about the continued generalized use of calcitriol in children with CRF. Recent studies have shown the critical roles of parathyroid hormone–related protein (PTHrP) and the PTH/PTHrP receptor in the regulation of endochondral bone formation. The PTH/PTHrP receptor mRNA expression has been shown to be down-regulated in kidney and growth plate cartilage of animals with renal failure. Differences in the severity of secondary hyperparathyroidism influence not only growth plate morphology but also the expression of selected markers of chondrocyte proliferation and differentiation in these animals. Such findings suggest potential molecular mechanisms by which cartilage and bone development may be disrupted in children with CRF, thereby contributing to diminished linear growth.
INTRODUCTION
Growth retardation and its profound impact on final adult height have been considered to be a significant obstacle to full rehabilitation of pediatric patients with chronic renal failure (CRF). In a recent report from the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS), the mean standard deviation score (SDS) for height of children with CRF (estimated glomerular filtration rate ≤75 ml/minute/1.73 m2) was –1.5 and almost 50% of those below 5 years of age had a height SDS ≤1.88 (below the third percentile).1 Long-term follow-up during the course of renal failure and dialysis demonstrated that height deficits progressively worsen in these children. At the time of transplantation, patients were severely retarded with mean height SDS of –2.16 and linear growth did not improve in children older than 5 years during the 4-year post-transplant follow-up.2 Furthermore, Hokken-koelaga et al. reported that more than 70% of patients who were transplanted before 15 years of age had an adult height below the third percentile.3
Since longitudinal bone growth in children occurs primarily through endochondral bone formation, the critical role of the epiphyseal growth plate must be considered in the analysis of growth retardation. Recent studies using gene ablation and overexpression strategies demonstrate potential roles for parathyroid hormone–related peptide (PTHrP), PTH/PTHrP receptor or the PTH type 1 receptor and Indian hedgehog (Ihh) in endochondral bone growth4-8; these observations have provided remarkable insights into specific gene products that control normal skeletal growth and development. As such, disturbances in the key regulators of chondrocyte proliferation and differentiation may contribute to impaired linear growth in renal failure.
Numerous factors have been implicated in the development of growth impairment in CRF; however, despite correction of some of the metabolic abnormalities, maintenance of aggressive nutritional support, initiation of dialytic therapy, and, after successful renal transplantation, a substantial proportion of patients attain an adult stature that is suboptimal to their genetic potential. In this review, the factors contributing to growth retardation will be considered and the therapeutic strategies in the management of this disorder in pediatric patients with renal disease will be discussed.
ENDOCHONDRAL BONE FORMATION
Normal bone growth
Endochondral bone formation is a complex process through which the growth plate cartilage, an avascular tissue located between the epiphyses and metaphyses of proximal and distal ends of long bones, is replaced by bone. The growth plate and bone front steadily advance away from the bone center, resulting in progressive elongation of bone.9 Longitudinal growth continues while the growth plate remains open; growth plate starts to close after puberty in humans. Growth in the diameter of the long bone, however, is accomplished by appositional growth beneath the periosteal surfaces of bone and by bone resorption at the endosteal surfaces.9, 10 These processes occur simultaneously with bone remodeling, which provides a mechanism for modification of bone shape and structure.9 For example, bone remodeling of the long bone results in reshaping of the wider metaphysis into the narrower diaphysis.
The cellular events at the epiphyseal growth plate cartilage are fundamental to longitudinal bone growth.11 During endochondral ossification, growth plate chondrocytes undergo an orderly programmed transition, creating four distinct zones based on morphological criteria: reserve or resting cartilage, proliferating chondrocytes, and upper and lower zones of hypertrophic chondrocytes12 (Fig. 1). Further characterization of the growth plate chondrocytes has been obtained through the localization of transcripts for selected markers of chondrocyte proliferation and differentiation to specific chondrocytes by in situ hybridization.13 Type II collagen is expressed in both proliferating and hypertrophic chondrocytes. Transcripts for PTH/PTHrP and alkaline phosphatase (ALP) are localized to the lower zone of proliferating and upper zone of hypertrophic chondrocytes. Type X collagen mRNA expression is seen throughout the hypertrophic cartilage whereas osteopontin is expressed only in the lower zone of hypertrophic chondrocytes13 (Fig. 1).
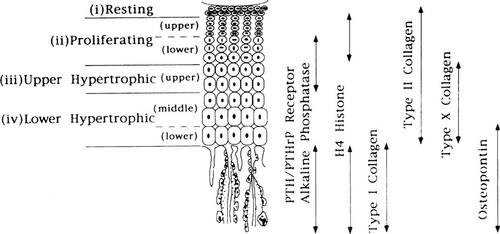
Localization of selected markers of chondrocyte proliferation and differentiation during endochondral bone development. (From Lee et al.13; © The Endocrine Society)
Mineralization is initiated at the lower hypertrophic zone, primarily involving the cartilage matrix of the longitudinal septae, whereas the matrix of the transverse septae remains generally uncalcified. Fully differentiated (hypertrophic) chondrocytes undergo apoptosis, although some studies have shown that terminal chondrocytes can differentiate into osteoblasts.14 Vascular invasion of the terminal chondrocyte lacunae ensues and ∼70% of the mineralized matrix adjacent to the vascular invasion front become resorbed by osteoclasts/chondroclasts.9 The remaining mineralized septae serve as scaffolds for deposition of bone matrix by osteoblasts, resulting in the formation of the primary spongiosum. Overall, the balance between processes which increase the growth plate width (chondrocyte proliferation, hypertrophy, matrix synthesis) and those that reduce it (vascular invasion and resorption) helps to maintain the width of the growth plate constant.
Several hormones (growth hormone [GH], thyroid, parathyroid, and steroids) and growth factors (insulin-like growth factor-I [IGF-I], fibroblast growth factor, transforming growth factor-β) have been known to influence cellular activities at the growth plate. Indeed, GH increases local production of IGF-I within the growth plate cartilage, and the latter acts in a paracrine manner to stimulate clonal expansion of proliferating chondrocytes. In addition, both GH and IGF-I have been shown to promote chondrocyte differentiation.
Regulation of endochondral bone formation
Over the past few years, considerable research has focused on identifying the major factors regulating endochondral bone formation and bone elongation. In addition to its role in regulating calcium homeostasis, gene deletion studies have recently established the critical roles of PTHrP and the PTH/PTHrP receptor in chondrocyte differentiation and bone growth. Mice with homozygous deletions of either the PTHrP or the PTH/PTHrP receptor genes die in midgestation or shortly after birth, and they manifest a skeletal phenotype characterized by misshapen skull and markedly shortened limbs due to accelerated differentiation of growth plate chondrocytes and premature initiation of endochondral ossification.4-8 In contrast, targeted overexpression of PTHrP to chondrocytes using the type II collagen promoter produces a substantial delay in chondrocyte differentiation and ossification such that transgenic mice are born with a cartilaginous endochondral skeleton.15 Likewise, misexpression of Ihh prevents the transition of proliferating chondrocytes into hypertrophic chondrocytes.5
Similar alterations in skeletal development are demonstrated in Jansen's metaphyseal chondrodysplasia, an autosomal dominant disease characterized by short-limbed dwarfism, hypercalcemia, and hypophosphatemia despite low or normal serum PTH and PTHrP levels. Genomic DNA studies have shown that this rare disorder is caused by activating mutations in the PTH/PTHrP receptor.16, 17 More recently, absence of functional PTH/PTHrP receptors has been described in Blomstrand chondrodysplasia, a rare lethal disorder characterized by profound skeletal abnormalities including short limbs, increased bone density, and markedly advanced skeletal maturation.18, 19 Overall, these results indicate that PTHrP, PTH/PTHrP receptor and Ihh interact through an autocrine/paracrine mechanism to delay chondrocyte differentiation within the epiphyseal growth plate of long bones. Moreover, such findings suggest potential molecular mechanisms by which epiphyseal growth plate cartilage development and bone elongation may be disrupted in children with CRF, thereby contributing to reductions in linear growth (vide infra).
PATHOGENESIS OF GROWTH RETARDATION IN CHRONIC RENAL FAILURE
Growth retardation associated with CRF is multifactorial in origin. Factors that have been thought to contribute to growth failure include protein and calorie malnutrition, metabolic acidosis, end organ GH resistance, anemia, and renal bone disease. In addition, linear growth is influenced by the age of onset of CRF and primary renal disease. Children who develop renal failure in infancy are more growth impaired than those who acquire renal disease later in childhood.20 Since 30% of the final adult height is attained during the first 2 years of life, onset of renal failure during this critical period has been associated with markedly diminished adult height. Data from the Growth Failure in Children with Renal Diseases Study and NAPRTCS indicate that younger children have larger height deficits at study entry than older children.1, 21 Similarly, a substantial proportion of children below 6 years of age at dialysis initiation is below the third percentile for height.2 These findings emphasize the need for aggressive intervention at an early age to maximize the growth potential of these children.
The etiology of the primary renal disease has also been suggested to influence skeletal growth in CRF. Patients with renal dysplasia and congenital obstructive disorders may develop growth retardation associated with tubular dysfunction, such as impaired urine concentrating ability and salt wasting. However, impaired growth may be a consequence of the early age of onset of renal disease and not essentially be due to the primary disease. The relative importance of each of these factors depends on the stage of development of the child.22 While nutrition is considered to be the primary determinant of growth during infancy, GH axis and gonadotrophic hormone axis are thought to contribute significantly during childhood and puberty, respectively.23 The main factors implicated in the pathogenesis of growth retardation are: malnutrition, metabolic acidosis, GH resistance, anemia, and renal osteodystrophy, as discussed below.
Malnutrition
Protein and calorie deficiency has been suggested to play a role in the growth of children with renal disease, particularly in infancy. Simmons et al. first reported growth velocity in children treated with maintenance hemodialysis that were comparable to those with intact renal function when calories were supplemented to ∼70% of the recommended dietary allowance (RDA).24 Betts and Magrath found a correlation between growth velocity and energy intake in children with CRF and concluded that diminished linear growth would occur when energy intake was < 80% of the RDA.20 In a follow-up study, however, growth rate did not improve despite caloric supplementation.25 However, Arnold et al. demonstrated increased growth velocity from 59% to 90% of expected when caloric supplementation was given to increase energy intake to 100% of the RDA for age. Although catch up growth was not observed during this period, supplementation prevented a further decline in SDS for height, which had been found in the preceding unsupplemented period.26 Rizzoni et al. likewise noted normal growth rates in infants with CRF when caloric intake >100% of the RDA was consumed.27
These results suggest that provision of calorie intake according to the RDA for age should be recommended to prevent further reductions in growth. The need for aggressive nutritional support and periodic nutritional assessment should be emphasized, particularly since recent data indicate that the mean caloric intake of children with CRF was 80 ± 23% of the RDA. Supplemental enteral feeding must be initiated, especially in infants, when voluntary intake does not consistently meet the caloric requirements for age.28 Although appropriate energy intake is needed for anabolism and to maximize growth and nutritional status, the present data do not support the contention for extra calorie supplementation beyond the RDA for age in both patients with CRF and those treated with maintenance dialysis.
With regard to dietary protein requirements, two multicenter clinical trials on dietary protein restriction (0.8–1.1 g/kg/day) in children with CRF showed no difference in both progression of renal failure and linear growth between children on a protein-restricted diet and children in the control group.29, 30 However, a feasibility study demonstrated diminished linear growth during treatment with low protein (1.4 g/kg/day) formula in infants with CRF.31 These results raise considerable concern regarding the safety of protein restriction, particularly in younger children with CRF. Current recommendations for protein intake should provide 100% of the RDA, using proteins of high biologic value. Further studies are warranted to define the optimal protein requirement for children with chronic renal disease.
Metabolic acidosis
Metabolic acidosis is one of the consequences of renal failure that has been associated with impaired growth. Support for its role in the development of growth retardation was derived from the study of children with primary renal tubular acidosis, in whom correction of metabolic acidosis with bicarbonate supplementation resulted in accelerated growth and attainment of normal height.32 McSherry et al. initially reported preliminary findings of inhibition of pituitary GH response to provocative tests with arginine in children with renal tubular acidosis.33 Subsequent experimental studies by Challa et al. demonstrated that metabolic acidosis decreased pulsatile GH secretion and reduced serum IGF-I levels associated with down-regulation of hepatic IGF-I and GH receptor mRNA expression.34, 35 However, alterations in hepatic IGF-I mRNA expression may be partly explained by insufficient caloric intake since pair-fed animals also showed decreased hepatic IGF-I expression. Nevertheless, these findings may account for preliminary reports of reduced expression of chondrocyte IGF-I mRNA in the epiphyseal growth plate cartilage and the lack of response to exogenous GH administration in the presence of severe metabolic acidosis.36, 37 In clinical studies, Brüngger et al. recently found that NH4Cl-induced metabolic acidosis resulted in lower serum IGF-I levels, presumably due to hepatic GH resistance as shown by diminished IGF-I response to GH administration.38 Accordingly, the enhanced GH response to GH-releasing hormone administration in acidotic states may be attributed to the loss of negative feedback to GH secretion by reduced IGF-I levels.38 Overall, these data suggest that metabolic acidosis contributes to the disturbances in the GH–IGF-I axis and thus must be appropriately treated in children with CRF. However, it should be emphasized that there is a lack of clinical studies that have carefully assessed the effects of acidosis on growth retardation in children with CRF.
Growth hormone resistance
Disturbances in the GH–IGF axis have been postulated to be one of the principal mechanisms of profound growth retardation in children with CRF. Serum GH levels are normal or elevated in children with CRF for the following reasons: increased pituitary GH secretion rate because of attenuated feedback of bioactive IGF-I, and prolonged GH half-life due to impaired renal clearance. Growth failure develops, however, despite such elevations in serum GH levels in children with CRF, and this disparity has been taken as evidence for end organ GH resistance in renal failure. Tönshoff et al. have demonstrated down-regulation of hepatic GH receptor gene expression in uremic animals and this was not reversed with GH treatment.39 More recently, circulating GH binding proteins (BP) levels, which have been used as noninvasive marker of GH receptors in the liver, were found to be decreased in children and adults with CRF, further providing evidence for the resistance of target tissues to the actions of GH, and consequent growth failure.40
Alterations in serum IGF and IGFBP concentrations have also been described in children and adults with CRF, and these may represent additional factors for GH insensitivity in uremia. In predialysis children, circulating IGF-I and IGF-II levels are normal but IGF bioactivity is reduced, suggesting the presence of IGF inhibitors in CRF. More recently, serum levels of IGFBP-1, IGFBP-2, low molecular weight fragments of IGFBP-3, and IGFBP-6 were found to be elevated41-43 and this excess of high-affinity IGFBP in uremic serum may account for the diminished IGF bioactivity in CRF (Fig. 2). As advanced renal failure develops, this discrepancy worsens since serum IGFBP levels progressively increase whereas serum IGF-I levels decline despite normal or elevated GH levels due to hepatic GH resistance. Accordingly, the treatment modalities that increase the ratio of serum IGF-I/IGFBP and thus raise the concentration of bioavailable IGF-I may result in accelerated linear growth in children with CRF. Indeed, the growth-promoting effects of supraphysiologic doses of recombinant human GH (rhGH) in children with CRF is associated with increased serum IGF-I and IGFBP-3 levels; IGF bioactivity improved because the increase in IGF-I levels was greater than the increase in IGFBP-3 levels. Moreover, serum IGFBP-1 levels were decreased most likely from insulin-mediated inhibition of hepatic IGFBP-1 synthesis.
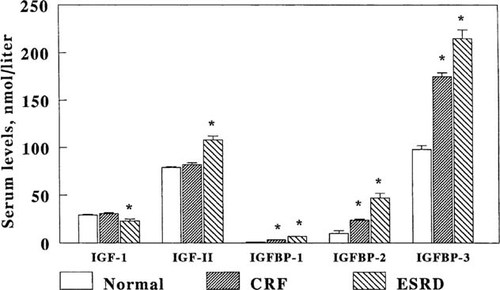
Serum concentrations of IGF-I, IGF-II, IGFBP-1, IGFBP-2, and IGFBP-3 in children with normal renal function, preterminal CRF, and ESRD. (Modified from Tonshoff et al.41; Reprinted by permission of Blackwell Science, Inc.)
Anemia
The anemia of CRF develops mainly from decreased erythropoietin production by the kidney and is associated with transfusion dependency and its attendant risks, decreased exercise tolerance and working capacity, growth retardation, and delayed neurologic development.44-46 The availability of recombinant human erythropoietin (rhEPO) has made a tremendous impact in the overall management of patients with CRF. Initial clinical trials in adult and pediatric patients with end-stage renal disease (ESRD) established the efficacy of rhEPO therapy in increasing hematocrit levels and eliminating transfusion requirements in most patients.47-50 With correction of anemia, pediatric patients reported improvements in appetite, physical activity, exercise tolerance, and cognitive function, overall leading to a better quality of life.44, 49, 51-55 Accordingly, increased linear growth was expected during rhEPO therapy in children with CRF as a result of improved tissue oxygenation and nutritional status. Although increments in height were observed in some patients, enhanced linear growth was not a consistent finding during rhEPO administration in such patients with CRF.52, 56-60 It should be noted, however, that the target hematocrit was 30% in the vast majority of these studies, thus, the potential beneficial effect of a hematocrit more close to the level present in subjects with normal renal function remains to be determined.
Renal osteodystrophy
The consequences of renal osteodystrophy may be devastating in pediatric patients because of the profound effects on skeletal growth and remodeling, which often result in disabling bone deformities and growth retardation. Growth failure in children with severe secondary hyperparathyroidism may be due to alterations of the normal architecture of the growth plate cartilage. Since longitudinal growth occurs through the process of endochondral bone formation in children, replacement of the primary and secondary spongiosa by dense fibrous tissue may impair bone growth.61 As such, correction of the skeletal lesions may maximize normal growth.
Renal osteodystrophy represents a spectrum of skeletal disorders ranging from high-turnover lesions of secondary hyperparathyroidism to low-turnover lesions of osteomalacia and adynamic bone62 (Fig. 3). The subtypes of renal bone disease represent different histopathologic manifestations with calcium, PTH, and vitamin D as the main regulators of osteoblastic activity and bone formation. In the past, secondary hyperparathyroidism was the predominant histologic lesion in adults and children with ESRD.63-66 Recent data, however, demonstrate increased prevalence of the adynamic lesion in the absence of aluminum deposition both in adults with CRF and those undergoing regular dialysis.67-70 In pediatric patients, the adynamic lesion of renal osteodystrophy became predominant after high-dose intermittent calcitriol therapy for the management of secondary hyperparathyroidism in patients treated with peritoneal dialysis.71 The change in distribution of skeletal lesions may be attributed to the use of large doses of vitamin D and increased calcium load from calcium-containing phosphate binders and dialysate solutions.67
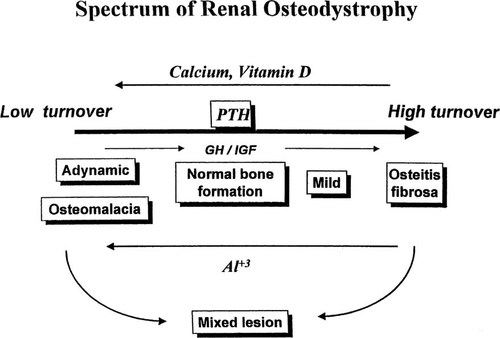
The spectrum of skeletal disorders of renal osteodystrophy. (Modified from Salusky and Goodman.62; Reprinted by permission of Blackwell Science, Inc.)
Several factors contribute to the development of high-turnover lesions of bone in patients with CRF.72 Among these are hypocalcemia, impaired renal calcitriol production, skeletal resistance to the calcemic actions of PTH, alterations in the regulation of prepro-PTH gene transcription, reductions in CaSR expression in the parathyroid glands, and hyperphosphatemia. Serum PTH levels represent a major regulator of bone formation and turnover in patients with CRF. The PTH/PTHrP receptor mediates the action of two distinct hormones: PTH and PTHrP. Its expression in bone and kidney accounts for the well established systemic calcium-regulating actions of PTH, whereas its abundance in epiphyseal growth plate cartilage mediates the localized actions of PTHrP on chondrocyte proliferation and differentiation. The expression of PTH/PTHrP receptor is reduced in renal failure and such changes may contribute to impairment of linear growth.
Recent studies from our laboratory indicate that differences in the severity of secondary hyperparathyroidism influence not only epiphyseal growth plate morphology but also the expression of selected molecular markers of chondrocyte proliferation and differentiation in rats with renal failure.73 Growth plate width was reduced and PTH/PTHrP receptor mRNA expression was diminished in uremic rats with advanced secondary hyperparathyroidism, but neither change was demonstrated in animals with mild secondary hyperparathyroidism. However, type X collagen expression was decreased in growth plate cartilage of rats with mild secondary hyperparathyroidism compared with animals with intact renal function; such difference did not occur between corresponding groups in animals with advanced secondary hyperparathyroidism.73
Whether GH and/or calcitriol modify the expression of PTHrP, the PTH/PTHrP receptor, Ihh, or other selected markers of endochondral bone development remain to be determined in children with CRF. But both GH and calcitriol up-regulated type X collagen expression in nephrectomized rats with mild secondary hyperparathyroidism. Moreover, GH increased expressions for type II and type X collagen and PTH/PTHrP receptor in uremic rats with severe secondary hyperparathyroidism; however, up-regulation of mRNA expression for type II and type X collagen was attenuated when both GH and calcitriol were administered. In addition, GH increased the width of the growth plate, whereas calcitriol blocked the expected increment in growth plate cartilage width during GH therapy73 (Fig. 4). These results indicate that the severity of the skeletal lesion of secondary hyperparathyroidism influences the expression of key regulators of endochondral bone formation. The recent advances in the understanding of the regulation of growth at the growth plate level will provide new insights into the pathogenesis and treatment of growth retardation in the context of renal insufficiency.
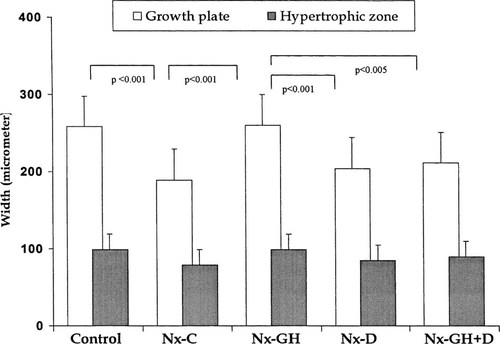
Measurements of the growth plate width in control animals and in four groups of rats with renal failure and severe secondary hyperparathyroidism. Nephrectomized animals were treated with saline vehicle (Nx-C), growth hormone (Nx-GH), calcitriol (Nx-D), or both hormones (Nx-GH + D). (Modified from Sanchez et al.73; Reprinted by permission of Blackwell Science, Inc.)
MANAGEMENT OF GROWTH RETARDATION
Impaired linear growth persists despite correction of acidosis, anemia, malnutrition, treatment with dialysis, and following successful renal transplantation. There is considerable interest therefore in therapeutic interventions that can prevent or reverse growth retardation in children with CRF. As such, rhGH and calcitriol or 1,25-dihydroxyvitamin D have been widely utilized for the treatment of growth retardation in pediatric patients with renal disease.74-77
Chesney et al. emphasized the significance of renal bone disease to linear growth more than 20 years ago, when they demonstrated improvement in growth velocity and bone disease during treatment with daily doses of calcitriol in children with CRF and secondary hyperparathyroidism.77 Similarly, Chan et al. reported enhanced growth during daily calcitriol therapy in children with CRF and ESRD.78, 79 Although the observations were made in a small number of children, these findings have provided the basis for the widespread use of calcitriol in pediatric patients with CRF. Subsequent studies, however, failed to demonstrate consistent improvement in linear growth during vitamin D therapy.80-82 Moreover, linear growth may be diminished in prepubertal patients with adynamic bone after intermittent calcitriol therapy.83
Calcitriol and other vitamin D analogs have been shown to be effective in suppressing PTH secretion in adults and children with secondary hyperparathyroidism, and several studies have described correction of the biochemical, radiographic, and histologic abnormalities associated with this disorder. High turnover skeletal lesions persist, however, in a proportion of children despite treatment with daily oral doses of calcitriol.63, 84 Consequently, high intermittent doses of calcitriol have been used to reverse the skeletal lesions of severe secondary hyperparathyroidism.85-89 However, recent data in pediatric patients undergoing continuous cycling peritoneal dialysis (CCPD) have shown markedly decreased bones formation rates and the development of adynamic lesion in a substantial proportion of patients during intermittent calcitriol therapy71, 88 (Fig. 5). Moreover, biochemical markers such as serum ALP and PTH levels may not necessarily reflect changes in bone formation and turnover nor correspond to the skeletal lesion during intermittent oral calcitriol therapy.71, 88 Therefore, serum PTH levels should be monitored regularly during intermittent calcitriol therapy, and the dose of calcitriol should be lowered or withheld when serum PTH levels fall to values four to five times the upper limit of normal to prevent oversuppression of PTH secretion and bone formation rates in these patients.
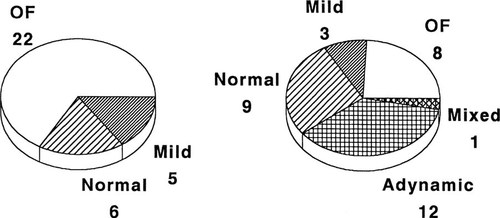
Changes in histologic subtypes of renal osteodystrophy during intermittent calcitriol therapy. Abbreviations are: OF osteitis fibrosa; mild, mild secondary hyperparathyroidism; normal, bone formation rate within the normal range. (Modified from Salusky et al.71; Reprinted with permission of Blackwell Science, Inc.)
The increasing prevalence of adynamic lesions of renal osteodystrophy and the known antiproliferative effects of calcitriol on different chondrocyte cell lines have raised concern about its continued generalized use in pediatric patients with CRF.90, 91 Indeed, diminished linear growth has been demonstrated in children with bone biopsy proven secondary hyperparathyroidism during intermittent calcitriol therapy; the greatest reductions in height were found in children who developed adynamic bone during treatment with large intermittent doses of calcitriol (Fig. 6).83 In addition, patients with low turnover bone lesions are particularly prone to develop hypercalcemia during vitamin D therapy. While high serum calcium levels per se have not been shown to increase mortality risk, Ca × PO4 product levels > 72 were associated with a significantly higher risk of death in adult hemodialysis patients.92 Moreover, preliminary observations in our laboratory showed decreased linear growth and alterations in growth plate morphology in rats given calcium supplementation to induce biochemical changes consistent with adynamic bone.93 Although little is known regarding the long-term consequences of low-turnover bone disease, these findings represent important potential implications on the indications for vitamin D therapy in children with CRF.
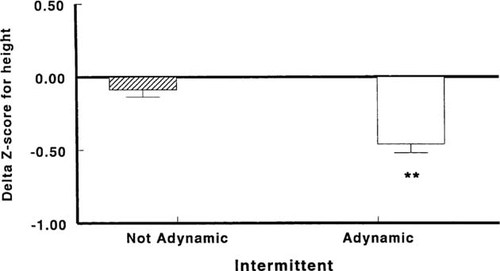
Greater decline in Z scores for height was seen in patients who developed adynamic bone lesions compared with those with normal or increased rates of bone formation, **p < 0.001. (From Kuizon et al.83; Reprinted by permission of Blackwell Science, Inc.)
Fifteen years ago, Mehls and Ritz first reported the beneficial effects of supraphysiologic doses of porcine GH on linear growth in uremic animals.94 A subsequent study using rhGH demonstrated improvements in weight gain, food utilization ratio, and growth velocity in uremic rats, confirming previous observations.95 These data suggest that the GH resistance state in renal failure may be reversed by exogenous GH treatment. Following these promising results, clinical trials were initiated to evaluate the efficacy and safety of rhGH therapy in growth retarded children with CRF.
Initial short-term studies in children with growth failure associated with CRF demonstrated accelerated linear growth during treatment with rhGH.74, 96-100 Data from subsequent placebo-controlled studies in the United States and Europe confirmed the results of previous uncontrolled rhGH trials.75, 101 A multicenter randomized double-blind placebo-controlled study in children with CRF demonstrated marked improvement in linear growth in the rhGH-treated group compared with the placebo-control group. Bone age advanced but it correlated with the advancement in chronologic age. In addition, serum IGF-I levels were elevated during rhGH therapy and this may be a contributing factor to the efficacy of exogenous GH.74, 99, 100 By augmenting the circulating levels of IGF-I, supraphysiologic doses of GH raises free IGF-1 levels, thereby overcoming peripheral resistance to the actions of GH.
The increment in height was greater in the initial year of rhGH treatment but the SDS for height continued to improve during the second year.75 Tonshoff et al.102 and Van Es et al.103 likewise reported reduced growth response during the subsequent year of rhGH therapy but the growth velocity during the second year of treatment was still significantly better compared with pretreatment period.102, 103 On long-term treatment with rhGH, continued improvement in SDS for height was observed, thus enhancing the potential for attaining the target final adult height.75
Although marked improvement in linear growth during rhGH therapy has been documented in children with CRF on conservative management, a less favorable response has been observed in those receiving maintenance dialysis and post-transplantation.97, 102-104 The reasons for the different responses to therapy with rhGH are not very clear at the present time. Differences in GH insensitivity, concomitant therapy with calcitriol, as well as concurrent steroid therapy in transplant recipients have been suggested.105
In addition to its actions on the growth plate, GH is well known to have significant effects on bone and mineral metabolism.106-108 Whether GH modifies the effects of calcitriol on bone formation and turnover is not known. Preliminary findings of our prospective randomized study in 22 pediatric patients undergoing CCPD demonstrate increased serum concentrations of phosphorus, ALP, PTH, and IGF-1 during combined rhGH and calcitriol therapy, whereas corresponding levels declined in control subjects given calcitriol alone. This disparity was seen despite progressive increases in the dose of calcitriol in both groups throughout the study period.109 These data suggest that GH modifies the skeletal response to calcitriol therapy. Biochemical indices of bone metabolism should be monitored regularly during rhGH treatment and the need for calcitriol determined based on these results. However, the optimal dose of calcitriol during rhGH therapy remains to be determined in children undergoing maintenance dialysis. Further studies are needed to determine the potential interactions between GH and calcitriol on linear growth within the spectrum of renal osteodystrophy, particularly since both hormones are extensively utilized in the management of children with renal failure.
Acknowledgements
This work was supported in part by USPHS grants DK-35423, RR-00865, and DK53655–01, UCLA Child Health Research Center P30 HD34610, Hilda Gershon Sugarman Young Investigator Grant of the National Kidney Foundation, and by funds from the Casey Lee Ball Foundation.




