Monoclonal Antibodies Against Synthetic Peptides Corresponding to the Extracellular Domain of the Human Ca2+ Receptor: Characterization and Use in Studying Concanavalin A Inhibition
Abstract
We generated monoclonal antibodies against two synthetic peptides corresponding to residues 214–235 (ADD) and 374–391 (LRG) of the human Ca2+ receptor (hCaR) extracellular domain (ECD). Although both antibodies reacted well with their respective immunizing peptides on peptide-based enzyme linked immunosorbent assay, ADD was much more strongly reactive with the hCaR than LRG in assays such as immunoblots done under denaturing conditions. The opposite pattern was seen in flow cytometry analysis of the native receptor stably expressed in transfected 293 cells. We speculate that the ADD epitope is unexposed in the native receptor while the reverse is true for the LRG epitope. The ability to measure cell surface expression of the hCaR under native conditions using flow cytometry with the LRG monoclonal allowed us to study the basis for Concanavalin A (Con A) inhibition of CaR activation by Ca2+. Our studies show that Con A inhibition is partially accounted for by receptor internalization but, additionally, Con A may prevent Ca2+ stimulation directly by binding to carbohydrate residues in the receptor ECD.
INTRODUCTION
Ca2+ RECEPTORS (CaR) are involved in regulation by extracellular Ca2+ of parathyroid hormone (PTH) secretion and urine Ca2+ excretion.1 cDNAs that encode these receptors have been cloned from bovine2 and human3 parathyroid, and rat kidney4 and brain.5 Cloning of CaR cDNAs has allowed the generation of polyclonal antisera against synthetic peptides which correspond to the cDNA-predicted protein sequence. Such polyclonal antisera have been used in immunoblot, immunocytochemical, and immunohistochemical studies of the CaR and have provided substantial information on the expression and distribution of the receptor.5-10 While polyclonal antisera are useful for many purposes, monoclonal antibodies have the theoretical advantage of an unlimited supply of an invariant reagent. We therefore attempted to generate monoclonal antibodies against the human CaR (hCaR). Also, since no antibodies have been described in the literature as being useful for measurement of the native CaR on intact cells, we used flow cytometry to test candidate monoclonal antibodies for such binding activity. Given the lack of a direct ligand binding assay for the CaR, an antibody capable of measuring cell surface expression of the native CaR would be very useful.
The human Ca2+ receptor (hCaR) cDNA encodes a 1078 amino acid protein with a putative signal peptide, a large (∼600 residue) aminoterminal extracellular domain (ECD), an integral membrane domain with seven membrane-spanning α-helices, and an ∼200 residue carboxy-terminal intracellular domain.3 We synthesized a series of peptides that correspond to sequences putatively within the hCaR ECD and used these to generate polyclonal antisera in rabbits. The two peptides yielding the highest titer polyclonal antisera were selected as immunogens for the generation of mouse monoclonal antibodies. We describe here the generation and characterization of these two monoclonal antibodies and their use in studying the basis for inhibition by Concanavalin A (Con A) of receptor stimulation by extracellular Ca2+.
MATERIALS AND METHODS
Monoclonal antibody production
Peptides with the sequences ADDDYGRPGIEKFREE AEERDI (ADD) and LRGHEESGDRFSNSSTAF (LRG) which represent amino acid residues 214–235 and 374–391, respectively, of the hCaR ECD were synthesized by fluoro-methoxy-carbonyl (FMOC) chemistry. Peptides were conjugated to keyhole limpet hemocyanin as previously described,11 and used for immunization of mice. The immunization and generation of monoclonal antibodies were done at Perimmune Inc. (Gaithersburg, MD, U.S.A.). To measure immunoreactivity of hybridoma supernatants during the cloning procedure, we used a peptide-based enzyme linked immunosorbent assay (ELISA). Peptides were conjugated to ovalbumin at a molar ratio of 6:1 using disuccinimidyl suberate (DSS) (Pierce Chemical Company, Rockford, IL, U.S.A.). For ELISA screening, 0.1 ml per well of peptide-ovalbumin conjugate at a concentration of 1 μg/ml in 0.1 M carbonate buffer, pH 9.1, was coated overnight at 4°C on polystyrene 96-well microtiter plates (Corning, Corning, NY, U.S.A.). Plates were washed three times with phosphate-buffered saline, containing 0.05% Tween-20 (PBS-T), and then blocked at room temperature for 1 h with 200 μl of PBS-T containing 1% bovine serum albumin (BSA). After an overnight incubation at 4°C with 0.1 ml/well of undiluted hybridoma supernatant, plates were washed three times with PBS-T. Second antibody incubations using 0.1 ml of goat anti-mouse IgG antibody conjugated to alkaline phosphatase (Kierkegaarde & Perry, Gaithersburg, MD, U.S.A.) at a 1:1000 dilution in PBS-T with 1% BSA were for 2 h at room temperature, followed by three washes with PBS-T. ELISA plates were read at a wavelength of 405 nM after a 15 minute incubation at room temperature using 100 μl/well of 1 mg/ml of α-napthol phosphate in 0.625 M 2-amino-2-methyl-1-propanol, pH 10.25 (Sigma, St. Louis, MO, U.S.A.) as a substrate solution. Those hybridomas with supernatants exhibiting the greatest optical densities were selected for subcloning and eventual ascites production (data not shown). Mouse monoclonal antibodies were isolated from ascites fluid by protein A affinity purification.
Stable transfection of HEK-293 cells with hCaR and rat CaR cDNAs
The full-length human parathyroid CaR cDNA3 and full-length rat kidney CaR cDNA4 were each subcloned into the pCEP4 expression vector (Invitrogen, San Diego, CA, U.S.A.). In addition, a mutant form of the hCaR cDNA in which cysteine 598 was changed to serine and isoleucine 599 was changed to a stop codon was also subcloned into pCEP4. HEK-293 cells were transfected using CaPO4 and selected in 200 μg/ml hygromycin. Resistant colonies were subcloned and screened for CaR expression by a solution hybridization assay.12 The clones selected for the present studies either express high levels of the full-length hCaR (clone 7) or rat CaR (clone 6) based on immunoblot analysis of cell membranes or secrete immunoreactive hCaR ECD protein into the medium (clone 32; see Results). These clones were routinely cultured in Dulbecco's modified Eagle's medium (DMEM; Life Technologies Inc., Gaithersburg, MD, U.S.A.) supplemented with 10% fetal bovine serum, 1% glutamine, 1% penicillin and streptomycin, and 200 μg/ml hygromycin at 37°C in a 5% CO2 environment.
ECD ELISA
ECD purified from the conditioned medium of clone 32 cells (P.K. Goldsmith et al., unpublished data) was used for ELISA and immunoblot analysis. For ECD ELISA, 96-well polystyrene microtiter plates were coated overnight at 4°C with 200 μl/well of ECD at 1 μg/ml in 0.1 M carbonate buffer, pH 9.1. Plates were washed three times with PBS-T and blocked for 1 h at room temperature with 200 μl of PBS-T with 1% BSA, followed by overnight incubation of 200 μl/well of purified antibodies appropriately diluted in PBS-T with 1% BSA at 4°C. Plates were washed three times with PBS-T followed by incubation with 200 μl/well of second antibody (same as peptide ELISA) at a dilution of 1:2000 for 2 h at 37°C. Plates were washed three times with PBS-T and developed with substrate solution (as for peptide ELISA) for 30–60 minutes before being read.
Immunoblots
Membranes were prepared from wild-type and stably transfected HEK-293 cells using the method described by Bai et al.9 Protein concentrations were determined using the BCA protein method (Pierce Chemical Co.). All membrane preparations and detergent extract samples were stored at −70°C. For immunoblot analysis, proteins were separated under denaturing conditions on 10% polyacrylamide gels containing 0.1% sodium dodecyl sulfate (SDS) or on 5–15% gradient polyacrylamide gels containing 0.1% SDS.13 To prevent temperature-induced receptor aggregation, membrane extracts were treated in sodium dodecyl sulfide (SDS)-containing buffer for 30 minutes at room temperature prior to sample loading and electrophoresis. Following overnight electrophoretic transfer to nitrocellulose membranes at 150 mA constant current, the nitrocellulose membranes were blocked with 5% horse serum in 50 mM Tris-HCl, pH 8.0, 500 mM NaCl, 0.1% Tween-20 (TBST) for 1 h at room temperature. All first and second antibodies were diluted in TBST containing 5% horse serum and 0.05% thimersol as a bacterial growth inhibitor. First antibody incubations were overnight at room temperature using the concentrations listed in the figure legends; second antibody incubations were for 2 h using 1 μg/ml of goat anti-mouse immunoglobulin G (IgG) conjugated with horseradish peroxidase (Kierkegaarde & Perry). Development of the blots was with 4-chloronaphthol as previously described.11
Immunohistochemistry
Bovine parathyroid glands or rat thyroid glands were sectioned at 10 μM on a cryostat and thaw mounted onto gelatin-coated glass slides. The sections were fixed for 10 minutes in 4% formaldehyde in Tris buffered saline (TBS: 0.9% NaCl and 50 mM Tris, pH 7.4), rinsed in TBS, and pretreated with DAKO peroxidase blocking reagent and DAKO protein block serum-free solution (DAKO Co., Carpinteria, CA, U.S.A.). Two micrograms per milliliter of ADD antibody diluted in TBS + 1% BSA was applied to the sections, and they were incubated in a damp chamber at 4°C overnight. The slides were rinsed in TBS containing 1% Triton-X and antibody binding was detected using the Vectastain Elite ABC kit according to the manufacturer's directions (Vector Laboratories, Inc., Burlingame, CA, U.S.A.). This system uses a biotinylated horse anti-mouse second antibody, avidin-conjugated horseradish peroxidase, and diaminobenzidine tetrahydrochloride as the peroxidase substrate. Stained sections were then dehydrated and coverslips applied. For the antigen preabsorption control, 2 μg/ml ADD antibody was preincubated with 4 ng/ml ADD peptide at 4°C overnight prior to applying it to the sections, and the assay proceeded as previously described. A “no first antibody” negative control was performed by incubating sections overnight in TBS + 1% BSA and processing with the Vectastain Elite kit as described above.
Measurement of phosphoinositide hydrolysis
Phosphoinositide (PI) hydrolysis was measured in wild type and transfected HEK 293 cells grown for 24 h at a cell density of 0.5 to 1 million cells per well in 24-well culture plates in supplemented DMEM media without antibiotics. The cells were then incubated with 1 ml of supplemented DMEM media containing 3.0 μCi3H-myo-inositol/ml (DuPont Co., NEN Research Products, Boston, MA, U.S.A.) for another 24 h. All subsequent treatments were also performed at 37°C in the culture plates. Cells were preincubated for 45 minutes with 1 ml of PI buffer: 109 mM NaCl, 5 mM KCl, 5.6 mM glucose, 0.4 mM MgCl2, 20 mM LiCl in 25 mM PIPES buffer, pH 7.2, containing 0.5 mM CaCl2, and when necessary, the appropriate concentration of the lectin Con A (United States Biochemical Corp., Cleveland, OH, U.S.A.). After gentle aspiration of the PI buffer, cells were incubated for another 30 minutes with 0.5 ml of PI buffer containing the concentrations of agonists and/or inhibitors indicated in the figure legends. Reactions were terminated by the addition of 1 ml of acid methanol (165 μl of concentrated HCl in 120 ml of methanol). Total phosphoinositides were determined by liquid scintillation counting of methanolic extracts washed over 0.33 ml columns of Dowex-1-X8 (formate form; BioRad, Richmond, CA, U.S.A.) with formate containing buffers.14
Flow cytometry
Flow cytometry was performed using an ELITE fluorescence cell sorter (Coulter Corp., Miami, FL, U.S.A.). First antibody incubations were performed at 4°C for 1 h with ADD and LRG antibodies diluted in 0.5 ml of supplemented DMEM media using wild-type and stably transfected HEK 293 cells at concentrations of 1–2 million cells/ml. Cells were subsequently washed twice with 1.5 ml of Dulbecco's phosphate buffer without calcium and magnesium (DPBS) and incubated with 0.5 ml of supplemented DMEM media containing a 1:100 dilution of reconstituted fluorescein-conjugated goat anti-mouse IgG antibody (FITC-antibody; Kierkegaarde & Perry) for 1 h at 4°C. Cells were washed twice with 1.5 ml DPBS and resuspended at their starting concentrations in 2% paraformaldehyde, PBS, pH 7.4 for flow cytometry. Mean fluorescence was measured on the gated live cell population for each experiment.
RESULTS
Synthetic peptides were used to generate two monoclonal antibodies, ADD and LRG, which were purified from ascites fluid and evaluated for relative reactivity by two different types of ELISA. Figure 1 shows a comparison of the reactivities of serial dilutions of the ADD and LRG monoclonal antibodies against their cognate ovalbumin-conjugated peptides. ADD shows approximately 2-fold greater reactivity than LRG in peptide-ELISA. The same dilutions of antibodies were tested for reactivity against the purified hCaR ECD by ELISA. Note that the ECD protein encompasses the full sequence of both synthetic peptides used to generate ADD and LRG monoclonal antibodies. The ECD ELISA results (Fig. 2) are strikingly different from those seen in Fig. 1; ADD reactivity against the ECD is of the same order of magnitude as its cognate peptide reactivity, whereas LRG reactivity against the ECD is barely measurable even at concentrations above 100 ng/ml.
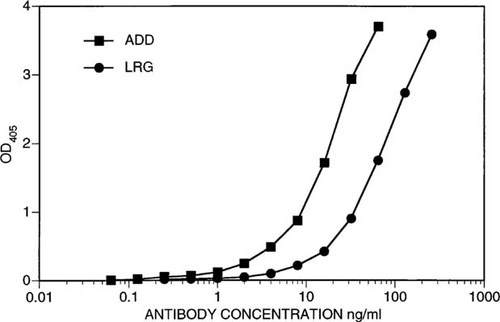
Reactivity of increasing concentrations of ADD and LRG monoclonal antibodies in an anti-peptide ELISA. Cognate peptides cross-linked to ovalbumin were used as described in Materials and Methods as solid phase antigens. Serial 1:2 dilutions in triplicate of each antibody were incubated overnight at 4°C and plates developed as described in Materials and Methods. The highest concentrations of monoclonal antibody used per well were 256 ng/ml for LRG antibody and 64 ng/ml for ADD antibody.
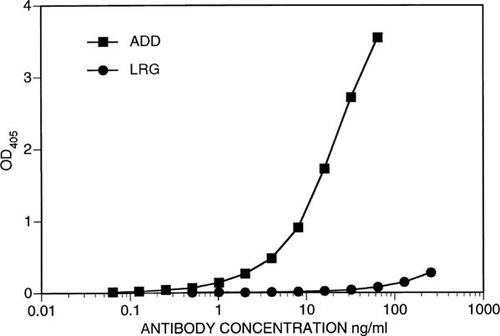
Reactivity of increasing concentrations of ADD and LRG monoclonal antibodies in an anti-ECD ELISA. Purified ECD protein was used as solid phase antigen, and ELISA performed as described in Materials and Methods. Antibody concentrations are as in Fig. 1.
Figure 3 shows a comparison of ADD and LRG reactivity against the hCaR ECD by immunoblot analysis. The secreted ECD is predicted to contain 579 residues after cleavage of the signal peptide and should give a protein in the 60 kD range, but the CaR ECD contains multiple glycosylation sites. Both antibodies stain broad bands in the 90 kD range which is consistent with a heavily glycosylated ECD. A broad doublet in the 60 kD range is seen with ADD. We believe this represents proteolytic fragments arising during ECD purification. As seen in Fig. 3, lower quantities of ADD antibody give substantially greater intensity staining than higher quantities of LRG antibody.
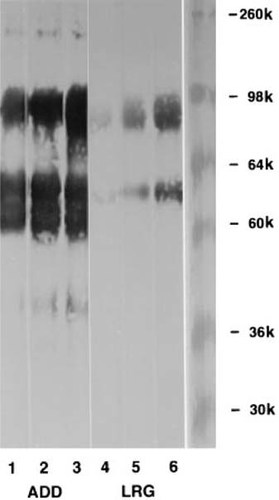
Immunoblot comparison of ADD and LRG reactivity to purified hCaR ECD. ECD protein, 1.7 μg/lane, was loaded. Individual lanes were cut out of the nitrocellulose membranes, and each strip was incubated with serial 1:4 dilutions of each antibody. Lanes 1–3, ADD at 0.156 μg/ml, 0.625 μg/ml, and 2.5 μg/ml, respectively. Lanes 4–6, LRG at 5 μg/ml, 20 μg/ml, and 80 μg/ml, respectively.
Figure 4 shows the immunoblot reactivity of both antibodies against 1% Triton X-100 extracts of membranes from wild-type HEK 293 cells which do not express the hCaR, clone 7 HEK 293 cells which stably express the hCaR, clone 6 HEK 293 cells which stably express the rat CaR, and bovine parathyroid glands. As seen in lanes in Fig. 4 containing the wild-type HEK 293 cell membrane extracts, nonspecific reactivity is minimal for both antibodies. As with the ECD immunoblot, ADD is very much more reactive than LRG against the remaining three membrane extracts. In clone 7 membrane extracts, ADD stains three major bands at approximately 150, 130, and 115 kD. These have been shown to represent the mature, fully glycosylated, incompletely processed, and nonglycosylated forms of the receptor, respectively.9 Staining of higher molecular size (>250 kD) material is also apparent and may represent aggregated or dimerized receptor.9 ADD stains bands of similar, but not identical, size in clone 6 and bovine parathyroid membrane extracts.
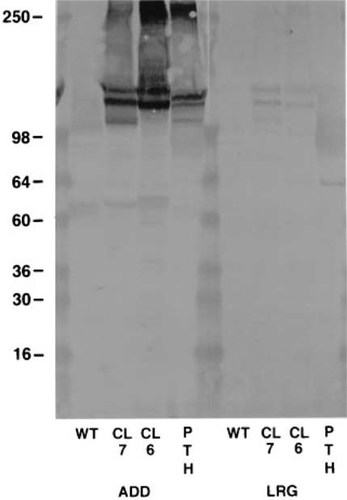
Immunoblot comparison of ADD and LRG reactivity to membrane extracts. One percent Triton-100 extracts, 100 μg/lane of membranes from wild-type HEK 293 cells (WT), clone 7 HEK 293 cells expressing hCaR (CL 7), clone 6 HEK 293 cells expressing rat CaR (CL 6), and bovine parathyroid glands (PTH) were loaded. Blots containing all four lanes with each membrane sample were incubated overnight with 1 μg/ml of ADD (left) or 40 ug/ml of LRG (right) antibodies. The positions of molecular size markers run simultaneously are labeled on the left.
Differences in size of immunoreactive bands and in relative intensity of these bands in the different membrane preparations likely reflect differences in processing of the receptor in different cell types as well as species differences related to number of glycosylation sites. For LRG, faint but definite bands at ∼150, 130, and 115 kD are seen with the clone 7 membrane extract, but in clone 6 and bovine parathyroid membrane extracts such bands are barely detectable. A prominent band at ∼64 kD seen with LRG in the bovine parathyroid membrane extracts could represent a proteolytic fragment of the receptor, but nonspecific reactivity with another parathyroid membrane protein cannot be excluded.
Figure 5 shows the immunohistochemical staining of bovine parathyroid (Fig. 5A) and rat thyroid (Fig. 5B) sections using the ADD antibody. A pattern of chief cell staining is seen in the parathyroid gland and of C cell staining in the thyroid gland. These staining patterns are the same as previously reported for immunohistochemistry using polyclonal antibodies to the CaR.15 No staining of the sections was seen in either the “no first antibody” (Fig. 5C) or peptide preabsorbed antibody (Fig. 5D) negative control sections. Immunohistochemistry using the LRG antibody did not produce a reaction product in either bovine parathyroid or rat thyroid sections (data not shown).
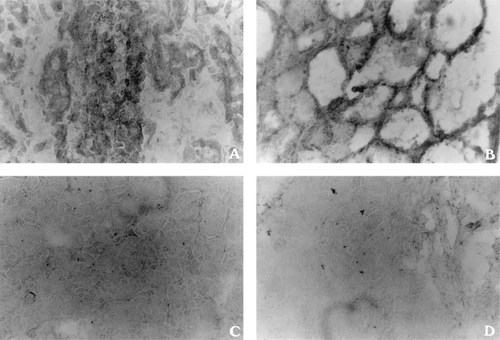
CaR immunohistochemistry using the ADD antibody. Bovine parathyroid and rat thyroid gland sections were prepared and immunohistochemistry performed as described in Materials and Methods. (A) Bovine parathyroid section stained with 2 μg/ml ADD (100× magnification); rat thyroid section stained with 2 μg/ml ADD (×50 magnification); (C) bovine parathyroid section, negative control omitting ADD antibody (×100 magnification); (D) bovine parathyroid section, negative control, 2 μg/ml ADD antibody preabsorbed with 4 ng/ml ADD peptide (×100 magnification).
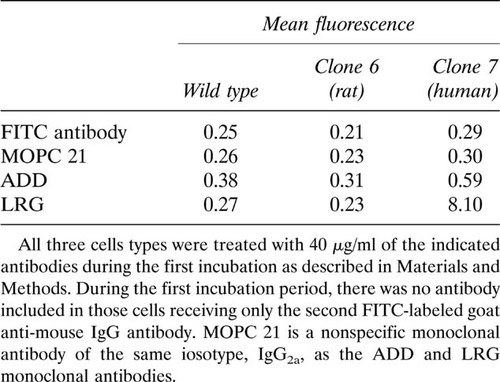
To assess the ability of ADD and LRG monoclonal antibodies to react with the native CaR expressed on the cell surface, the antibodies were evaluated by flow cytometry using clone 7 and clone 6 cells stably expressing the human and rat CaRs, respectively. The results of an experiment comparing the mean fluorescence of wild-type, clone 7 HEK 293 cells, and clone 6 HEK 293 cells, as measured by flow cytometry after incubation with ADD and LRG monoclonal antibodies, are shown in Table 1. Cells were incubated only with FITC-labeled goat anti-mouse antibody as a control to determine levels of autofluorescence for gating on live cells. Cells were also incubated with MOPC 21 myeloma protein as a nonspecific antibody control. As seen in Table 1, the mean fluorescence of all three cell types treated with only FITC second antibody is similar and not different from the mean fluorescence of all three cell types when incubated with MOPC 21. The mean fluorescence of cells incubated with ADD antibody was somewhat greater in each cell type than seen with the controls; the clone 7 cells gave a slightly higher mean fluorescence. With LRG, however, the mean fluorescence of clone 7 cells was nearly 14-fold higher than that of cells incubated with ADD. Note that there appears to be no demonstrable LRG reactivity against clone 6 cells.
To quantitate further ADD and LRG reactivity on flow cytometry, we incubated clone 7 cells with serial dilutions of both antibodies. It is clearly evident (Fig. 6A) that LRG reactivity is much greater than that for ADD. Figure 6B shows the relative cell distributions measured by flow cytometry of clone 7 cells incubated with 160 μg/ml of either ADD (peak 2) or LRG (peak 3) compared with that of cells treated with only the FITC-labeled second antibody (peak 1). The mean fluorescence for peak 2 is 0.43 compared with 0.21 for peak 1, which indicates that even at this elevated antibody concentration there is little ADD binding. The mean fluorescence of peak 3 is 10.6, indicating that LRG reactivity at this antibody concentration is nearly 25 times that for ADD.
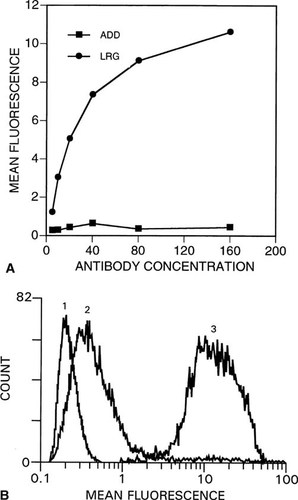
(A) Binding of increasing concentrations of ADD and LRG antibodies to clone 7 HEK 293 cells measured by flow cytometry. Cells were treated as described in Materials and Methods with decreasing serial 1:2 dilutions of ADD and LRG monoclonal antibodies. (B) Distributions of fluorescence of clone 7 HEK 293 cells incubated with 160 μg/ml of ADD antibody (peak 2) or LRG antibody (peak 3) followed by FITC second antibody compared with FITC second antibody only (peak 1).
The ability to quantitate cell surface expression of the hCaR by using flow cytometry with LRG monoclonal antibody provided us the opportunity to study the basis for CaR inhibition by Con A. As shown in Fig. 7, Ca2+ stimulates PI hydrolysis in transfected clone 7 HEK 293 cells in a concentration-dependent manner. No stimulation by Ca2+ is observed in wild-type HEK 293 cells (data not shown), thus this action is mediated by the hCaR stably transfected in clone 7 cells. The higher Ca2+ concentrations required for stimulation of PI hydrolysis in clone 7 cells (half-maximal effect at about 4.5 mM) compared with those effective in inhibiting PTH release in parathyroid cells (half-maximal effect at about 1.2 mM) have been noted by others10, 16 and likely reflect the different parameters being measured and the nonphysiologic context of the CaR transfected into HEK 293 cells. Ca2+ stimulation of PI hydrolysis is inhibited by preincubation of cells with 400 ug/ml of Con A (Fig. 7). Lower concentrations of Con A cause proportionately smaller degrees of inhibition at any given Ca2+ concentration, but Con A does not inhibit PI hydrolysis stimulated by fluoride (data not shown). Con A, 400 μg/ml, shifts the Ca2+ dose response to the right and leads to approximately 50% inhibition even at the highest concentration of Ca2+ tested (16 mM). Six millimolar Ca2+ stimulation of PI hydrolysis is 86% inhibited by preincubation with 400 μg/ml Con A (Fig. 8). When α-methyl mannoside (αmm), a known inhibitor of Con A binding, is added at 10 or 50 mM with 6 mM Ca2+ to cells preincubated with Con A, it is only partially able to reverse Con A inhibition. Including αmm in the preincubation with Con A prior to stimulation with 6 mM Ca2+ is more effective in preventing Con A inhibition (50 mM > 10 mM αmm), but does not completely block it.
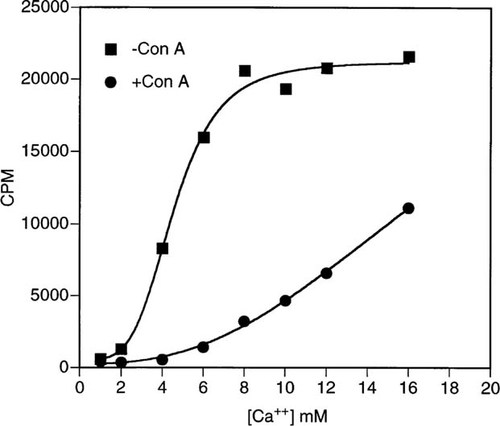
Concentration-dependent stimulation of phosphoinositide hydrolysis by Ca2+ in clone 7 HEK 293 cells. Cells were preincubated as described in Materials and Methods with or without 400 μg/ml of Con A.
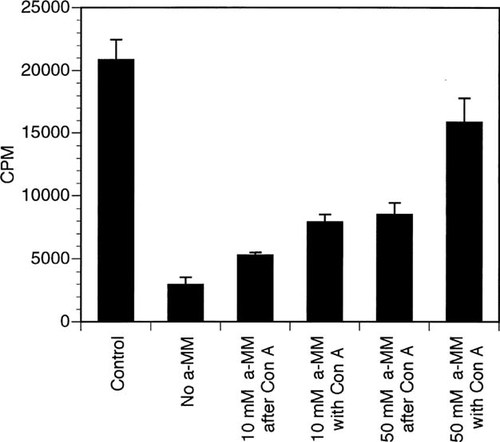
Reversal of Con A inhibition by α-methyl mannoside. All cells except those whose value is shown in the bar labeled “Control” were preincubated for 45 minutes with 400 μg/ml Con A. Following this, all cells were incubated with 6 mM Ca2+ and PI hydrolysis measured as described in Materials and Methods (results are shown as counts per minute and are averages ± SE of triplicate wells). α-methyl mannoside (aMM) was added at 10 or 50 mM final concentration either with or after Con A preincubation as indicated in labels.
By using flow cytometry with the LRG antibody to measure the mean fluorescence of clone 7 HEK 293 cells as an index of cell surface CaR expression, we tested the hypothesis that Con A inhibition of CaR stimulation is due to lectin-promoted receptor internalization. We allowed Con A to bind to cells at either 4 or 37°C during a 1 h of preincubation prior to the flow cytometry protocol described in the Materials and Methods section. At 4°C, no difference in mean fluorescence was observed between clone 7 cells incubated with or without Con A (data not shown) consistent with a lack of internalization at 4°C. To exclude the possibility that LRG antibody could be sterically hindered from binding to its epitope by bound Con A after pretreatment, cells were preincubated at 4°C with decreasing concentrations (400–12.5 μg/ml) of Con A followed by a fixed amount (40 μg/ml) of LRG antibody during the first incubation step of the flow cytometry protocol. There was no difference in mean fluorescence at any concentration of Con A preincubation, indicating that Con A did not interfere with the binding of the LRG antibody (data not shown). Preincubation with Con A at 37°C (Fig. 9) leads to different results than those observed at 4°C. Con A preincubation (Fig. 9C) causes a shift to the left in mean fluorescence, 2.91 compared with 5.72 for cells preincubated without Con A (Fig. 9B). When cells are preincubated with αmm in addition to Con A (Fig. 9D), mean fluorescence shifts to the right with a value of 6.21. The mean ± SE for mean fluorescence in three separate flow cytometry experiments performed at 37°C were: (a) LRG antibody only = 6.99 ± 1.59; (b) 400 μg/ml Con A preincubation = 3.84 ± 0.59; (c) 400 μg/ml Con A + 10 mM αmm preincubation = 7.49 ± 0.57.
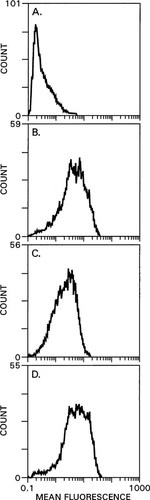
Con A effects on cell surface expression of hCaR in clone 7 HEK 293 cells examined by flow cytometry with LRG monoclonal antibody. Cells were preincubated for 1 h at 37°C before flow cytometry analysis as described in Materials and Methods. (A) FITC-labeled second antibody only. (B) LRG antibody followed by FITC-labeled second antibody. (C) Preincubation with 400 μg/ml Con A followed by LRG antibody and FITC-labeled second antibody. (D) Preincubation with 400 μg/ml Con A and 10 mM α-methyl mannoside followed by LRG antibody and FITC-labeled second antibody.
DISCUSSION
In an effort to obtain an antibody capable of quantitating cell surface expression of the native CaR, we generated monoclonal antibodies against two synthetic peptides corresponding to different regions of the hCaR ECD. We selected the ADD and LRG peptides as immunogens based on predicted antigenicity using the PEPTIDESTRUCTURE algorithm (GCG package, University of Wisconsin, Madison, WI, U.S.A.) which takes into account measures of hydrophilicity, chain flexibility, and probability of surface exposure.
Since the three-dimensional structure of the CaR ECD is unknown, we cannot ascertain the location of either the ADD or LRG peptides within the tertiary structure of the native receptor ECD protein. The PEPTIDESTRUCTURE secondary structure prediction algorithm (according to either Chou-Fasman or Garnier) suggests that much of the ADD peptide forms an α-helix, whereas the LRG peptide is predicted to form a flexible turn. Based on lack of binding on flow cytometry, we speculate that the ADD monoclonal antibody reacts with a linear epitope that is unavailable in the native receptor protein. Although not useful for measuring expression of native CaR at the cell surface, ADD is a very useful reagent for both immunoblots and immunohisto- and immunocytochemistry performed under conditions that allow antibody access to its epitope.
Note that human,3 bovine,2 and rat4, 5 CaR all contain the identical ADD peptide sequence, thus the ADD monoclonal should be equally reactive against the receptor from all three species (see Figs. FIG. 4., FIG. 5.). This enables the use of this reagent for CaR studies in each species, and likely other mammals as well, presuming equivalent conservation of this epitope. The peptide used to generate the ADD monoclonal antibody (214–235) is nearly identical to one (215–237) used to generate a polyclonal antiserum, “4641,” already used extensively in immunoblot and immunohistochemistry studies of the CaR.6-10 The advantage of the ADD monoclonal compared with polyclonal antisera such as 4641 is its theoretically unlimited supply and its lack of bleed-to-bleed variability.
Despite the use of a linear peptide immunogen to generate the LRG monoclonal antibody, this antibody is capable of recognizing the native receptor ECD and indeed is poorly reactive with denatured forms of the protein. We speculate that the LRG synthetic peptide is capable of assuming a conformation similar to the conformation of the corresponding region of the native receptor, and that the LRG monoclonal antibody selected shows specificity for this conformation rather than a simple linear epitope. The predicted secondary structure of this region, a flexible turn, is consistent with but certainly does not prove this hypothesis. LRG reactivity on peptide ELISA could reflect the ability of the ovalbumin-conjugated peptide used for ELISA to mimic this conformation. Under denaturing conditions, e.g., immunoblot, this conformation as well as LRG reactivity would be lost. The pattern of ADD and LRG reactivity (ADD ≫ LRG) on ECD ELISA performed under nondenaturing conditions deserves comment. Although not denatured, immobilization of the ECD for ELISA may alter native structure sufficiently to expose the ADD epitope and obscure that for LRG. Neither the LRG nor the ADD monoclonal showed functional effects (stimulation of PI hydrolysis or inhibition of Ca2+-stimulated PI hydrolysis) when tested on clone 7 cells (P.K. Goldsmith and G. Fan, unpublished observations). This is not surprising for ADD which does not recognize the native receptor, but suggests that the epitope to which LRG binds in the native receptor is not critical for Ca2+ signal transduction.
Unlike ADD, the LRG peptide shows significant species differences in amino acid sequence. The LRG peptide derived from the hCaR (LRGHEESGDRFSNSSTAF) differs at the six underlined residues (VRSHEEGGNRLLNSSTAF) in the rat CaR and at the four underlined residues (LRGHEEGGARLSNSPTAF) in the bovine CaR. The importance of these for antibody reactivity on immunoblot is not clear given the overall low reactivity of the LRG monoclonal, but for flow cytometry there is a striking difference in reactivity between the hCaR and rat CaR. This indicates that one or more of the six differences in amino acid sequence between rat and human have a critical effect on antibody reactivity.
Prior to molecular characterization of the CaR, Brown and coworkers had shown that Con A blocks Ca2+ inhibition of cAMP accumulation, Ca2+ stimulation of PI hydrolysis, and Ca2+ inhibition of PTH release in bovine parathyroid cells.17, 18 Brown suggested that the inhibitory effect of Con A, which is known to bind to extracellular sugars, implicates an extracellular Ca2+-“sensing” receptor in mediating the effects of Ca2+ on parathyroid cells. This suggestion was substantiated by cloning of the CaR with its large, glycosylated ECD.2 However, the basis for Con A inhibition—Con A–promoted receptor internalization, steric hindrance of Ca2+ binding and receptor activation, or a combination of both—remained undefined.
We found that Con A inhibits Ca2+-stimulated PI hydrolysis in clone 7 cells stably expressing the hCaR. As Brown and coworkers had shown in bovine parathyroid cells,18 Con A does not inhibit effects of fluoride, which bypasses the CaR and directly activates G proteins coupled to the receptor. This indicates that Con A inhibition does not involve some nonspecific effect on the cell membrane. Reversal of Con A inhibition by αmm further indicates that the effects of Con A are due to binding to cell surface glycoproteins, presumably the heavily glycosylated ECD of the CaR itself. However, our experiments do not exclude effects due to binding to other cell surface carbohydrate-containing molecules.
Our observations that: (a) Con A inhibits when preincubated with cells at 37°C but not at 4°C (Con A binds at both temperatures but receptor internalization only occurs at the higher temperature) and (b) that αmm reversal of Con A inhibition was greater when the competing sugar was added with Con A during preincubation rather than after, when significant receptor internalization had presumably already taken place, suggest that a major part of the inhibition observed with Con A preincubation is caused by CaR internalization. Flow cytometric analysis with LRG monoclonal antibody provided direct evidence for loss of CaR from the cell surface after Con A preincubation at 37°C.
Con A inhibition of Ca2+-stimulated PI hydrolysis cannot, however, be explained entirely by receptor internalization. Concentrations of αmm that completely prevent receptor internalization (Fig. 9) do not completely block Con A inhibition even when added to cells with Con A during preincubation (Fig. 8). We speculate that amounts of Con A binding to the CaR insufficient to promote receptor internalization may still block Ca2+-stimulation. According to this model, Con A binding to critical carbohydrate residues in the CaR ECD would either directly impede Ca2+ binding or indirectly block Ca2+-stimulation by stabilizing an inactive conformation of the CaR ECD. Further studies on the structure of the CaR and the mechanism of Ca2+-stimulation will be necessary to test this hypothesis. The availability of the monoclonal antibodies described in this paper should facilitate these and other studies of the CaR.
Acknowledgements
We are grateful to Regina Collins for expert assistance in the culture of 293 cells, to Joyce Muthoni Njorge for assistance with flow cytometry, to Christine Dunn for assistance in the production of CaR-expressing HEK 293 cells, and to Nousheen Alasti for assistance with immunohistochemistry.