Signal Transduction of Mechanical Stimuli Is Dependent on Microfilament Integrity: Identification of Osteopontin as a Mechanically Induced Gene in Osteoblasts†
Portions of these studies were presented at the 1996 American Society for Bone and Mineral Research.
Abstract
Mechanical perturbation has been shown to modulate a wide variety of changes in second message signals and patterns of gene expression in osteoblasts. Embryonic chick osteoblasts were subjected to a dynamic spatially uniform biaxial strain (1.3% applied strain) at 0.25 Hz for a single 2-h period, and osteopontin (OPN), an Arg-Gly-Asp (RGD)-containing protein, was shown to be a mechanoresponsive gene. Expression of opn mRNA reached a maximal 4-fold increase 9 h after the end of the mechanical perturbation that was not inhibited by cycloheximide, thus demonstrating that mechanoinduction of opn expression is a primary response through the activation of pre-existing transcriptional factors. The signal transduction pathways, which mediated the increased expression of opn in response to mechanical stimuli, were shown to be dependent on the activation of a tyrosine kinase(s) and protein kinase A (PKA) or a PKA-like kinase. Selective inhibition of protein kinase C (PKC) had no effect on the mechanoinduction of osteopontin even though opn has been demonstrated to be an early response gene to phorbol 12-myristate 13-acetate (PMA) stimulation. Mechanotransduction was dependent on microfilament integrity since cytochalasin-D blocked the up-regulation of the opn expression; however, microfilament disruption had no effect on the PMA induction of the gene. The microtubule component of the cytoskeleton was not related to the mechanism of signal transduction involved in controlling opn expression in response to mechanical stimulation since colchicine did not block opn expression. Mechanical stimulus was shown to activate focal adhesion kinase (FAK), which specifically became associated with the cytoskeleton after mechanical perturbation, and its association with the cytoskeleton was dependent on tyrosine kinase activity. In conclusion, these results demonstrate that the signal transduction pathway for mechanical activation of opn is uniquely dependent on the structural integrity of the microfilament component of the cytoskeleton. In contrast, the PKC pathway, which also activates this gene in osteoblasts, acts independently of the cytoskeleton in the transduction of its activity.
INTRODUCTION
BASIC TO ALL LIVING ORGANISMS is the ability to respond to their physical environment, including electrical, chemical, temperature, light, and mechanical stimuli. While the molecular mechanisms that transduce these external stimuli into a cellular response have been extensively examined, mechanotransduction is one of the least understood. Within vertebrates, skeletal tissues serve as probably one of the best examples of the reciprocal relationship between biological function and structure,1 and skeletal cells must be responsive to their mechanical environments to meet their physiological function. Mechanical stimuli have been shown to provide necessary cues for the appropriate differentiation of secondary articular cartilage tissue of the mandibular joint surfaces during embryogenesis2 and influence the form and density of almost all bone during postnatal tissue development and maintenance of adult bone homeostasis.3-5 While in recent years there has been an increased definition of the specific biochemical responses in bone composition which are induced by mechanical stimuli, including changes in numerous second signal molecules6-11 and in the expression of various genes,12-15 the signal transduction mechanisms which mediate specific genomic effects in osteoblasts in response to a mechanical stimuli have not yet been defined.
Osteopontin initially was characterized as one of the predominant noncollagenous proteins that is accumulated in the extracellular matrix (ECM) of bone in a wide variety of vertebrates,16,17 and in several recent studies osteopontin expression was shown to be modulated in response to mechanical stimulation of bone cells in vitro.13,15,18 The expression of osteopontin is not restricted solely to skeletal tissues, but it has been shown to be expressed in many other tissues as well. It was identified as an early response gene during both T-cell18 and macrophage activation,19 and it has also been shown to be an immediate early response gene to protein kinase C (PKC) activation through phorbol ester tumor promoter treatment in a wide variety of cells.20-22 It has been identified as a serum marker of malignant cell growth and was shown to be present within metastatic tumors from a wide variety of primary tissue origins.23 Sequence analysis of opn from various species has identified a number of conserved functional domains within this protein's primary structure of which the most unique to this molecule are: its numerous (>5) phosphorylation sites,24 which are recognized by factor-independent protein kinase;25-27 its polyaspartate domain of seven to nine consecutive asp; its Arg-Gly-Asp (RGD) recognition site for integrin-mediated cell adhesion and spreading; and its chemotaxis through a non-RGD domain.28 Recently, osteopontin was also shown to have the potential to interact with a variant form of the CD44v receptor.28
The identification of the RGD sequence in the osteopontin molecule and its interaction with CD44v has led to major research efforts directed toward identifying the role that this molecule plays in cell matrix interactions and cell migration29 during normal and pathological processes. Studies have demonstrated that osteopontin promotes cellular adherence in vitro of a wide variety of cells including osteoblastic,30,31 fibroblastic,32-34 and osteoclastic35-37 cell types, and available data indicate that this protein specifically interacts with the αvβ3 integrin isotype.30,36,37 A number of hypotheses have been put forward, suggesting that osteopontin mediates osteoclast attachment to the mineralized ECM during bone resorption, thus playing an important role in this process.37,38 Other data also suggest that both osteopontin as well as other RGD-containing extracellular matrix proteins such as fibronectin and bone sialoprotein may play important roles in directing cellular migration during skeletal growth or in directing or initiating the spatial deposition of mineral in the extracellular matrix.39-41 The expression of specific RGD-containing adhesion proteins by osteoblasts and their interaction with these matrix ligands through specific cell surface integrin receptors therefore is of central importance in understanding how these cells sense and respond to both positional information within the extracellular matrix and mechanical stimuli.
The integrins are a heterodimeric family of transmembrane receptors composed of α and β subunits that bridge extracellular proteins to the cytoskeleton.42 In addition to their structural function in anchoring cells, the integrins are also intimately involved in generating intracellular signals. Such conclusions are supported by studies that demonstrate that ligation of the integrins with either antibodies against them or by binding to their ligands leads to changes in the intracellular pH, intracellular calcium levels, and the phosphorylation of tyrosines on several proteins.43,44 Cellular interaction of integrins with their ligands, receptor clustering, and/or cytoskeletal rearrangement that the receptor ligand interaction produce has been shown to lead to specific genomic effects.45 A growing body of data also suggests that the cytoskeleton itself may be important in the signal transduction process.46 While the nature of many of the transmitted signals and the downstream effects of these signals remains unidentified, one of the primary proteins that is known to be activated as a result of integrin engagement is focal adhesion kinase (FAK). Phosphorylation of FAK increases its activity and initiates a phosphorylation cascade that includes the phosphorylation of paxillin and tensin, thus FAK appears to be a prime candidate molecule which mediates the transduction of a wide variety of signals.47 A growing body of data also suggests that integrins may also be of central importance to the signal transduction mechanisms by which cells mediate their responses to mechanical stimulation.48
In the studies presented here, we demonstrate that within osteoblasts osteopontin is a mechanically responsive gene. These data demonstrate that the mechanism of mechanosignal transduction that mediates this gene's expression is unique from other known forms of signal transduction; based on the findings that microfilament structure is needed for the signal transduction process and its dependence on a tyrosine kinase and possibly a PKA-like kinase.
MATERIALS AND METHODS
Materials
All tissue culture supplies, chaetoglobosin C, cytochalasin D, colchicine, cheryelthrine, phorbol 12-myristate-13-acetate (PMA), forskolin, cycloheximide, protein A antitubulin, and anti-mouse and rabbit IgG fluorescent antibodies were from Sigma Chemical Company (St. Louis, MO, U.S.A.). Rhodamine-conjugated phalloidin was from Molecular Probes, Inc. (Eugene, OR, U.S.A.) H89 genistein and quercetin were from LC Laboratories (Woburn, MA, U.S.A.). Antibodies to chicken FAK and phosphotyrosine were from UBI (Lake Placid, NY, U.S.A.). ECL chemiluminesence detection system membranes and film were from Amersham Corp. (Arlington Heights, IL, U.S.A.). Nylon membranes for Northern blots were from Biotrans ICN Corp. (Aurora, OH, U.S.A.).
Cell culture
Osteoblasts were isolated by three sequential trypsin-collagenase treatments of 12-day-old chicken calvaria as previously described.49,50 Only the cells released from the third sequential digest were used for experimental determinations. For all experiments, 2 × 106 cells were plated per 100-mm diameter flexible membrane surface, which was coated with fibronectin as previously described.51 Cultures were grown for 2 weeks until reaching confluency in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) with media changes every 3 days. At the time that the cultures reached confluency, they were switched to BGJb media supplemented with 10% FBS. After 2 days, this media was supplemented with 10 mM β-glycerophosphate; subsequently, after an additional 2 days, this media was further supplemented with 12.5 μg of ascorbic acid. This media is denoted as “complete media,” and all experiments were initiated at 3 days after the cell cultures were switched to complete media. All analyses were performed on at least three separate preparations of cells, and all data are presented as a percent increase in expression over that of the controls which were determined from parallel cell cultures that were grown under identical conditions. All error bars represent the SD of the determinations from the separate preparations of cells, and in some experiments multiple determinations were carried out on samples from a given preparation of cells. The number of replicates used for each measurement is denoted in each figure.
Mechanical perturbation
The mechanical perturbation apparatus used for these experiments was as previously described.51 The device design imposes a verified temporal and spatial displacement profile to an optically transparent elastomeric membrane in which the strain magnitude was experimentally demonstrated to be homogeneous and isotropic (i.e., radial strain = circumferential strain = constant over the culture surface). While previous experiments used a special formulation silicone elastomer, due to manufacturing limitations of the silicone-based elastomer (Dow Corning Corporation, Corning, NY, U.S.A.), in the experiments reported here a polyurethane-based membrane (a generous gift of Dow Chemical Corporation, Midland, MI, U.S.A.) was used. Verification of the strain pattern utilizing the polyurethane membrane was carried out as described previously and demonstrated to be identical to the silicone membranes.51 For all the experiments reported here, the cells were mechanically perturbed at 1.3% uniform biaxial strain at 0.25 Hz for a single 2-h period. For each experiment, controls were carried on cultures at the same times from the same preparation of cells grown under identical conditions as the mechanically perturbed cultures.
Second signal inhibitor studies
Second signal transduction mechanisms were examined through the use of specific chemical inhibitors. The final concentration at which each of these compounds was used was 50 μM for cycloheximide, 1 μM for H89, 20 μg/ml for genistein, 50 μM for chelerythrine, and 6 μM for quercetin. In all experiments using these compounds, cells were preincubated for 0.5 h with the compounds before the mechanical stimulation was carried out. Microfilament disruption was carried by preincubation of the cell cultures for 1 h in 50 μM cytochalasin D. In other experiments, 1 μM of chaetoglobosin C, a cytochalasin analog which binds to microfilaments but does not cause microfilament depolymerization,52 was used to test for pharmacological effects of cytochalasins independent of f-actin polymerization. In these studies, preincubation was for 1 h. Microtubule dissociation was carried out by preincubation of the cultures for 6 h in 1 μM colchicine. All compounds were suspended in either dimethylsulfoxide (DMSO) or absolute ethanol and were added to the culture media by making a 1:1000 dilution to the final working concentration. Controls were separately determined for each compound in cultures treated identically with the various compounds but in which the cells were not mechanically stimulated.
In separate experiments in which PKC and PKA were chemically activated, 50 ng/ml phorbol 12-myristate 13-acetate and 105 M of forskolin were used, respectively. In these experiments, treatments were for 2 h, and mRNAs were analyzed immediately after the cultures had been treated with these compounds. Controls in these experiments were carried out with vehicle alone, in which the compounds had been suspended.
Isolation and analysis of RNA
Total RNA and DNA were isolated using Tri-Reagent (Molecular Research Center, Cincinnati, OH, U.S.A.) according to the manufacturer's instructions. RNA was resolved on 1% agarose gels containing 2.2 M formaldehyde,53,54 and 5 mg of total RNA based on OD260 was loaded per gel lane. Equal loading of the RNA was verified by ethidium bromide staining of the gel before blotting onto nylon membranes. The cDNA (MMPP2) encoding the complete sequence of chicken osteopontin21 was used to examine levels of expression for mRNA.32P-radiolabeled cDNA probes were synthesized,55 and hybridization was carried out at 65°C in 2.5 × SSC, 50 mM NA phosphate buffer, made 100 μg/ml single-stranded salmon sperm DNA, and for 18–24 h in a rotating hybridization oven (Robins Scientific, Sunnyvale, CA, U.S.A.). Underexposed autoradiograms were used for all quantification and were quantified using an LKB Ultra II scanning densitometer (LKB, Broma, Sweden). All values were then normalized to 18 S ribosomal RNA obtained by hybridization of each blot to a conserved nucleotide sequence probe of the 18 S ribosomal subunit (Ambion Corp., Austin, TX, U.S.A.). In all experiments except that presented in Fig. 1, all determinations of RNA levels were carried out 6 h after the end of a 2-h period of mechanical perturbation.
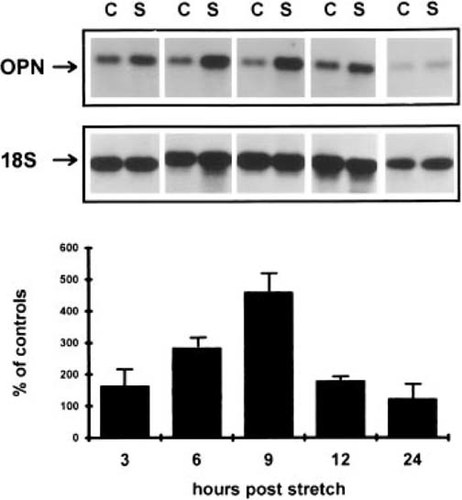
Time course of induction of osteopontin expression in response to mechanical perturbation. Northern blot analysis of osteopontin mRNA levels after mechanical perturbation. Time points are denoted as the total time from the initiation a single 2-h period of mechanical perturbation. Control RNA samples are from parallel time points of cultures which were unperturbed. Graphic analysis shows the percent induction of osteopontin mRNA expression relative to the control samples. Error bars are the SD of three experiments. In all figures, C = control. S = mechanically strained.
Cytoskeletal analysis
Immunochemical staining for tubulin was carried out with a rabbit polyclonal antibody directed toward chicken β-tubulin, while f-actin was visualized with rhodamine conjugate-phalloidin. For the phalloidin reactions, cell layers were pretreated for 15 minutes at room temperature with 0.1% Triton in phosphate-buffered saline (PBS). Cell layers were washed in PBS followed by a 20-minute fixation in 4% paraformaldehyde. After fixation, cultures were reacted with the primary antibody to tubulin or with rhodamine-conjugated phalloidin at predetermined dilutions in PBS for either 90 or 15 minutes, respectively. Antitubulin reactions were visualized by secondary antibody reactions carried out for 120 minutes with fluorescein-conjugated anti-rabbit IgG used at 1:250 dilution in PBS. Background fluorescence was reduced by extensive washing in PBS, samples were then air dried and cover slipped. Photomicroscopy was carried using an Olympus Microscope OM-2 (Olympus Co., Lake Purchase, NY, U.S.A.) on Kodak Tmax p3200 film (Eastman Kodak Co., Rochester, NY, U.S.A.). Rhodamine was visualized with an R610 ex/em barrier filter while fluorescein samples were visualized using a G520 ex/em barrier filter.
Analysis of focal adhesion kinase activation by mechanical strain
Osteoblasts were grown on flexible membranes as described above. In two different sets of experiments, the cells were either mechanically stimulated in the presence or absence of genistein at the same concentrations as described above. After 15 minutes of mechanical stimulation, the cells from each 100 mm dish were lysed in 1 ml of buffer A (10 mM Tris-HCl buffer, pH 7.2, containing 300 mM sucrose, 100 mM KCl, 1% Triton X-100, 5 mM MgCl2, 10 mM EGTA, 1 mM NaF, 0.1 mM sodium orthovanadate, 0.1 M ε-amino-n-caproic acid, 5 mM benzamidine, 1 mM p-hydroxymercuribenzoate, 5 μg/ml pepstatin, 1 μg/ml leupeptin) at 4°C. After 10 minutes, the lysate which contained the soluble intracellular proteins was removed, and the remaining cytoskeleton was washed 2× with 1 ml of buffer A. The cytoskeletal proteins and the tightly associated proteins, which were bound to it, were then solubilized by incubating the dishes in 1 ml of 10 mM Tris buffer, pH 7.6, containing 10 mM KCl, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 5 mM MgCl2, 10 mM EGTA, 1 mM NaF, 0.1 mM sodium orthovanadate, 0.1 M ε-amino-n-caproic acid, 5 mM benzamidine, 1 mM p-hydroxymercuri-benzoate at 4°C for 1 h. Both the soluble fraction and the cytoskeletal fraction were stored at −20°C until use.
For immunoprecipitation of FAK, 25 μl of each of the cellular fractions were used. The cell fraction lysates were precleared by incubation with 100 μl of insoluble protein A at 4°C for 1 h. After this incubation period, the suspension was centrifuged in a microcentrifuge at 5000 g for 10 minutes. The resultant supernatant was incubated with 0.1 mg of either anti-mouse or chicken affinity purified or anti-FAK antibody overnight at 4°C. The immune complexes were collected by incubation with 10 ml of insoluble protein A for 1 h at 4°C followed by centrifugation at 5000 g for 10 minutes. The protein A–immuno complexes were washed five times with lysis buffer, then once with 20 mM Tris-HCl. The immune complexes were released from the protein A beads by boiling the beads in 20 ml of SDS sample buffer containing 0.1% fresh 2-mercaptoethanol. For direct analysis of FAK, 5 μl of each of the cell fraction lysates were adjusted with SDS sample buffer and directly resolved by gel electrophoresis. The samples were resolved on an 8% SDS-polyacrylamide gel.56 Proteins were electrophoretically transferred to ECL membrane for 1 h at 400 mA using a semidry Bio-Rad blotting device (Bio-Rad Laboratories, Hercules, CA, U.S.A.) using conditions as described by the manufacturer. The blots were then reacted with antiphosphotyrosine antibody, and reactions were visualized using chemiluminescence secondary antibody reaction (ECL Western blotting detection reagents, Amersham, Little Chalfont, Buckinghamshire, U.K.) according to the manufacturer's protocol.
RESULTS
Characterization of the signal transduction pathway which mediates the mechanoinduction of osteopontin expression
Mechanical stimulation of cells has been shown to activate a wide variety of second signal transduction pathways, including PKA, PKC, and Ca2+ transients.57 Initial experiments therefore were directed at defining which of the signal transduction pathway(s) were used in the induction of the opn gene expression and whether the opn gene induction was pretranslationally controlled as a primary or secondary event in response to the signal transduction. These questions were addressed by first examining the temporal induction of the steady-state levels of opn mRNAs within primary calvaria osteoblasts over a 24-h period in response to a single 2-h period mechanical perturbation (Fig. 1). An initial moderate increase was seen in opn expression by 3 h with a continuing increase in its levels of expression observed for up to 9 h, at which time a maximal 4-fold increase was seen. After reaching its peak expression by 9 h, the levels of opn mRNAs began to decrease, returning to baseline by 24 h.
The relatively slow response by which mechanical perturbation mediated its pretranslational effects on opn mRNA levels suggested that the altered expression of opn was a secondary event to the signal transduction process. To test this, cycloheximide was used to inhibit de novo protein synthesis. Cultures were concurrently treated with cycloheximide at the time of mechanical perturbation, and opn mRNA levels were assessed. Surprisingly, cycloheximide did not ablate the induction of the opn mRNA expression in response to the mechanical perturbation (Fig. 2). Because opn is known to be an immediate early response gene to PKC activation by PMA,16,21 the next studies examined whether PKC was involved in the signal transduction processes mediating the induction of opn expression in response to mechanical perturbation. PKC inhibition with cheryelthrine also had no effect in blocking the actions of mechanical stimulation, demonstrating that mechanical induction of this gene's expression were not mediated by PKC (Fig. 2).
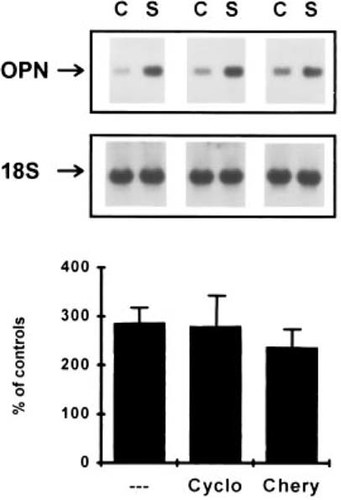
The effect of inhibition of new protein synthesis and PKC activation on the induction of osteopontin expression in response to mechanical perturbation. Northern blot analysis of osteopontin mRNA levels at 6 h after a single 2-h period of mechanical perturbation in the presence of cyclohexamide (Cyclo) or cheryelthrine (Chery). Graphic analysis shows the percent induction of osteopontin mRNA expression relative to control cultures treated identically with each inhibitor but that are mechanically unperturbed. Error bars denote the SD of three experiments.
Subsequent investigation then focused on whether the PKA and/or tyrosine phosphorylation-dependent signal transduction processes were involved in mediating the induction of the opn mRNA expression. In these experiments, all three inhibitors (genistein which is a specific inhibitor of tyrosine phosphorylation, H89, which is a specific inhibitor of PKA-like kinases, and quercetin, which inhibits a broad spectrum of kinases including the casein kinases and tyrosine kinases) ablated mechanoinduction of opn expression (Fig. 3). In the absence of mechanical stimulation, none of these compounds by themselves affected the baseline expression of opn, indicating that the activation of these kinases played a specific role in the mechano–signal transduction process independent of their role in maintaining the basal level of expression of this gene.
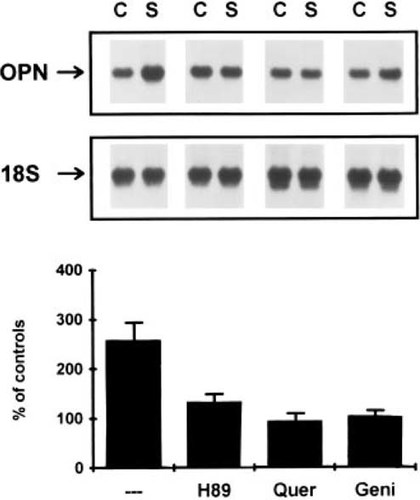
The effect of various second signal inhibitors on the induction of osteopontin expression in response to mechanical perturbation. Northern blot analysis of osteopontin mRNA levels at 6 h after a single 2-h period of mechanical perturbation in the presence of the PKA inhibitor H89 (H89) the general protein kinase inhibitor quercetin (Quer) or the specific tyrosine kinase inhibitor genistein (Geni). Graphic analysis shows the percent induction of osteopontin mRNA expression relative to control cultures treated identically with each inhibitor but that are mechanically unperturbed. Error bars denote the SD of three experiments.
Mechano–signal transduction, but not PKC or PKA signal transduction which mediate osteopontin gene expression, is dependent on microfilament integrity
The relationship between the biochemical processes that mediate signal transduction and the cytoskeletal structural elements of the cell were examined. Microfilament or microtubule structures were separately disrupted with cytochalasin D or colchicine, respectively, in control and mechanically perturbed cultures of osteoblasts. As can be seen in Fig. 4A, while the disruption of microfilament integrity blocked the increased expression of opn mRNAs, the disruption of the microtubule structure had only a marginal effect on the mechanoinduction of opn expression. Interestingly, while the disruption of the microfilament structure in the absence of mechanical stimulation did not effect the baseline expression of opn, microtubule disruption had a stimulatory effect.
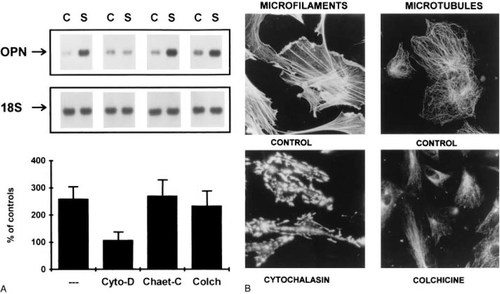
Relationship of microfilament but not microtubule structural integrity to the signal transduction process involved in the regulation of osteopontin expression in response to mechanical perturbation. (A) Northern blot analysis of osteopontin mRNA levels at 6 h after a single 2-h period of mechanical perturbation in cultures in which either microfilament structure was disrupted with cytochalasin D (Cyto-D) or microtubule structure was disrupted with colchicine (Colch). In a separate control, cultures were treated with chaetoglobosin (Chaet-C), a chemical analog to cytochalasin-D which binds to F-actin but does not disrupt microfilament structure. Graphic analysis shows the percent induction of osteopontin mRNA expression relative to control cultures treated identically with each compound but mechanically unperturbed. Error bars denote the SD of three experiments. (B) Immunofluorescent microscopic analysis of microfilament and microtubule disruption by cytochalasin-D and colchicine was carried out for confirmation of the effects of these drugs on osteoblasts. The nature of the immunofluorescent micrographs is presented. Magnification is ×800.
Since cytochalasin D has both known biochemical and genomic effects on cells independent of its actions on microfilaments, an analog to cytochalasin, chaetoglobosin-C, which binds to the barbed ends of actin polymers but does not block polymerization and mimics other pharmacological effects of cytochalasin,52 was used as a control. These experiments specifically support the conclusion that the effects of microfilament disruption are unique to the signal transduction process and are not a trivial pharmacological effect of cytochalasin D binding to F-actin. As a further control to these experiments, the disruption of the microfilament and microtubule structures in the osteoblasts with cytochalasin D and colchicine was confirmed by microscopic examination of these cytoskeletal elements (Fig. 4B).
The relationship of microfilament structural integrity to other known signal transduction processes that mediate the pretranslational regulation of opn gene activity was examined by activating PKC or PKA with PMA or forskolin, respectively. Osteopontin expression was then examined in control cultures and cultures treated with cytochalasin D (Fig. 5) to establish whether these signal transduction processes were also dependent on the cytoskeletal integrity or whether mechanical stimulation was unique in this respect. As was previously shown, PMA has a very strong effect causing an ∼5-fold stimulation in opn gene expression in immature osteoblasts,17 which is consistent with studies in other cell types, which have demonstrated that it is an immediate early response gene to PMA activation.20,21 Interestingly, forskolin also had a very moderate ∼2.0-fold stimulatory effect. In contrast to the ablation in induction of opn mRNA expression by cytochalasin D, which was seen in the mechanically perturbed cultures (Fig. 4), microfilament disruption had no effect on the mediation of either the PKA and PKC signal transduction processes which stimulated opn expression in response to the PMA and forskolin within osteoblasts.
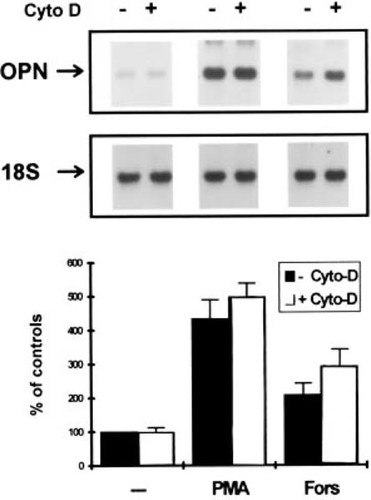
Relationship of microfilament disruption to PKC and PKA signal transduction mechanisms independent of mechanical perturbation. Northern blot analysis of osteopontin expression in osteoblasts treated with either Phorbol 12-myristate-13-acetate (PMA) or forskolin (Fors) in the presence (+) or absence of (−) of microfilament disruption with cytochalasin-D. Graphic analysis shows the percent induction of osteopontin mRNA expression relative to control cultures treated identically with each compound but in the absence of microfilament disruption. Error bars denote the SD of three experiments.
Activation of FAK by mechanical stimulation
Since the signal transduction was shown to be mediated through the activities of a tyrosine kinase(s), the final series of studies was directed at determining the immediate events at the cytoplasmic side of the membrane that were involved in the mechano–signal transduction process. In these studies, the activation of FAK was examined after a 15 minute period of mechanical stimulation. A further aspect of these studies was to determine whether there was specific translocation of the activated protein to the cytoskeleton. As can be seen from these results, mechanical stimulation induced the tyrosine phosphorylation of FAK based on the increased level of immunoreactivity with the antiphosphotyrosine antibody that was seen in both the cytosolic and cytoskeletal fractions (Fig. 6, top panel). Moreover, there was a clear increase in the quantities of activated FAK associated with the cytoskeleton after mechanical stimulation which were diminished after the cells were treated with cytochalasin D. To determine if FAK association with the cytoskeleton was dependent on its phosphorylation, the cultures were mechanically stimulated in the presence or absence of genistein. This analysis was carried out in an identical manner as that depicted in the top panel of Fig. 6 with FAK first being immunoprecipitated followed by Western blot analysis with the antiphosphotyrosine antibodies (Fig. 6, middle panel). As can be seen from Fig. 6, genistein treatment inhibited FAK association with the cytoskeleton, and a relative comparison of the amount of phosphorylated FAK that is found in the various fractions is identical to that seen in the top panel.
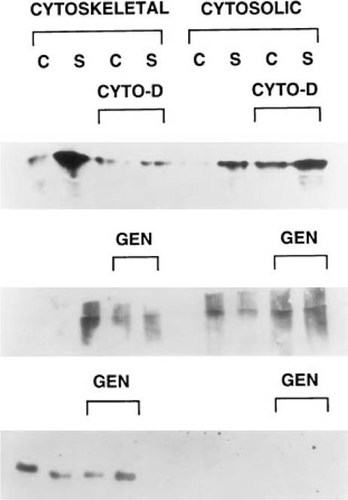
Mechanical stimuli induces the tyrosine phosphorylation of focal adhesion kinase (FAK). Lanes are the same for the cytoskeletal and cytosolic fractions for all panels. (Upper panel) Western blot analysis with antiphosphotyrosine antibody of immunoprecipitated FAK in the control (C) or mechanically strained (S) fractions. Cyto-D indicates fractions isolated from Cytochalasin-D treated cells. (Middle panel) Western Blot analysis with antiphosphotyrosine antibody of immunoprecipitated FAK in the cytosolic or cytoskeletal fractions in the absence or presence of genistein (Geni) inhibition. (Bottom panel) Analysis of total FAK protein content in the cytoskeletal and cytosolic fractions after inhibition with genistein. This panel depicts Western blot analysis of the cytoskeletal and cytoplasmic fractions before immunoprecipitation using chicken anti-FAK antibody.
These results again demonstrate that mechanical perturbation will stimulate FAK phosphorylation and specific cellular compartmentalization (cytosolic vs. cytoskeletal) is dependent on the state of FAK phosphorylation. This analysis, however, does not address whether the absolute state of FAK phosphorylation is inhibited by genistein treatment. To assess if FAK phosphorylation was variable between each of the cellular fractions, the total amount of FAK in each of the fractions in comparison with that which was phosphorylated was assessed by Western blot analysis of the various cell lysates before immunoprecipitation, using chicken anti-FAK antibody. As can be seen from this analysis, its state of phosphorylation is variable in comparison with the total FAK protein found in each of the cellular fractions as seen by comparing the bottom and middle panels of Fig. 6. These data also indicate that the total amount of FAK in the cytoplasm is several-fold less than that associated with cytoskeleton since the levels of FAK in the cytoplasm are below that of detectability by Western blot analysis of the total lysate without first concentrating the FAK by immunoprecipitation. Thus, these results demonstrate that mechanical stimulation activates FAK, that phosphorylated FAK is selectively associated with the cytoskeleton, and suggest that FAK overall is associated with the cytoskeleton.
DISCUSSION
opn as a mechanically responsive gene
In the present studies, opn expression was shown to be responsive to mechanical stimuli. This result corroborates the finding of several other studies, in which opn expression was shown to be up-regulated in response to various forms of mechanical perturbation. In vivo studies in rat using a four-point bending device applied externally to rat tibias showed that opn expression was stimulated 3- to 4-fold within periosteal tissue 12 h after mechanical loading.58 Similar studies to those reported here using cultures of primary human osteoblasts and rat osteosarcoma cells which were mechanically strained through growth on flexible membranes were also shown to induce osteopontin expression.13,15 Thus, within skeletal tissues in particular and possibly many other tissues in general, opn expression appears inducible in response to mechanical stimuli. The response of this gene to mechanical stimuli in widely divergent species and within various tissues also suggests that there are both conserved and unique mechanisms which both transduce mechanical signals and regulate this gene's expression in response to mechanical stimuli.
The demonstration that opn is a mechanically responsive gene might suggest that certain genes that encode integrin ligands might contain regulatory elements that are responsive to mechanical perturbation. Such a suggestion is consistent with the observation that fibronectin is also up-regulated in response to mechanical stimulation in osteoblasts.59 Thus, mechanical signal response through ECM-integrin interactions may be autoregulatory through changes in the composition of the specific integrin ligands within the ECM. It is intriguing to speculate that the integrin-specific ligands might themselves act in an analogous fashion to autocrine factors, but which are produced in response to specific structural changes in the ECM. It is of further interest to note that the nature of the osteopontin gene response to mechanical perturbation was dependent on the duration of the mechanical perturbation. The increased expression of osteopontin in response to short durations of mechanical perturbation is in contrast to its subsequent down-regulation with longer durations of mechanical stimulation.59 Such a temporal pattern of response to the duration of the stimulus is similar to how the expression that a number of mechanically stimulated genes in vascular endothelia are regulated as their expression changes as a chronic adaptive response.60 Finally, the transient increase in the expression of opn in response to a short duration of mechanical perturbation may reflect its role in mediating osteoclast or osteoblast interactions with the bone matrix and its function in the coupling process during resorption. This later conclusion is consistent with the demonstration that osteopontin binding to osteoclast cell surface receptors mediates calcium transients which subsequently leads to osteoclast activation.36,37 Such conclusions are also consistent with the emerging picture that opn is an early immediate response gene in T-cells, macrophages, and many other cell types in response to a wide variety of autocrine/paracrine regulators16 and may be part of the inflammatory response.
The role of specific kinases in the mechanical signal transduction process
A general understanding has emerged that integrins mediate their actions through the activation of specific tyrosine kinases, one of which is FAK.43,44,47,61 The central role of integrins in physically mediating the cellular communication processes with the extracellular matrix has led to the hypothesis that these receptors are involved in mechanical signal transduction.62 Through the use of selective kinase inhibitors, the data presented here demonstrate the involvement of both an H89 inhibitable PKA-like enzyme and a genistein inhibitable tyrosine kinase but no involvement of PKC in the signal transduction process mediating the opn gene response to the mechanical stimuli. Both the involvement of a tyrosine kinase in the signal transduction process and the demonstration that mechanical stimulation activates FAK provides strong evidence in support of the hypothesis that mechanical signal transduction is mediated through an integrin or some other cell surface receptor and that FAK is one of the intracellular transducers.
While the identification of a PKA-like enzyme in the signal transduction process is consistent with many studies that have shown elevated cAMP levels in osteoblasts in response to mechanical stimulation,8 in these cells cAMP was not elevated as an immediate response to the mechanical perturbation either after 15 or 30 minutes of mechanical stimulation or after the 2 h period used in these studies (data not shown). In the studies presented here, forskolin also only partially mimics the degree of opn activation seen with mechanical stimulation, and the mechanisms of forskolin activation of opn are clearly different from that of mechanical activation, based on the involvement of the cytoskeleton in the latter but not the former signal transduction processes. These data suggest that the inhibitor itself is not acting on PKA but is acting on another kinase which is sensitive to H89 and which is involved in the signal transduction process. The data presented here are also similar to the demonstration that cAMP levels are not involved in mediating the effects of lysophosphatidic acid (LPA) and bombesin, two compounds which have been shown to stimulate rapidly the formation of focal adhesions and stress fibers. LPA and bombesin were shown to activate the small guanine triphosphate (GTP) binding protein Rho through a genistein-inhibitable tyrosine kinase which then caused stress fiber formation and clustering of phosphotyrosine containing proteins at focal adhesions, but neither process was dependent on cAMP even though these two compounds led to diminished cAMP levels.63 It is interesting to note that in studies with LPA and bombesin that the role of PKA was indirectly extrapolated from the lack of effect that forskolin treatment had on the actions of these compounds. Thus, while the role of PKA or a PKA-like enzyme does appear to be involved in the mediation of the mechanical signal transduction, its independence from the levels of cAMP at present is not fully resolved.
Mechano–signal transduction process is uniquely dependent on the cytoskeleton
The unique dependency of the mechano–signal transduction processes, which activates opn, on the microfilament integrity was demonstrated by the ablation of the mechanical stimulation of opn expression by cytochalasin D but the lack of an effect of cytochalasin D on the PMA/PKC-mediated stimulation of opn expression. Since several studies within different cell types have demonstrated that integrin-mediated cell adhesion activates PKC,64 these results suggest the signal transduction processes that are mediated by integrin ligation and/or integrin clustering alone, may be unique from the signal transduction processes that mediate mechanical stimuli. The lack of inhibition by a specific PKC inhibitor on the mechanical stimulation of opn expression also suggests that the PKC signal transduction pathway is not involved in mechano–signal transduction regulation of osteopontin within osteoblasts. It is interesting to note that the unique nature of the signal transduction process may reside within the changes of the cytoskeletal organization itself, which occur in response to mechanical perturbation. Other studies examining the cytoskeletal structure of these osteoblasts in response to mechanical stimulation, showed that mechanically strained cells had much more prevalent microfilament structures based on the appearance of the F-actin organization into numerous elongated filaments, while the cells microtubule structures became dissociated (unpublished data). This would suggest that the cells' response to mechanical perturbation was to increase its rigidity as compensatory response, which increased its tensile strength in response to mechanical perturbation. This reciprocal alteration in microtubule structure relative to increased microfilaments is consistent with the proposals that cells maintain cellular tensegrity through the coordinated modulation of different components of cytoskeleton.65 Finally, these data suggest that at the nuclear level there may be unique trans and/or cis elements that are responsive to mechano–signal transduction, different from those that are responsive to PKC-mediated signal transduction or that the same elements are in some way uniquely activated by the mechano–signal transducer process.
An important consideration in the interpretation of these results, however, are variations in PKC association with the cytoskeleton and variations in the activation of PKC through alterations in cytoskeletal structure, which have been demonstrated for various cell types.66,67 It is also clear that there are multiple but interdependent signal transduction pathways that activate opn, which may be independently associated with the cytoskeleton. In studies within C3H10T½ cells depletion of PKC from cells resulted in down-regulation of opn expression which could be overcome by activation of p21ras.68 The opn promoter also contains a ras responsive element that is distinct from the PMA responsive element that binds to PEA-1/AP-1 transcription factor.69 Thus, inhibition of PKC alone may be insufficient to inhibit opn expression in the presence of activated p21ras. It may be speculated that since src activates p21ras and mechanical stimulation may activate src, even if PKC was localized in the cytoskeleton of osteoblasts and was activated, that the effects of its inhibition on opn expression would be masked.
In relating these findings to those of others, several studies have demonstrated that both disruption and polymerization of actin microfilaments lead to specific changes in the expression of a number of genes including collagenase,45 and urokinase-type plasminogen activator,70 glucokinase,71 and endothelin-1.72 In studies of endothelin-1 regulation within aortic endothelial cells, a direct relationship between microfilament structure and the regulation of this gene's expression by hemodynamic shear stress has also been demonstrated,72 while in the studies on glucokinase expression in hepatocytes cytochalasin D was shown to enhance the insulin-induced expression of this gene.71 Therefore, even though microfilament disruption had an opposite effect in mediating the activities of these different genes, these studies all demonstrate the involvement of the microfilaments in the signal transduction processes and mechanisms of regulation in the expression of specific genes.
In considering the relationship between the cytoskeleton and the signal transduction process, two sets of mechanisms may be speculated. One set of mechanisms would involve the imposition of effects from mechanically induced alterations of the cytoskeleton to the cell surface receptors, whereby cytoskeletal alterations would facilitate either the formation and/or activation of specific signal transducers within the focal adhesion complex. A second possibility is that the mechanical signal through its physical actions on the ECM alters the integrin conformation that facilitates the focal adhesion complex formation and/or activation of the signal transducers in the focal adhesion complex which then leads to cytoskeletal changes, and these changes in and of themselves are involved in the signal transduction process. In the present studies, the latter possibility seems to be more favored based on the rapid activation of FAK (on the order of minutes) and its selective association with the cytoskeleton, in comparison with the time frame in which maximal osteopontin gene expression was achieved (6–9 h). The time frame of opn gene activation is unusual in that it was not cycloheximide inhibitable and as such was not a secondary event. It is also interesting to note that the induction of endothelin-1 by shear stress also takes 6–9 h.72 These longer time frames in which some genes specifically respond to mechanical signals may therefore reflect the longer periods during which cytoskeletal changes take place as an adaptation to the mechanical stimuli. These genes then are induced not as a secondary response to the activation of specific transcription factors but as a secondary response to alterations in the cytoskeleton which imposes its effects directly on the transcription of specific genes.
Acknowledgements
We wish to acknowledge the expert technical assistance of Ms. Vivian Towe and Irina Simkina in the preparation and maintenance of the osteoblast cell cultures. We thank Becton Dickinson Labware and Dow Chemical Company for their generous support of this project. We thank Don Ingber for his critical reading of the manuscript and his comments.




