Abstract
There are few data on the relative effects of calcium supplementation with or without extra vitamin D on BMD in patients selected for low vitamin D status. The aim of this study is to evaluate the relative importance of vitamin D and calcium treatment on BMD and bone-related chemistry in elderly women with vitamin D insufficiency. Three hundred two elderly women (age, 77.2 ± 4.6 yr) with serum 25(OH)D concentrations <60 nM participated in a 1-yr randomized, double-blind, placebo-controlled trial. All subjects received 1000 mg calcium citrate per day with either 1000 IU ergocalciferol (vitamin D2) or identical placebo (control). The effects of time and time treatment interactions were evaluated by repeated-measures ANOVA. At baseline, calcium intake was 1100 mg/d, and 25(OH)D was 44.3 ± 12.9 nM; this increased in the vitamin D group by 34% but not the control group after 1 year (59.8 ± 13.8 versus 45.0 ± 13.3 nM, p < 0.001). Total hip and total body BMD increased significantly, and procollagen type I intact N-terminal propeptide (PINP) decreased during the study with no difference between the treatment groups (hip BMD change: vitamin D, +0.5%; control, +0.2%; total body BMD change: vitamin D, +0.4%; control, +0.4%; PINP change: vitamin D, −3.9%; placebo, −2.8%). Although the fasting plasma and urine calcium increased in both groups equally, there was no detectable change in serum PTH. The increase in 25(OH)D achieved with vitamin D supplementation had no extra effect on active fractional intestinal calcium absorption, which fell equally in both groups (vitamin D, −17.4%; control, −14.8%). In patients with a baseline calcium intake of 1100 mg/d and vitamin D insufficiency, vitamin D2 1000 IU for 1 year has no extra beneficial effect on bone structure, bone formation markers, or intestinal calcium absorption over an additional 1000 mg of calcium. Vitamin D supplementation adds no extra short-term skeletal benefit to calcium citrate supplementation even in women with vitamin D insufficiency.
INTRODUCTION
Older people are at increased risk of inadequate vitamin D production in the skin because of reduced sun exposure and the reduced ability of the skin to synthesize vitamin D.1 The resulting low vitamin D status may have negative effects on the maintenance of bone mass. Vitamin D plays a role on bone health through effects on skeletal mineralization, serum calcium and phosphorus concentrations, and regulation of parathyroid growth and PTH production.2, 3 However, studies of vitamin D supplementation without calcium supplementation in elderly women have been disappointing in that there has been little or no effect on BMD.4
On the other hand, several studies have shown that supplementation with both calcium and vitamin D reduces the rate of bone loss compared with placebo.5, 6 However, few studies have examined the additional effects of vitamin D in the presence of high calcium intake. Trials comparing supplementation of calcium plus vitamin D and supplementation of calcium alone have yielded conflicting results that could be related to the difference in calcium intakes of participants of these two studies.7, 8 Furthermore, there are few data on the relative effects of calcium supplementation with or without extra vitamin D in patients selected for low vitamin D status. Thus, the aim of this study was to evaluate these effects in elderly community-dwelling women with vitamin D insufficiency. The effects of the intervention on falls risk have been published separately.9
MATERIALS AND METHODS
Subjects
Women between 70 and 90 yr of age were recruited between April 2003 and October 2004 in Perth, Australia (latitude 32° S) from three sources: patients attending the emergency departments of teaching hospitals, patients receiving services from the local community home nursing service (Silver Chain) for management of falls, and the Electoral Roll, which lists >98% of women of this age. The inclusion criteria were a plasma 25(OH)D concentration <60 nM and a history of at least one fall in the previous 12 mo. Exclusion criteria were current vitamin D consumption; current consumption of bone or mineral active agents apart form calcium; a BMD Z-score at the total hip site of less than −2.0; medical conditions or disorders that influence bone mineral metabolism including laboratory evidence of renal insufficiency (creatinine > 2-fold above the normal value); a fracture in the past 6 mo; and a Mini Mental State Examination score <24 or the presence of significant neurological conditions likely to substantially impair balance or physical activity such as stroke and Parkinson's disease.
The study was approved by the Human Research Ethics Committee of the Sir Charles Gairdner Hospital. Written informed consent was obtained from each woman. The study was conducted in compliance with the ethical principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practices Guidelines and registered with the Australian Clinical Trials Registry (registration no: ACTRN012606000331538).
Treatment
Participants received 1000 mg/d of calcium as calcium citrate (Citracal; Mission Pharmacal, Key Pharmaceutical, Rhodes, Australia) for 1 yr as two calcium tablets in the morning with breakfast and two calcium tablets with the evening meal. They were randomized to receive 1000 IU ergocalciferol (vitamin D2) per day or identical placebo (Ostelin; Boots Healthcare, North Ryde, Australia) consumed with the evening meal for 1 yr.
The randomization schedule to vitamin D or placebo was generated by an independent research scientist and was kept in the Pharmacy Department of the Sir Charles Gairdner Hospital, who labeled the bottles and dispensed the study medications to subjects. The randomization procedure used a random number generator with a block size of 10 to assign participants to vitamin D or placebo in a ratio of 1:1. The study subjects and the study staff remained blinded to the treatment code until all the data had been entered, evaluated for accuracy, and the a priori hypotheses reviewed. Adherence to the study medications was established by counting tablets returned at the clinic visits at 6 and 12 mo.
Bone measurements
DXA BMD was measured at baseline and 12 mo at the hip and total body with a Hologic Delphi fan beam densitometer (Hologic, Waltham, MA, USA) using an identical protocol. The CV at these sites was <2% in our laboratory.
Biochemistry analysis
At baseline and 12 mo, venous blood and second morning void urine samples were collected between 8.30 and 10.30 a.m. after overnight fasting. Serum 25(OH)D concentrations were assessed by radioimmunoassay (DiaSorin, Stillwater, MN, USA). Serum PTH concentrations were measured by chemiluminescent enzyme-labeled immunometric assay (Immulite 2000 Intact PTH; DPC, Los Angeles, CA, USA). Serum procollagen type I intact N-terminal propeptide (P1NP) concentrations were measured using an automated electrochemiluminescent assay using the Roche Elecsys 2010 system. Serum and urinary concentrations of total calcium and creatinine were measured by routine laboratory method.
Intestinal calcium absorption
Gut calcium absorption was measured using Ca45 as calcium chloride as previously reported.10, 11 At baseline and 1 year, 10 mg of calcium chloride containing 5 uCi of calcium chloride tracer was administered with 140 ml deionized water. The 10-mg calcium load was selected to measure the active fraction of gut calcium absorption.
A blood sample was collected 1 h later and counted in a β-counter. The absorbed fraction was calculated from the total counts administered and measured in the circulation at 1 h corrected for the specific activity and the extracellular volume estimated as 15% of the individual's body weight.12
Other assessments
At screening, demographic information including smoking history, use of community services, medications, patient recall of prevalent morbidity, and socioeconomic status was obtained. The standard Mini Mental State Examination was used to assess the subject's mental status at baseline. Calcium intake was assessed by a food frequency questionnaire developed in a previous study.13 This questionnaire includes 39 food items and uses the NUTTAB 90 database, a nutritional database that uses chemical analysis of Australian foods.14 Activity levels were calculated in kilocalories per day using a validated method using body weight, questions on the number of hours and type of physical activity, and energy costs of such activities.15, 16 Weight and height were measured at baseline and 12 mo with light clothes and without shoes.
Adverse event recording
Details on adverse recording have been reported elsewhere.9 In brief, participants were asked to fill out an adverse event diary during the study in which each contact with a health care provider was recorded.
Sample size calculation and statistical analysis
Power calculations were performed before the start of the study. Calculations for changes in BMD showed that, given a 2% improvement in BMD and a within-group SD of 4%, a sample size of 85 was required at 90% power and 5% level of significance. Because the study was also aimed to evaluate the effects of vitamin D supplementation on risk of falling,9 and 147 subjects in each group was needed to detect a relative risk reduction of 0.37 in a population with a 1-yr fall risk of 0.6 allowing for 30% drop out, the sample size was decided to be 150 per group.
Descriptive statistics are reported as means ± SDs and differences as means ± SEs for all variables, unless otherwise stated. Baseline values between the two groups were compared by Student t-test. Changes in BMD and biochemical variables were expressed as the percentage from baseline. A two-factor repeated-measures ANOVA was used to test time × treatment interactions and main effects of time. Significant treatment by time interaction represents significant between group differences from baseline. All tests were two tailed, and the significance value was set at p < 0.05. The data analyses were performed with SPSSPC for Windows (version 15; SPSS, Chicago, IL, USA).
RESULTS
Recruitment, retention, and compliance
A total of 3968 subjects responded to letters asking them to join the study and were contacted by telephone; of these, 827 attended the clinic screening visit. Of the subjects attending the clinic, 558 had serum 25(OH)D concentrations measured, and 215 (38%) were excluded because of concentrations >60 nM; 302 patients entered the study (Fig. 1). Withdrawals after recruitment were not significantly different between the two groups. Excluding 10 subjects who started to take other bone active agent during the trial, results on 123 and 133 patients in the vitamin D and control groups, respectively, who had a hip and total body DXA BMD at both baseline and 12 mo form the data for this paper (Fig. 1). Medication discontinuations after recruitment were not significantly different between the two groups. In subjects who remained on medication, compliance rates, as determined from tablet counting, were similar in the vitamin D and the control groups at 86.7% and 86.8%, respectively.
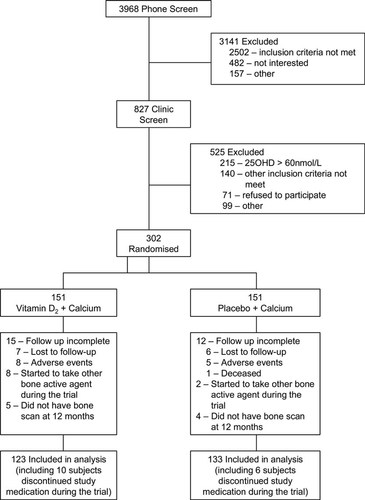
Participants flow through the study.
Participant characteristics
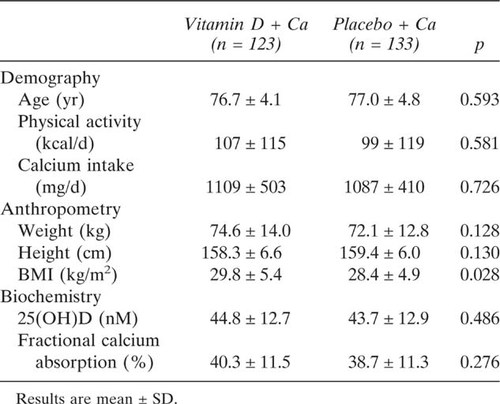
There were no significant differences between the vitamin D and control groups in baseline characteristics, except that the vitamin D group had significant higher BMI than the control group (Table 1). The mean baseline 25(OH)D concentration was 44.3 ± 12.9 nM, and 14% subjects had levels <30 nM. Mean calcium intake was 1097 ± 495 mg/d, and only 37% of study participants had a calcium intake >1200 mg/d.
Effects on BMD and bone metabolism–related chemistry
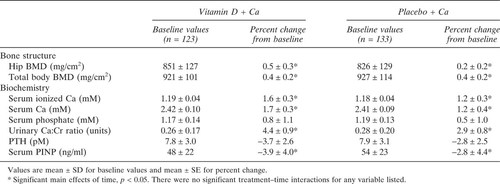
The vitamin D group had significantly higher serum 25(OH)D concentrations than the control group at 12 mo, increasing 34% over the baseline levels in that group (Fig. 2). There were significant main effects of time for total hip BMD and total body BMD, indicating an increase in hip and total body BMD over the 1-yr study period (Table 2). There were also significant main effects of time for serum calcium and P1NP concentrations and urinary calcium/creatinine ratios (Table 2). There were no significant treatment group × time interactions for all the bone and bone metabolism related biochemistry variables listed in Table 2, indicating that there were no significant vitamin D treatment effects on these parameters.
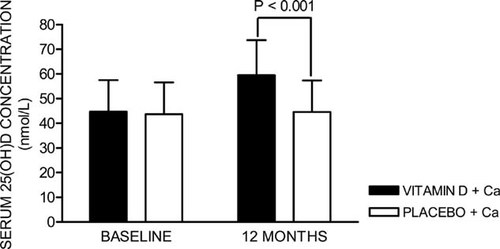
Vitamin D status during the study. Error bars represent SD.
Effects on calcium absorption
There were significant reductions in fractional rate of calcium absorption at 12 mo compared with baseline in both groups (change over 12 mo: vitamin D, −17.4 ± 2.4%; placebo, −14.8 ± 2.8%) but no between-group differences (Fig. 3).
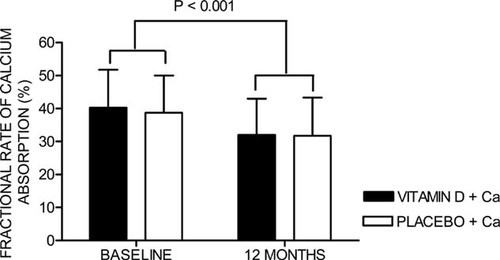
Fractional rate of calcium absorption during the study. Error bars represent SD.
Adverse events
During the study period, there were no significant differences between the vitamin D and the control groups in the rate of incident cancer and vascular disease (ischemic heart disease and stroke). One participant in the vitamin D group had mild asymptomatic hypercalcemia on one occasion. No case of renal calculus was reported.
DISCUSSION
In elderly women with vitamin D insufficiency, vitamin D and calcium supplementation had no extra benefit on bone structure or bone formation markers compared with calcium alone. This finding is consistent with another study from our group of a 5-yr study of calcium supplementation compared with calcium and vitamin D supplementation11 and a previous study of postmenopausal black women, which showed that there was no effect of 800 IU vitamin D supplementation on bone loss or bone turnover markers in calcium-replete subjects.8 In contrast, another study showed that 400 IU of vitamin D in addition to calcium supplementation could improve spine BMD after 1 yr.7 However, the calcium supplementation given to subjects in the latter study was only 377 mg/d. Another study also suggested that vitamin D supplementation may have a greater effect in calcium-deficient women.4
The lack of extra vitamin D treatment effects on improving bone structure and reducing bone turnover is likely caused by the high level of calcium intake in these patients. Certainly, vitamin D supplementation did not have any extra effect on active calcium absorption, which fell in both groups presumably because of a fall in 1,25(OH)2D. We did not measure this hormone because of the difficulties in the precision of the assay. Nevertheless, it is accepted that 1,25(OH)2D is a major determinant of calcium absorption when measured with a low carrier load.17, 18 Interestingly, these data suggest that a previous cross-sectional study suggesting that 25(OH)D is more important than 1,25(OH)2D in stimulating calcium absorption are probably incorrect10 at high calcium intake in view of the fact that 25(OH)D levels rose significantly in the vitamin D group compared with control, but this had no effect on the suppressed calcium absorption.
It should be noted that, although active calcium absorption fell, the net amount of calcium absorbed increased compared with baseline because of the calcium supplementation. This contention is supported by the increase in serum and urinary calcium concentrations and the reduced rate of bone turnover as shown by the reductions in the levels of circulating P1NP, a biomarker of bone formation, which were equal in both the vitamin D and control groups.
The increase in BMD with increased calcium intake is consistent with findings of previous studies that showed that calcium supplementation has beneficial effects on bone mass maintenance in the elderly.13, 19-21
A limitation of the study is that the effects of vitamin D alone, in the absence of calcium, was not tested. It may be that, in these patients, the treatment effect of vitamin D alone may have had similar beneficial effects to the calcium alone effect. Indeed, one study of elderly men and women with a dietary calcium was 742 mg/d treated with 100,000 IU oral cholecalciferol (vitamin D3) every 4 mo showed a reduction in fractures.22 Next, it could be argued that the increase in 25(OH)D concentration in the vitamin D group was relatively small and that the 60 nM achieved was less than the 75 nM or higher suggested to be ideal.23 The evidence in this study argued against this because there was no extra effect of the vitamin D dose on any outcome, and thus it could be argued that no extra vitamin D is needed in these subjects if the calcium intake is high enough. The suggestion that there is bone benefit to higher 25(OH)D levels is based on studies of maximal suppression of PTH concentrations23 and epidemiological data from a hip DXA BMD study suggesting a gain of about one third of an SD of hip BMD if 25(OH)D levels are >80 nM.24 In these papers, the mean calcium intakes were substantially less than in this study. Thus, we would argue that there is no conflict between the various studies and that as reviewed by others25 that calcium and vitamin D need to be understood as two aspects of the same issue: optimal delivery of calcium to bone and optimal suppression of PTH induced bone loss occurring as a result estrogen deficiency increasing urine calcium loss and decreasing intestinal calcium absorption. In view of the concerns of some that vitamin D2 may not be as active as vitamin D3, it should be noted that a recent paper reported that vitamin D2 is as effective as vitamin D3 in maintaining 25(OH)D levels.26 In addition, the increase in 25(OH)D levels in our study of 15 nM is similar to the level of increase in a previous study that used vitamin D3 as the supplement.27 Finally a limitation of all clinical trials is that, strictly speaking, the data can only be applied to patients with the same entry characteristics as the patients studied. Thus, a caveat applies to generalizing our findings to individuals with more severe vitamin D deficiency, because these subjects were not included in our study.
In conclusion, 1000 IU/d vitamin D2 supplementation adds no extra short-term skeletal benefit to 1000 mg/d calcium citrate supplementation, even in women with vitamin D insufficiency. Thus, calcium supplementation should be recommended for all elderly women with vitamin D insufficiency as first line treatment to improve bone structure. However, we have recently shown that the addition of vitamin D treatment to these women reduced their falling propensity, a potent cause of fractures in the elderly.9 These findings may explain the success of combined treatment with calcium and vitamin D in reducing fracture rates in the elderly.28
Acknowledgements
We are grateful to all the study participants for their cooperation. The Boots Company of Australia supplied the vitamin D preparation (Ostelin) and identical placebo free of charge. Mission Pharmaceutical supplied the calcium citrate free of charge. This study was supported by a research grant from the National Health and Medical Research Council of Australia (Project Grant 353638). None of the funding agencies had any role in the conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.