Abstract
Berberine (BBR) has been implicated in bone biology. Although BBR reduces osteoporosis by enhancing BMD and inhibiting osteoclast activity, the effects of BBR on osteoblasts during the process of osteogenesis have not been thoroughly studied. In osteoblastic cells, BBR enhanced the expression of osteogenic marker genes including osteopontin and osteocalcin and promoted the transcriptional activity of the key osteogenic transcription factor Runx2. In osteoblasts, BBR increased the binding of Runx2 to the promoter region of osteopontin. The recruitment of co-factors such as p300 and HDAC1 to the promoter regions of osteopontin and osteocalcin was regulated by BBR, resulting in an enhancement in the expression of those genes. Furthermore, BBR activated p38 mitogen-activated protein kinase (MAPK) and increased cyclooxygenase 2 (COX2) expression, which are key factors in osteoblast differentiation. Consistently, a p38 MAPK-specific inhibitor attenuated the effect of BBR on osteogenesis, whereas p38 MAPK overexpression augmented BBR-induced osteogenic gene expression. Moreover, BBR stimulated bone area formation in calvarial organ culture. Taken together, these findings indicate that BBR promotes osteoblast differentiation through activation of Runx2 by p38 MAPK. Therefore, BBR may be a potential therapeutic agent to treat bone-related disorders including osteoporosis.
INTRODUCTION
Osteoblasts originate from mesenchymal stem cells and are located on bony surfaces.1 They synthesize matrix proteins that subsequently become mineralized during the process of bone formation.2 Osteoblasts are regulated by several transcription factors including Runx2 and osterix.3, 4 Runx2 is exclusively expressed in mineralized tissues and osteoblasts and is a key transcription factor for osteogenesis and bone formation.5 Runx2 overexpression in mesenchymal stem cells stimulates osteoblast differentiation.6 Inactivation of one Runx2 allele causes the cleidocranial dysplasia syndrome, a disease characterized by delayed osteoblast differentiation for bone forming through intramembranous ossification in mice and humans.4, 7 Homozygous Runx2 knockout or Runx2 carboxy terminus–truncated mice exhibit a complete lack of functional osteoblasts, mineralized bone, and hypertrophic cartilage.8, 9 However, there is a poor correlation between the level of Runx2 expression and the degree of osteoblast marker gene expression, at least in vitro studies. For example, the Runx2 protein level is not significantly induced during osteogenesis in MC3T3-E1 cells, whereas the expression of other osteoblast marker genes such as osteocalcin and osteopontin is dramatically enhanced.10 Furthermore, it has been reported that extracellular matrix (ECM) production induces osteoblast differentiation through increase in the Runx2 transcriptional activity, but it is not accompanied by a significant change in Runx2 mRNA or protein levels.10, 11 Thus, it seems that Runx2 would also regulate osteoblast differentiation by alteration of its activity, probably at the level of post-translation but not by alteration in the Runx2 amounts.
Prostaglandin E2 (PGE2), a major prostaglandin produced by osteoblasts, stimulates bone formation by increasing both osteoblast replication and differentiation.12 The rate-limiting enzymes that regulate the conversion of arachidonic acid from membrane phospholipids into PGE2 are cyclooxygenases (COXs), including COX1 and COX2.13, 14 COX1 is constitutively expressed, whereas COX2 shows the inducible expression by various hormones and cytokines such as TNF-α and BMP-2.13, 15 In osteoblasts, BMP-2 promotes COX2 expression in a Runx2-dependent manner, which seems to be crucial for osteogenic gene expression and bone formation.15 Although COX2 is important for bone metabolism, the regulation of COX2 in osteoblasts is poorly understood.
Berberine (BBR) is an isoquinoline alkaloid compound extracted from several medicinal herbs, including Rhizoma coptidis and Cortex phellodendri. Recent studies have shown that BBR inhibits the expression of adipogenic transcription factors such as peroxisome proliferator-activated receptor γ (PPARγ), resulting in the suppression of adipocyte differentiation.16, 17 Although the direct cellular target of BBR is yet unidentified, BBR potently activates AMP-activated protein kinase (AMPK) and mitogen-activated protein kinases (MAPKs) for the modulation of lipid metabolism in myocytes and adipocytes.17, 18 Therefore, it is probable that BBR can regulate the differentiation and/or metabolism of certain cell type through AMPK or MAPKs.
Recently, it has been reported that BBR reduces osteoporosis by enhancing BMD and inhibiting osteoclast activity.19 However, the effects of BBR on osteoblasts during the process of osteogenesis have not been thoroughly studied. In this report, we showed that BBR stimulated the phosphorylation of Runx2 and increased its transcriptional activity, which were sensitively inhibited by SB203580, a p38 MAPK-specific inhibitor. Moreover, BBR increased the COX2 expression through Runx2 and p38 MAPK signaling and augmented new bone area formation. Together, these data suggest that BBR could be a therapeutic target for bone disease, probably by the increase in osteoblast differentiation.
MATERIALS AND METHODS
Cell culture and differentiation of osteoblasts and adipocytes
Primary bone marrow cells were isolated from the femur and tibia of 4-wk-old C57BL/6J mice and maintained in αμϵμ (Gibco BRL, Carlsbad, CA, USA) with 20% horse serum and 100 nM hydrocortisone. For osteoblast differentiation, primary bone marrow cells were cultured in αMEM with 10% FBS, 50 μM ascorbic acid, and 10 mM β-glycerophosphate. C3H10T1/2 murine mesenchymal stem cells and MC3T3-E1 calvarial cells were maintained in DMEM (Gibco BRL) supplemented with 10% FBS. Osteoblast differentiation was induced with DMEM containing 10% FBS, 50 μM ascorbic acid, and 10 mM β-glycerophosphate. Adipocyte differentiation was induced with DMEM containing 10% FBS, 500 μM 3-isobutyl-1-methylxanthine, 1 μM dexamethasone, and 5 μg/ml insulin for 60 h. The culture medium was replaced every alternate day with DMEM containing 10% FBS and 5 μg/ml insulin.
Alkaline phosphatase staining and Oil red O staining
Differentiated osteoblast cells were stained for alkaline phosphatase (ALP) activity using the BCIP/NBT color development substrate (Promega, Madison, WI, USA). Staining with Oil red O was performed as previously described.20
Constructs, DNA transfection, and reporter assay
For luciferase assays, the HEK293 cells were cultured in 12-well plates 2 days before transfection. We transiently transfected pCS4-3 Myc-Runx2, pII1.3-Luc (1.3 kb of OG2 promoter), or pXP-2-COX2-Luc using the calcium phosphate method.21 At 24 h after transfection, cell lysates were analyzed for luciferase activity. The pCMV-β-galactosidase plasmid was used as an internal control for transfection efficiency.
Immunoprecipitation and Western blotting
Cells were lysed on ice by using TGN buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1% Tween- 20, 0.2% NP-40, 1 mM phenylmethylsulfonyl fluoride, 100 mM NaF, 1 mM Na3VO4, and 1 protease inhibitor cocktail tablet; Roche, Basel, Switzerland). Lysates were incubated with anti-p38 MAPK or anti-Myc antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) as indicated for 12 h at 4°C. Immune complexes were precipitated with Protein A-Sepharose CL-4B (Amersham-Biosciences, Piscataway, NJ, USA) for 2 h at 4°C. The beads were washed three times with TGN buffer. Proteins were eluted from the beads by boiling in 5× SDS sample buffer for 5 min, separated by electrophoresis on SDS-polyacrylamide gels, and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Temecula, CA, USA). After transfer, the membranes were blocked with skim milk and probed with primary antibodies at 1:1000 dilutions. Antibodies against Myc, p38 MAPK, COX2, FLAG, actin, β-tubulin (Santa Cruz Biotechnology), and phosphorylated p38 MAPK (BD Biosciences, Franklin Lakes, NJ, USA) were used. Western blot analyses were visualized with horseradish peroxidase–conjugated secondary antibodies (Sigma Aldrich, St Louis, MO, USA) and enhanced chemiluminescence.
Real-time PCR
Total RNA was isolated with Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. After isolation of total RNA, cDNA generated by M-MuLV Reverse Transcriptase (Fermentas, Burlington, Ontario, Canada) was analyzed by real-time PCR (Bio-Rad, Hercules, CA, USA) with SYBR Green (BioWhittaker Molecular Application, Rockland, ME, USA). All reaction products were normalized to the expression level of mRNA. PCR primer sets used are as follows: Runx2 forward, 5′-GAAGGAAAGGGAGGAGGGGT-3′; reverse, 5′-TCTGTCTCTCCTTCCCTTCC-3′; osteocalcin forward, 5′-CGCTCTCAGGGGCAGACACT-3′; reverse, 5′-GCACCCTCCAGCATCCAGTA-3′; osteopontin forward, 5′-TGCCTGACCCATCTCAGAAGCA-3′; reverse, 5′-TGAGAGGTGAGGTCCTCATC-3′; osterix forward, 5′-TTTCAGCCCCCAAAACCATGGG-3′; reverse, 5′-AGATGGGTAAGTAGGCAGCT-3′; COX2 forward, 5′-AGAAGGAAATGGCTGCAGAA-3′; reverse, 5′-GCTCGGCTTCCAGTATTGAG-3′; PPARγ forward, 5′-TTGCTGAACGTGAAGCCCATCGAGG-3′; reverse, 5′-GTCCTTGTAGATCTCCTGGAGCAG-3′; ADD1 forward, 5′-GGGAATTCATGGATTGCACATTTGAA-3′; reverse, 5′-CCGCTCGAGGTTCCCAGGAAGGGT-3′; FAS forward, 5′-TGCTCCCAGCTGCAGGC-3′; reverse, 5′-GCCCGGTAGCTCTGGGTGTA-3′; aP2 forward, 5′-CAAAATGTGTGATGCCTTTGTG-3′; reverse, 5′-CTCTTCCTTTGGCTCATGCC-3′; and GAPDH forward, 5′-TGCACCACCAACTGCTTAG-3′; reverse, 5′-GGATGCAGGGATGATGTTC-3′.
Orthophosphate labeling
HEK293 cells transfected with pCS4-3 Myc-Runx2 were incubated in phosphate-free DMEM (Gibco BRL) for 2 h, [32P] orthophosphate (PerkinEImer Life Sciences, Waltham, MA, USA) was added, and the cells were incubated for 2 h.22 After incubation, SB203580 was pretreated for 1 h, and cells were treated with BBR for 2 h, and the total cell lysates were extracted with TGN buffer. Radiolabeled Myc-Runx2 proteins were obtained by immunoprecipitation with anti-Myc antibodies. Immunoprecipitates were separated by SDS-PAGE and subjected to autoradiography.
p38 MAPK in vitro kinase assay
The total cell extract (500 μg) was immunoprecipitated with anti-p38 MAPK antibodies and washed with 20 mM HEPES (pH 7.4). Kinase assays were performed for 30 min at 30°C using 2 μg glutathione S-transferase-activating transcription factor 2 (GST-ATF2) as the substrate in the reaction buffer (20 mM HEPES [pH 7.4], 10 mM MgCl2, 12 mM β-glycerophosphate, 1 mM Na3VO4, 2 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, and 1 μCi [γ-32P] ATP).23 The reactions were terminated using 5× SDS sample buffer, the products were resolved by SDS-PAGE, and the level of incorporated [32P] was detected by autoradiography. Furthermore, the membranes were immunoblotted using anti-p38 MAPK and phosphorylated-p38 MAPK antibodies.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were performed as described previously.21 PCR primer sets used in this study are as follows: osteopontin promoter forward, 5′-GGCCAACCTAAGCTACCGAA-3′; reverse, 5′-CCACCAATCAGGAGGTGGAG-3′; osteocalcin promoter forward, 5′-CGCTCTCAGGGGCAGACACT-3′; reverse, 5′-GCACCCTCCAGCATCCAGTA-3′; and COX2 promoter forward, 5′-AGCTGTGTGCGTGCTCTGA-3′; reverse, 5′-TCGCAGTTTGACAACTGGC-3′.
Retroviral infection
BOSC cells were transfected with either pWZL or pWZL-Flag-p38 MAPK using the calcium phosphate method and retroviral particles infected in MC3T3-E1 cells. Retrovirally infected cells were selected using geneticin (400 μg/ml; Gibco BRL).
Mouse calvarial organ culture
Neonatal calvarial bones were obtained from 4-day-old pups of ICR mice, and analysis of new bone formation was performed as previously described.24 The amount of new bone area formation was assessed morphologically using Bioquant Osteo II software (Bioquant Image Analysis, Nashville, TN, USA).
Statistics
Data were analyzed using Student's t-test. p < 0.05 and p < 0.01 were considered significant.
RESULTS
BBR inhibits adipogenesis and enhances osteogenesis in mesenchymal stem cells
To study the effect of BBR on the differentiation of mesenchymal progenitor cells, we treated the mesenchymal stem cell line C3H10T1/2 with or without BBR during adipogenic and osteogenic differentiation. As previously reported,17 we observed that adipocyte differentiation was significantly repressed by BBR (Figs. 1A and 1B). BBR dramatically reduced lipid droplet accumulation and suppressed the expression of PPARγ and adipogenic marker genes including ADD1, FAS, and aP2. On the contrary, we observed the stimulatory effects of BBR on osteogenesis. In mesenchymal stem cells, including C3H10T1/2 and primary bone marrow cells, BBR greatly enhanced ALP+ cells (about 2- to 3-fold; Figs. 1C and 1D). Furthermore, the expression of osteogenic marker genes, including osteopontin and osteocalcin, was elevated by BBR (Fig. 1E). These results suggest that BBR might negatively regulate adipocyte differentiation, whereas it could promote osteoblast differentiation in mesenchymal stem cells.
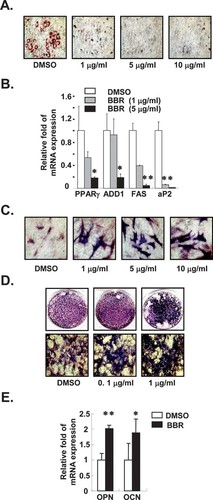
BBR inhibits adipogenesis and promotes osteogenesis. (A and B) C3H10T1/2 cells were differentiated into adipocytes with or without BBR (1∼10 μg/ml) for 5 days. (A) Oil red O staining was used for detection of accumulated lipid droplets. (B) Relative amounts of each mRNA for PPARγ, ADD1, FAS, and aP2 genes were determined using real-time PCR. Values are normalized to the levels of GAPDH mRNA. (C) C3H10T1/2 cells and (D) primary bone marrow cells were differentiated into osteoblasts with or without different amounts of BBR for 6 and 8 days, respectively. Differentiated osteoblasts were stained for ALP+ cells using ALP staining. (E) C3H10T1/2 cells were differentiated into osteoblasts with or without BBR (1 μg/ml) for 6 days. Relative amounts of OPN and OCN mRNAs were determined using real-time PCR. Values are normalized to the levels of GAPDH mRNA. Values are expressed as the mean ± SD. *p < 0.05; **p < 0.01. BBR, berberine; PPARγ, peroxisome proliferator-activated receptor γ; ADD1, adipocyte determination and differentiation dependent factor 1; FAS, fatty acid synthase; aP2, adipocyte fatty acid-binding protein; OPN, osteopontin; and OCN, osteocalcin.
BBR stimulates osteoblast differentiation in MC3T3-E1 cells
To further study the effect of BBR on osteoblast differentiation, we treated the osteoblast cell line MC3T3-E1 with BBR during osteogenesis. As shown in Fig. 2A, BBR promoted the numbers of ALP+ cells in a dose-dependent manner (at least >2-fold). In addition, the expression of osteopontin and osteocalcin mRNA was greatly induced by BBR (Fig. 2B), and the expression level of these genes was proportional to the BBR treatment periods (Fig. 2C). However, other osteogenic genes such as Runx2 and osterix were moderately enhanced by BBR (Fig. 2B). These results suggest that BBR is able to induce osteogenic gene expression and eventually promote osteogenesis not only in mesenchymal stem cells but also in osteogenic cell lines.
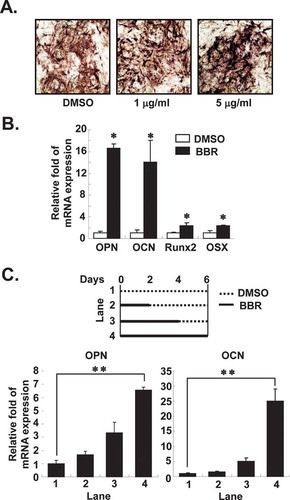
BBR enhances osteoblast differentiation in MC3T3-E1 cells. (A) MC3T3-E1 cells were treated with different amounts of BBR for 6 days during osteoblastic differentiation. ALP+ cells were monitored by the ALP staining. (B) MC3T3-E1 cells were differentiated for 6 days in the presence or absence of BBR (5 μg/ml). Relative amounts of mRNA such as OPN, OCN, Runx2, and OSX were determined using real-time PCR. Values are normalized to the levels of GAPDH mRNA. (C) MC3T3-E1 cells were treated with BBR (5 μg/ml) as indicated time periods during differentiation. Relative amounts of mRNA were determined using real-time PCR. Values are normalized to the levels of GAPDH mRNA. Runx2, Runt-related transcription factor 2 and OSX, osterix. Values are expressed as the mean ± SD. *p < 0.05; **p < 0.01.
BBR stimulates transcriptional activity of Runx2 with co-factors
Tissue-specific activation of the osteogenic marker gene is often associated with chromatin modification and recruitment of transcriptional co-factors.21, 25 In accordance with these results, we recently reported that p300 recruitment to the osteocalcin promoter is significantly increased during osteogenesis, whereas HDAC1 recruitment is decreased, resulting in hyperacetylation of that region.21 Because both osteocalcin and osteopontin are well-known target genes of Runx2, we decided to examine the effects of BBR on the transcriptional activity of Runx2 with these genes. As shown in Fig. 3A, BBR significantly promoted Runx2 transcriptional activity at the osteocalcin promoter when we conducted the reporter assays with HEK293 cells. Because it has been reported that Runx2 phosphorylation stimulates its transcriptional activity at the osteocalcin promoter,26 we assessed whether BBR would affect Runx2 phosphorylation or not. Interestingly, BBR enhanced Runx2 phosphorylation without change of Runx2 protein amounts in HEK293 cells (Fig. 3B). To decipher the mechanism by which BBR-mediated Runx2 phosphorylation augments its target gene expression, we performed ChIP assays. It is interesting to observe that BBR increased the recruitment of Runx2 to the osteopontin promoter (Fig. 3C). Furthermore, BBR promoted the recruitment of p300, a HAT co-activator, to the osteopontin and osteocalcin promoters, whereas it greatly decreased the recruitment of HDAC1, a co-repressor, to the promoters of target genes (Fig. 3C). Consistently, osteopontin and osteocalcin mRNA expressions were upregulated by BBR with no change of endogenous Runx2 protein amounts in MC3T3-E1 cells (Fig. 3D). Taken together, these results clearly indicate that BBR would accelerate osteogenesis by increasing the Runx2 transcriptional activity, probably by increasing Runx2 phosphorylation and modulating co-factor recruitments at its target genes during osteoblast differentiation.
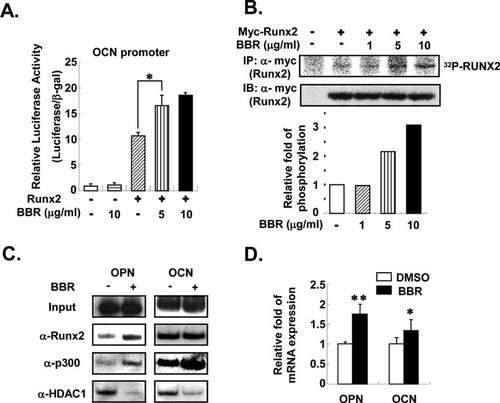
BBR increases transcriptional activity of Runx2 by phosphorylation and co-factor recruitments. (A) A reporter construct containing the OCN promoter region was cotransfected with Myc-tagged Runx2 in HEK293 cells. Runx2 transcriptional activity was determined with or without BBR. Luciferase activities were determined 24 h after transfection. Transfection efficiency was normalized with β-gal activity. (B) Degree of Runx2 phosphorylation was measured with or without BBR. Myc-tagged Runx2 was transfected into HEK293 cells. After incubating with [32P] orthophosphate in phosphate-free DMEM medium, Myc-Runx2 protein was immunoprecipitated with anti-Myc antibodies (α-Myc) and resolved in SDS-PAGE. Western blot analysis was carried out using antibodies against Myc (α-Myc). Relative amounts of phosphorylated Runx2 levels were normalized to the level of Myc-Runx2 without BBR. Phosphorylation of Runx2 was quantified with PhosphorImager (BAS2500; Fuji). (C and D) MC3T3-E1 cells were differentiated into osteoblasts for 2 days and treated with BBR (10 μg/ml) for 12 h. (C) ChIP assays were performed for detection of co-factor recruitment to osteogenic marker gene promoters. MC3T3-E1 cells were cross-linked with formaldehyde, and isolated nuclei were immumoprecipitated with the indicated antibodies. Immunoprecipitated DNA fragments were amplified by PCR at the promoter regions of the indicated genes. Input represents 10% of the total input chromatin. (D) Relative amounts of mRNA for OPN and OCN were determined by real-time PCR in MC3T3-E1 cells after treatment with BBR for 12 h. Values are normalized to the levels of GAPDH mRNA. Values are expressed as the mean ± SD. *p < 0.05; **p < 0.01.
BBR signaling is dependent on p38 MAPK pathway
Recent studies have shown that BBR is capable of activating MAPK pathways, including p38 MAPK and extracellular signal–regulated kinase (ERK) MAPK.17, 27 To study whether BBR stimulates MAPK signaling in osteoblasts, MC3T3-E1 cells were challenged with or without BBR, resulting in the increase in p38 MAPK phosphorylation by BBR (Fig. 4A). Furthermore, BBR augmented phosphorylation of activating transcription factor 2 (ATF2), a substrate for p38 MAPK in HEK293 cells (Fig. 4B), indicating that BBR would stimulate p38 MAPK kinase activity in osteoblasts.
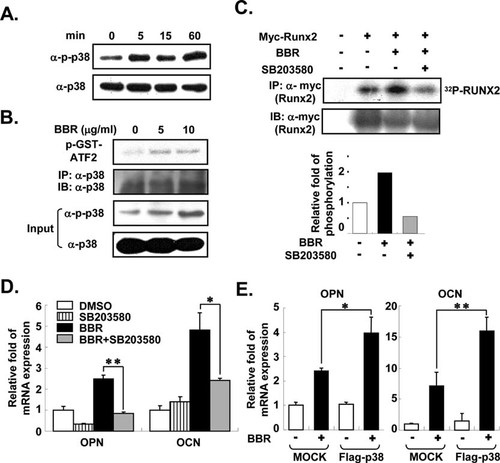
BBR stimulates osteogenesis in a p38 MAPK-dependent manner. (A) BBR (5 μg/ml) was treated for the indicated time periods in MC3T3-E1 cells. Western blot analyses were conducted using antibodies against phosphorylated p38 (α-p-p38) and p38 (α-p38). (B) p38 MAPK kinase activity was determined in the absence or presence of BBR. HEK293 cells were treated with several doses of BBR for 2 h. Whole cell lysates were immunoprecipitated with anti-p38 MAPK antibody (α-p38), and in vitro kinase assays were carried out using GST-ATF2 peptide substrates at 30°C for 30 min. Western blot analyses were performed using antibodies against phosphorylated p38 (α-p-p38) and p38 (α-p38). The level of p38 MAPK was determined as the loading control. Input represents 10% of the total input immunoprecipitation. (C) In vitro Runx2 phosphorylation was determined as described above after pretreatment of HEK293 cells with SB203580 (10 μM), a p38 MAPK-specific inhibitor. SB203580 was pretreated for 1 h before treatment with BBR (10 μg/ml). Western blot analysis was conducted using antibodies against Myc (α-Myc). Relative amounts of phosphorylated Runx2 levels are normalized to the level of Myc-Runx2 without BBR. Runx2 phosphorylation was quantified with PhosphorImager (BAS2500; Fuji). (D and E) Relative amounts of mRNA for OPN and OCN were determined using real-time PCR. Values are normalized to the levels of GAPDH mRNA. (D) MC3T3-E1 cells differentiated into osteoblasts for 6 days with or without BBR (5 μg/ml) and SB203580 (10 μM). (E) MC3T3-E1 cells were infected with mock or Flag-p38 MAPK retrovirus and induced for osteoblast differentiation for 8 days with or without BBR (5 μg/ml). Values are expressed as the mean ± SD. **p < 0.01. GST-ATF2, glutathione S-transferase-activating transcription factor 2; MOCK, pWZL vector-infected cells; and Flag-p38, Flag-p38 MAPK-infected cells.
Because MAPK signaling pathways have been shown to play a key role in the phosphorylation of Runx2,26, 28 we studied whether activation of p38 MAPK is involved in Runx2 phosphorylation by BBR. To investigate this, SB203580, a p38 MAPK-specific inhibitor, was co-treated with BBR, and great inhibition of Runx2 phosphorylation was observed (Fig. 4C), implying that p38 MAPK is required for BBR-induced Runx2 phosphorylation. Furthermore, SB203580 prevented the BBR-dependent increase in osteopontin and osteocalcin gene expression (Fig. 4D). To directly elucidate whether p38 MAPK is important for effects of BBR on osteoblast differentiation, we examined the effect of BBR on p38 MAPK-overexpressing MC3T3-E1 cells. BBR significantly elevated the mRNA expression of osteopontin and osteocalcin to 60% and 33%, respectively, in p38 MAPK-overexpressing osteoblasts (Fig. 4E). Thus, these results indicate that enhanced osteoblast differentiation with BBR is primarily mediated by the p38 MAPK pathway.
COX-2 expression is increased by BBR
To comprehend the mechanism by which BBR promotes osteogenesis, we analyzed the expression of subsets of osteogenic marker genes including Runx2, osterix, ALP, and COXs in MC3T3-E1 cells with or without BBR using real-time PCR analyses. Interestingly, we observed that BBR remarkably increased COX2 expression. BBR treatment on the MC3T3-E1 cells promoted both the mRNA and protein levels of COX2 (Figs. 5A and 5B). Moreover, COX2 expression was significantly increased by BBR during osteoblast differentiation (Fig. 5C). It has recently been shown that the promoter of the COX2 gene has a Runx2-binding site and is regulated by Runx2 transcriptional activity.29 To determine whether BBR increases COX2 expression by Runx2, we conducted a luciferase reporter assay using the COX2 promoter in the absence or presence of Runx2 or BBR in HEK293 cells. Similar to a previous report,29 Runx2 stimulated COX2 promoter activity and BBR additionally enhanced Runx2 transcriptional activity at the COX2 promoter (Fig. 5D), implying that COX2 is the target gene of Runx2 and its expression is regulated by BBR. Next, we studied the recruitment of Runx2 and co-factors onto the promoter regions of COX2 with or without BBR. Similar to the osteopontin gene, recruitments of Runx2 and p300 to the COX2 promoter were markedly increased by BBR, whereas the recruitment of HDAC1 to that promoter region was decreased (Fig. 5E). In addition, the COX2-specific inhibitor Dup697 repressed the effect of BBR on osteogenic marker gene expression (Fig. 5F), implying that BBR-induced COX2 activity would be involved in osteoblast differentiation.
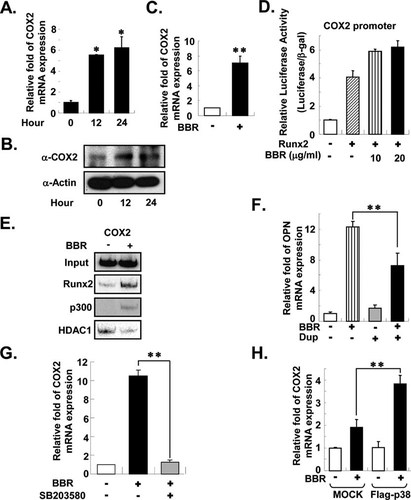
COX2 expression is induced by BBR through p38 MAPK. (A and B) BBR (5 μg/ml) was treated for the indicated time periods in MC3T3-E1 cells. (A) The level of COX2 mRNA was measured by real-time PCR. Values are normalized to the levels of GAPDH mRNA. (B) The protein level of COX2 was detected by western blot analysis. Antibodies against actin (α-Actin) were used as the loading control. (C) MC3T3-E1 cells were differentiated for 6 days in the presence or absence of BBR (5 μg/ml). Relative amounts of COX2 mRNA were determined using real-time PCR. Values are normalized to the levels of GAPDH mRNA. (D) A reporter construct containing the COX2 promoter was co-transfected with Myc-tagged Runx2 in HEK293 cells. Runx2 transcriptional activity was determined with or without BBR. Luciferase activities were determined 24 h after transfection. Transfection efficiency was normalized using β-gal activity. (E) MC3T3-E1 cells were differentiated into osteoblasts for 2 days and treated with BBR (10 μg/ml) for 12 h. ChIP assays were performed as described above. Immunoprecipitated DNA fragments were amplified by PCR at the promoter regions of COX2. Input represents 10% of the total input chromatin. (F and G) MC3T3-E1 cells were incubated with osteogenic differentiation media for 6 days with or without BBR (5 μg/ml), Dup (10 μM), a COX2-specific inhibitor, and SB203580 (10 μM). Relative amounts of mRNA for OPN and COX2 were determined using real-time PCR. Values are normalized to the levels of GAPDH mRNA. (H) MC3T3-E1 cells overexpressing p38 MAPK were differentiated with or without BBR for 8 days. Relative amounts of mRNA were determined using real-time PCR. MOCK-infected MC3T3-E1 cells were used as control. Values are normalized to the levels of GAPDH mRNA. Values are expressed as the mean ± SD. **p < 0.01. Dup, Dup697. MOCK, pWZL vector-infected cells; Flag-p38, Flag-p38 MAPK-infected cells.
In addition, we studied whether the BBR-induced increase in COX2 expression is dependent on p38 MAPK. The effect of BBR on COX2 mRNA expression was decreased when p38 MAPK was inhibited with SB203580 (Fig. 5G); however, this effect was significantly augmented on overexpression of p38 MAPK (Fig. 5H). Taken together, it is feasible to speculate that BBR-induced COX2 expression seems to be mediated by p38 MAPK and Runx2, and BBR would stimulate osteoblast differentiation through the activation of p38 MAPK as well as Runx2.
BBR increases new bone area
To confirm the effect of BBR on new bone area formation, we treated ex vivo calvarial organ cultures from new born mice with BBR. Similar to simvastatin, which has been known to induce bone area formation,30 BBR remarkably increased new bone area (Fig. 6). Therefore, these results indicate that BBR could induce new bone area formation by stimulating osteoblast differentiation.
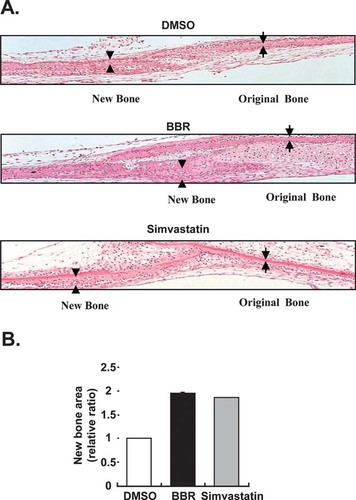
Effects of BBR on neonatal murine calvaria. (A) Parietal skullcaps were explanted and cultured for 4 days with or without BBR (0.05 μg/ml) and simvastatin (1 μM). Sections of cultured neonatal calvarial bones were stained with H&E. (B) Relative widths of new bone area by BBR and simvastatin were presented. Original and new bone areas were indicated as arrow and arrowhead, respectively.
DISCUSSION
Osteoporosis is a major public health problem that most commonly occurs in elderly women as a result of increased bone resorption caused by estrogen deficiency after menopause. Indeed, most bone therapies such as selective estrogen receptor modulators (SERMs) and bisphosphonates are currently available to repress bone resorption by osteoclasts. Moreover, it is also important to fill up the resorbed bone regions that show increased osteoblast differentiation. Recently, the development of effective stimulators for bone formation, such as statins (inhibitors of HMG-CoA reductase), has been in the limelight because these stimulators augment the expression and activity of BMP-2 and promote mineralization in osteoblasts.31, 32 Thus, it is probable that the development of anabolic drugs, which can induce osteoblast differentiation, would be beneficial to cure for bone-related diseases such as osteoporosis by recovering the resorbed bone regions.
Fatty marrow, which contains accumulated adipocytes in the bone marrow, is clinically observed in patients with bone diseases such as osteoporosis.33, 34 Because adipocytes and osteoblasts originate from mesenchymal stem cells,35 it would be clinically and biologically important to understand the regulatory mechanism for adipogenesis and osteogenesis. PPARγ is a master regulator of adipogenesis that represses Runx2 transcriptional activity, and PPARγ ligands such as rosiglitazone inhibit osteoblast differentiation.36, 37 Thus, there is probably a counterbalanced relationship between osteogenesis and adipogenesis, implying that PPARγ might be a potential therapeutic target for osteoporosis. Recently, several groups including our group have shown that BBR strongly inhibits adipocyte differentiation through repression of PPARγ activity.16, 17 In this study, we provided the first evidence that BBR promotes osteoblast differentiation with an increase in the phosphorylation and activity of Runx2 through the p38 MAPK pathway in vitro, and we verified the effects of BBR by an ex vivo bone formation study.
Runx2 activity is regulated by several covalent modifications including ubiquitination, acetylation, and phosphorylation.28, 38, 39 Among these, the role of Runx2 phosphorylation has been well characterized. Runx2 has multiple phosphorylation sites, and its phosphorylation events at different residues would confer inhibitory or stimulatory effects. For instance, Runx2 is negatively regulated by the phosphorylation at two serine residues (S104 and S451).40 On the other hand, fibroblast growth factor 2 (FGF2) induces Runx2 phosphorylation through ERK1/2 MAPK at the C-terminal region and increases Runx2 activity.26 Furthermore, FGF2 promotes Runx2 phosphorylation through PKCδ and elevates Runx2 transcriptional activity.41 Here, we showed that BBR greatly increased Runx2 phosphorylation and transcriptional activity, which were potently repressed by SB203580, suggesting that BBR would induce phosphorylation and activation of Runx2 through p38 MAPK activation. Furthermore, it has been reported that inhibition of Runx2 phosphorylation results in a significant loss of the Runx2 DNA binding activity,42 implying that Runx2 phosphorylation might enhance its DNA binding ability. Consistent with these results, we showed that Runx2 recruitment on osteopontin and COX2 promoters was increased by treatment with BBR. However, it is unclear which Runx2 protein residue(s) is phosphorylated by the action of BBR-mediated p38 MAPK. Indeed, we cannot rule out the possibility that unknown modifications of Runx2 other than phosphorylation may be induced by BBR to affect its DNA-binding ability. To answer this question, further studies are needed to identify the exact Runx2 phosphorylation site(s) induced by BBR and to study the effects of Runx2 mutants on the DNA binding affinity as well as transcriptional activity.
To confer osteoblast differentiation, Runx2 activity is also tightly regulated by association with co-factors. For instance, co-repressors including HDACs and TLE interact with Runx2 and repress Runx2 transcriptional activity.21, 43, 44 On the contrary, on osteogenic signals, it seems that co-activators, including p300 and TAZ, replace co-repressors from Runx2 and stimulate its transcriptional activity.45, 46 Surprisingly, we discovered that BBR treatment on MC3T3-E1 cells enhanced the association of Runx2 and p300 at the osteopontin and osteocalcin promoters, as well as COX2 promoters, whereas recruitment of HDAC1 to these regions was dramatically reduced (Figs. Figure 3, Figure 5). Therefore, it is feasible to suggest that Runx2 phosphorylation by BBR would lead to conformational changes that might change association between Runx2 and co-factors at the target gene promoters. Thus, the idea that phosphorylated Runx2 might have higher affinity to co-activators than co-repressors should be investigated in future studies.
To date, activation of p38 MAPK has been implicated in the differentiation of a variety of cell types such as myocytes and adipocytes.47, 48 Regarding osteogenesis, it has been shown that p38 MAPK is required for BMP2-induced osteogenesis in osteoblasts49 and that p38 MAPK-specific inhibitor potently inhibits osteoblast differentiation in primary calvarial osteoblasts.50 In this work, we showed that BBR stimulated the phosphorylation of p38 MAPK and its kinase activity, which eventually promoted the execution of osteoblast differentiation. Several lines of evidence suggest that Runx2 may be a substrate for BBR-activated p38 MAPK in osteoblasts. First, inhibition of p38 MAPK with SB203580 dramatically reduced Runx2 phosphorylation. Second, overexpression of p38 MAPK in MC3T3-E1 cells reinforced the effects of BBR on the expression of Runx2 target genes such as osteopontin and osteocalcin. Although there is a possibility that BBR might affect other MAPKs (or kinases) in osteoblasts, it is plausible to propose that involvement of p38 MAPK in Runx2 activation is at least one of the critical steps for the BBR-dependent increase in osteoblast differentiation.
Regarding BBR-induced signaling pathways including MAPKs, BBR potently activates AMPK in several tissues including liver and fat tissues.17 In osteoblasts, BBR increased AMPK phosphorylation after 1 h, whereas increase in p38 MAPK phosphorylation by BBR was monitored within 15 min (data not shown), implying that p38 MAPK seems to be a more upstream responding kinase than AMPK. Moreover, we observed that AMPK inhibitor (AraA) and activator (AICAR) did not significantly influence osteogenesis (data not shown), which suggests that AMPK activation by BBR would not contribute to osteoblast differentiation.
COX2, which produces PGE2, is considered as an important regulator in bone formation.12, 51 Our data clearly indicated that BBR in osteoblasts activates p38 MAPK, which elevates COX2 expression by stimulation of Runx2 activity. SB203580 consistently repressed the transcriptional activity of Runx2 on COX2 gene expression, whereas overexpression of p38 MAPK enhanced the effects of BBR on COX2 expression. Although it has been reported that COX2 could be regulated by MAPKs,14 this is the first study that showed that the p38 MAPK-Runx2 pathway governs COX2 expression in osteoblasts. On the whole, it is likely that BBR stimulates osteoblast differentiation not only by enhancing osteogenic marker gene expression but also by inducing COX2 expression through the p38 MAPK-Runx2 pathway (Fig. 7).
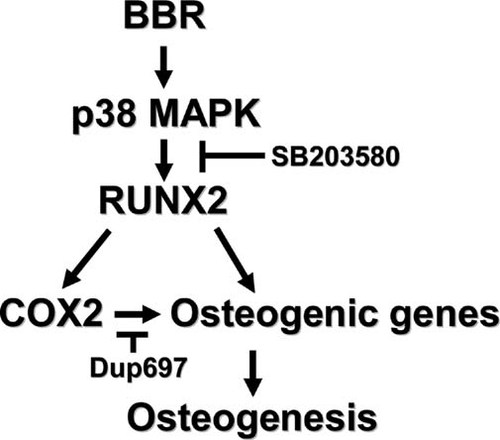
Model for BBR-mediated osteoblast differentiation. In osteoblasts, BBR activated p38 MAPK, which increased Runx2 phosphorylation. BBR-induced Runx2 activation would promote its target gene expressions as well as COX2. Multiple effects of BBR on osteogenesis are potently repressed by specific p38 MAPK-specific inhibitors.
Collectively, BBR activated the p38 MAPK-Runx2 pathway, which enhanced osteoblast differentiation and resulted in bone area formation in an ex vivo calvaria culture model. Although future studies will show whether BBR induces bone formation in vivo, the data presented here suggest that BBR could be a potential therapeutic agent by regulating osteoblast differentiation in osteoporotic bone regions.
Acknowledgements
The authors thank Yun Sok Lee and Joo-Won Lee for critically reading the manuscript and Carol Pilbeam for the pXP-2-COX2-Luc plasmid. This work was supported in part by grants from the Stem Cell Research Center of the 21st Century Frontier Research Program (SC3230), the National Research Laboratory Program of the Korea Science and Engineering Foundation (ROA-2004-000-10359-0), the Research Center for Functional Cellulomics of Science Research Center Program (R11-2005-009-01002-0) (to JBK), and the Ministry of Health and Welfare of Korea (01-PJ3-PG6-01GN11-0002) (to JYC). HWL, JHS, AYK, and JBK are supported by the BK21 Research Fellowship from the Ministry of Education and Human Resources Development.




