Abstract
To understand the function of human hydroxysteroid (17β) dehydrogenase 2 (HSD17B2) in the peripheral tissues in vivo, we studied the bone development in transgenic male mice ubiquitously expressing human HSD17B2. Bones of HSD17B2TG and WT males (26 days and 2 and 6 mo old) were analyzed by pQCT and histomorphometry, and data were correlated with serum testosterone (T), IGF-I, and osteocalcin concentrations. At the age of 26 days, the body weight of HSD17B2TG males was significantly lower, and the lengths of the tibia and femur of the HSD17B2TG males were significantly shorter. Histomorphometric and pQCT analyses showed lower trabecular and cortical BMD, a markedly smaller area of cortical bone at both of the diaphyses, and a smaller percentage of trabecular bone volume and thickness in the HSD17B2TG males. The data suggested slower osteoblast differentiation and a slower bone formation rate of femoral diaphysis on the periosteum but faster on the endocortical surface in HSD17B2TG males. The altered bone parameters were correlated with low serum T, IGF-I, and osteocalcin concentrations at the prepubertal age. Interestingly, after puberty, the bone parameters analyzed in the adult HSD17B2TG males were mostly normal, consistent with the normal body weight and normalized serum concentrations of IGF-I and T. In conclusion, HSD17B2TG males presented with growth retardation and a decreased bone formation rate at prepubertal age. These changes were associated with lower serum IGF-I, osteocalcin, and T concentrations. It is concluded that the enforced constitutive expression of HSD17B2 disturbs the coordinated action of IGF-I and sex steroids essential for pubertal bone growth.
INTRODUCTION
Sex steroids are essential for normal skeletal development and for maintenance of bone remodeling in both sexes.1 They participate in the regulation of cortical and cancellous bone metabolism and stimulate the acquisition of peak bone mass and inhibit bone loss in males and females.2, 3
The skeleton is one of the target organs for estrogens, whereas the pubertal growth spurt of both sexes is driven primarily by estrogen.4 Men with a homozygous inactivating mutation in the estrogen receptor 1 gene (Esr1, ERα) or with Cyp19a1 (P450 aromatase) deficiency present with increased bone turnover, osteoporosis, unfused epiphyses, delayed bone age, and continued linear growth into adulthood.5-8 There is, therefore, a compelling evidence for an important role of estrogens in the maintenance of the male skeleton.9, 10 Observations have shown that age-associated decline in male BMD is more directly related to declining estrogen level than to declining androgen level. This suggests that the control of bone turnover in older men is predominantly controlled by estrogens. Accordingly, in the testicular feminized (Tfm) rat, cancellous bone volume is similar to that of normal male littermates.11 However, orchidectomy, which removes the testosterone (T) that is the precursor for estradiol (E2) production, prevents the attainment of normal cancellous bone volume, suggesting a role for estrogens in bone development in males.11, 12
Despite the central role of estrogens in the skeletal development and maintenance, the role of androgens in growth of the skeleton during puberty is supported by several observations. Administration of T to young men with overt hypogonadism before epiphyseal closure leads to increases in bone mass,13 and T administration to prepubertal boys results in increased bone calcium accretion.14 Animal studies in vivo have shown that the effects of androgens on bone growth are manifested particularly by increasing bone size, with male animals having both larger bones and thicker cortices than their female counterparts.15 Hypogonadism in men is associated with increased bone turnover, which may result in osteoporosis, and T supplementation increases BMD in eugonadal osteoporotic men.16, 17 Androgen replacement is also associated with significant reduction in bone resorption markers.18 An imbalance between bone formation and resorption results in the loss of cancellous and cortical bone in the orchidectomized male rats.19 The skeletal effects of castration in male mice can be prevented by the administration both of T and nonaromatizable androgens, indicating that aromatization of androgens to estrogens could not be totally responsible for androgenic effects on the skeleton.20, 21
HSD17B2 is one of the several hydroxysteroid (17β) dehydrogenases (HSD17Bs) identified thus far.22 HSD17B2 has been characterized in several mammals including humans.23 The catalytic properties of HSD17B2 have been studied in a variety of cell lines in vitro. These studies have shown that the enzyme predominantly catalyzes the inactivation of the 17β-hydroxy forms of sex steroids to the 17β-keto forms.24 The predominant oxidative activity of HSD17B2 with broad tissue distribution and broad substrate specificity25 indicates that the enzyme could play an essential role in the inactivation of highly active 17β-hydroxy steroids at target tissues of hormone action, thereby protecting the tissues against excessive sex hormone influence. Despite several studies on enzyme characteristics in vitro, little is known about the physiological role of HSD17B2 in vivo. To study its function further and to analyze the role of HSD17B2 in the regulation of local steroid availability in the target tissues in vivo, we generated transgenic mice (TG) ubiquitously expressing human HSD17B2 under the CMV-enhanced chicken β-actin promoter (HSD17B2TG). The TG male mice showed delayed eye opening at postnatal age and disrupted spermatogenesis at adulthood in the presence of normal serum and intratesticular T concentrations.26 In this study, we investigated the bone phenotype in HSD17B2TG males.
MATERIALS AND METHODS
Animals
The generation of HSD17B2TG mice and the testicular phenotype with disrupted spermatogenesis has been described elsewhere.26 In this study, eight young (26 days old), six young adult (2 mo old), and eight adult (6 mo old) HSD17B2TG males together with eight wildtype (WT) FVB/N male littermates at each age group were studied. These age groups were selected based on the preliminary data indicating that, at the age of 26 days and 2 mo, the body weight of the TG mice was significantly lower than WT males, and at the age of 6 mo, the TG males had gained a normal body weight.26 Genotyping of the HSD17B2TG mice was carried out by two different PCR analyses using DNA extracted from the ear biopsies.
The young male mice at the age of 26 days were given intraperitoneal injections of oxytetracycline (20 mg/kg; Pfizer) and calcein (10 mg/kg; Sigma, St Louis, MO, USA) 5 and 2 days, respectively, before the mice were killed. The same injections for the adult mice at the age of 2 and 6 mo were given 8 and 2 days, respectively, before death. Animals were housed one to four per cage in controlled conditions of light and temperature with commercial soy-free mouse chow (SDS, Witham, UK) and tap water ad libitum. The animals were handled in accordance with the institutional animal care policies of the University of Turku, with appropriate permissions (Turku, Finland).
Onset of male puberty
For analyzing the onset of male puberty, HSD17B2TG and WT males of the same litters were examined daily for the balanopreputial separation between 25 and 40 days of age. Puberty was defined as the day of complete balanopreputial separation.
Measuring hormones and growth factors
The mice were anesthetized by intraperitoneal injection of 300–600 μl 2.5% avertin (2,2,2-tribromoethyl alcohol + tert-amyl alcohol), and blood was collected by cardiac puncture. Serum samples were separated by centrifugation and stored at –20°C until used for hormone measurements. Serum T concentrations were measured by a radioimmunoassay after diethyl ether extraction,27 and intratesticular T concentrations were measured with the same method after homogenizing the tissue in PBS buffer, followed by diethyl ether extraction.
Serum osteocalcin and IGF-I levels were measured with commercially available ELISA methods (Biomedical Technologies and R&D Systems, respectively) according to the manufacturer's instructions. The sensitivity of the osteocalcin and IGF-I measurements was 1 ng/ml and 3.5 pg/ml, respectively.
pQCT
For BMD measurement, the right tibias and femora were stored at –20°C and measured as described previously.28 Shortly thereafter, the tibias and femora were thawed at room temperature for 1 h. After removing the soft tissue, the bones were fixed in a plastic tube and scanned by pQCT (XCT 540, Stratec; Norland Medical Systems). For tibial measurements, three cross-sections, with a 0.5-mm distance between each other, were first measured 1.5 mm below the proximal end of the tibia. Thereafter, two additional sections with a distance of 0.5 mm were measured, 3 mm above the tibiofibular junction. For femur, two sections were first measured starting 1.8 mm above the distal end, and one additional section was measured 6 mm from the distal end.
Bone histomorphometry
For histomorphometric study, the left tibias and femora were stored in 96% ethanol at 4°C, dehydrated, degreased, and embedded into methylmethacrylate as described previously.28 From tibias, 4- and 8-μm-thick longitudinal undecalcified sections were prepared, and cross-sections of 80 μm in thickness were harvested 6 mm above the distal end of the femur by a low-speed saw. The 4-μm-thick sections were stained using the Masson-Goldner-trichrome method, and the histomorphometric data of trabecular bone in proximal tibia were measured. An area of 0.75 mm2, locating 0.5 mm below the growth plate of young mouse bones and just below the growth plate in the adult mice, was used for the measurements. The 8- and 80-μm unstained sections were used to measure the dynamic parameters of bone formation. All histomorphometric parameters were based on the nomenclature recommended by the American Society of Bone and Mineral Research and were preformed with the OsteoMeasure system (Version 3.21; OsteoMetrics, Atlanta, GA, USA).
RT-PCR analysis
Primary osteoblasts were obtained from neonatal mice according to a method described elsewhere.29 The calvaria (frontal and parietal bones of skull) were collected from 2- or 3-day-old neonatal mice. The periostea were removed from the calvaria, and the collected calvaria were subjected to collagenase-dispase digestion with 0.1% collagenase-I (Worthington Biochemical) and 0.2% dispase (Invitrogen) for 7 min at 37°C. The first two cell fractions containing mesenchymal cells were discarded, whereas the last three cell fractions were collected and used as the primary osteoblasts.
Total RNA was isolated from the primary osteoblasts with the RNeasy Mini Kit (Qiagen, Hilden, Germany), and 1 μg of total RNA was treated with DNase I (Invitrogen). cDNA was synthesized using reverse transcriptase (Finnzymes Oy, Espoo, Finland), and PCR was performed using the primers for Cbfa1/AML3 core binding factor α/acute myelogenous leukemia (Runx2), osteopontin (OPN), osteocalcin (OCN), and collagen type 1a (COL) with 30 cycles. The sequences of primers were as follows: Runx2 (NM_009820), forward 5′-CCAACCGAGTCATTTAAGGCT-3′, reverse 5′-GCTCACGTCGCTCATCTTG-3′ (56°C, 207 bp); OPN (NM_009263), forward 5′-AGCAAGAAACTCTTCCAAGCAA-3′, reverse 5′-GTGAGATTCGTC-AGATTCATCCG-3′ (56°C, 134 bp); OCN (NM_001032298), forward 5′-CGGCCCTGAGTCTGACAAA-3′, reverse 5′-GCCGGAGTCTGTTCACTACCTT-3′ (56°C, 68 bp); COL (NM_007742), forward 5′-CCTGGTAAAGATGGTGCC-3′, reverse 5′-CACCAGGTTCACCTTTCGCACC-3′ (59°C, 223 bp). Mouse ribosomal protein L19 (L19), a housekeeping gene, was used as a control, and the specific primer for human Hsd17b2 was used to detect the expression of transgene in the osteoblasts.26
Statistical analysis
Statistical analyses were performed using the SigmaStat program (SYSTAT Software). The results were analyzed by t-test or Mann-Whitney rank sum test. All values are expressed as mean ± SD, and p < 0.05 was considered significant.
RESULTS
Body weight and bone growth
The growth of HSD17B2TG male mice was slower after birth, and the body weight was significantly lower at the age of 26 days and 2 mo (Fig. 1). Accordingly, the tibia and femur length of HSD17B2TG males was significantly shorter compared with those of the WT controls at the age of 26 days (Fig. 2A). Also, the ash weight of the tibia and femur were much lower in HSD17B2TG males (Fig. 2B). However, at the age of 2 and 6 mo, the differences in tibia and femur length between the TG and WT animals were obviously decreased, and the differences of the bone ash weights had disappeared.
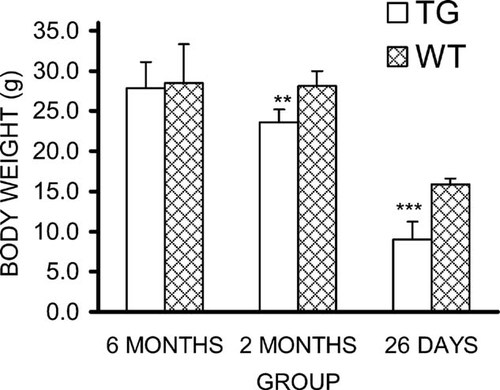
Body weight of HSD17B2TG (TG) and wildtype (WT) male mice at different ages. HSD17B2TG males showed significantly lower body weight than WT males at the age of 26 days and 2 mo. **p < 0.01; ***p < 0.001.
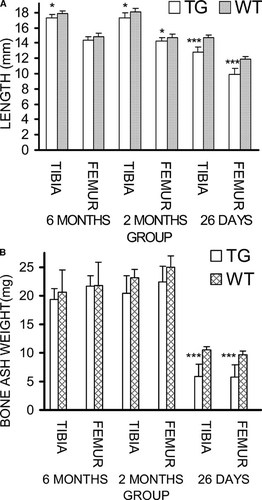
(A) Length of tibia and femur and (B) bone ash weight in HSD17B2TG (TG) and wildtype (WT) male mice at different ages. At the age of 26 days, bone length and ash weight were markedly lower, but the parameters recovered rapidly thereafter. *p < 0.05, ***p < 0.001.
pQCT
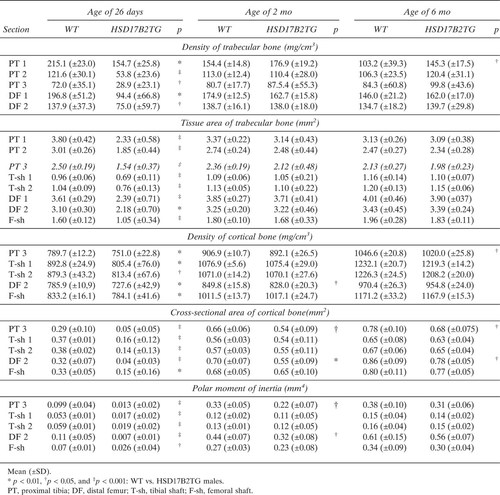
The results of pQCT measurements are shown in Table 1. In the 26-day-old mice, density of both trabecular and cortical bones from all measured bone sites was significantly lower in HSD17B2TG mice than in WT animals. The bone tissue area, cortical bone area, and polar moment of inertia of HSD17B2TG mice were also much smaller compared with WT animals. Interestingly, at adulthood, most parameters in HSD17B2TG mice were normalized in the TG mice. The trabecular BMD in section 1 the of proximal tibia at the age of 6 mo was the only parameter found to be significantly higher in TG males compared with that of WT mice.
Bone histology and morphometry
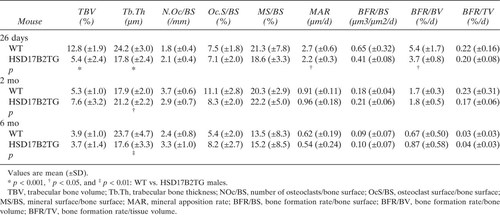
Histomorphometric data of the proximal tibia showed that, in young mice, the percentage of trabecular bone volume and the trabecular bone thickness were significantly decreased in HSD17B2TG males compared with WT controls (Table 2). In adult HSD17B2TG males, trabecular bone volume returned to WT level, but the difference was maintained in the trabecular bone thickness between the animals. However, the indicators of trabecular bone growth were not different between HSD17B2TG and WT mice at the adult age (Table 2). At the age of 26 days, the thickness of the growth plate of the proximal tibia was reduced in HSD17B2TG males compared with the WT controls, but at adult age, the thicknesses were identical (Figs. Figure 3, Figure 4). The growth rate of the growth plate was slower in the HSD17B2TG male mice at the age of 26 days, but at the age of 2 mo, it was faster in TG males (Fig. 3B).
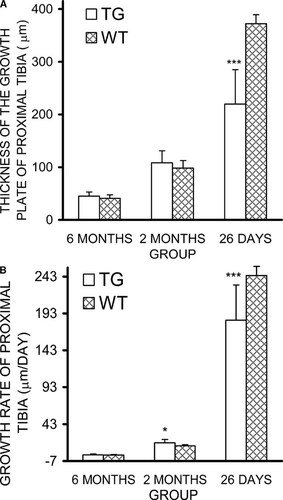
(A) Thickness of the growth plate in HSD17B2TG (TG) and wildtype (WT) male mice at the different ages. (B) The growth rate of the growth plate in mice at the different ages. At the age of 26 days, the growth plate was thinner in TG males compared with WT males, and the growth rate was slower. However, at the age of 2 mo, the growth rate of growth plate was faster than in WT males. The calculation of the growth rate is based on double fluorescence labeling,28 and the distance (μm) between the two fluorescence markers divided by labeling time (days) is defined as the growth rate. *p < 0.05, ***p < 0.001.

Structure of the growth plate of proximal tibia in the wildtype (WT) (A–C) and HSD17B2TG (TG) (D–F) male mice. At the age of 26 days (26D), the width of the growth plate of WT mice was reduced in the TG mice, whereas no difference was detected at adult age, at 2 (2M) and 6 mo (6M). The borders of the growth plate are indicated by the arrows.
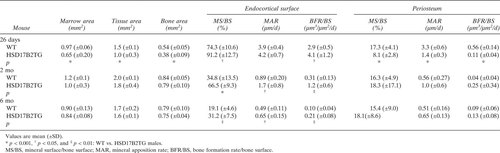
Table 3 shows that HSD17B2 expression in the males induced a thinner femoral shaft in young 26-day-old mice that was normalized at the age of 2 and 6 mo. The obviously decreased bone formation rate at the periosteum after puberty in HSD17B2TG males was returned to the WT level at the adulthood. At the endocortical surface, the bone formation rate was much higher in HSD17B2TG males than in WT animals in the three age groups studied.
Osteocalcin, IGF-I, and T concentrations
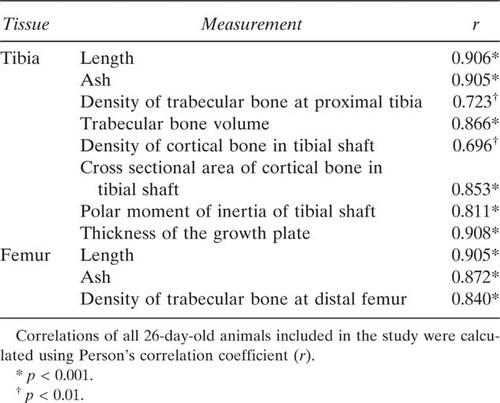
Serum osteocalcin concentration is a marker for bone formation, and in the HSD17B2TG males, the osteocalcin levels were decreased by 44% (p < 0.001; Fig. 5A) at the age of 26 days but were not significantly different in the older age groups. Because the growth hormone (GH)/IGF-I axis is highly sensitive for the changes in overall skeletal size and development, serum IGF-I concentration was measured in HSD17B2TG males at the age of 26 days and found to be 54% lower than in WT males (p < 0.001; Fig. 5B). Interestingly, the serum IGF-I concentration in the HSD17B2TG mice at the age of 2 mo was found to be higher than in WT males (p < 0.05; Fig. 5B). The serum IGF-I levels also correlated well with length, ash weight, BMD, cortical cross-sectional area, polar moment of inertia, and thickness of the growth plate (Table 4). We also measured the serum and intratesticular T concentrations and found those to be lower in HSD17B2TG males compared with WT males at 26 days of age (Fig. 6), but the values were normalized at adulthood. Furthermore, the data indicated that HSD17B2TG male mice presented with delayed onset of puberty. The day of the complete balanopreputial separation was 29.0 ± 0.7 days in WT males and 34.9 ± 1.7 days in HSD17B2TG males.
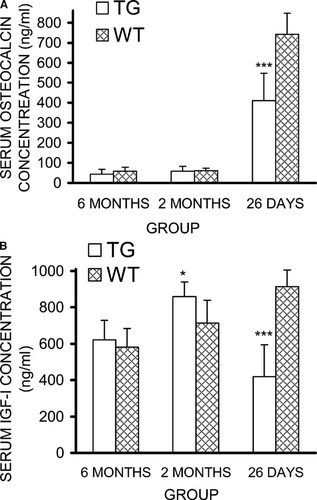
(A) Osteocalcin concentrations and (B) serum IGF-I concentrations in HSD17B2TG (TG) and wildtype (WT) male mice at different ages. At the age of 26 days, TG males showed significantly lower serum IGF-I and osteocalcin concentrations compared with WT males. Furthermore, serum IGF-I concentration in TG males, at the age of 2 mo, is higher than that of WT males. *p < 0.05, ***p < 0.001.
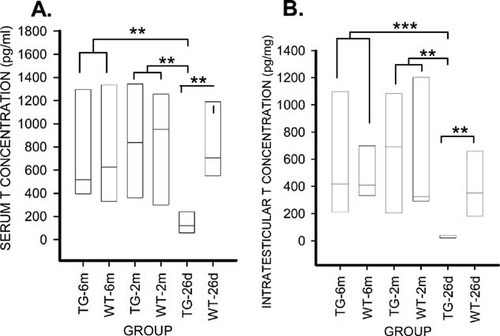
(A) Serum and (B) intratesticular testosterone (T) concentrations in the HSD17B2TG mice (TG) and wildtype (WT) male mice. TG males showed significantly lower serum and intratesticular T levels compared with the WT males at the age of 26 days (26d), whereas no difference was detected at adult age at 2 (2m) and 6 mo (6m). **p < 0.01, ***p < 0.001.
RT-PCR analysis of osteoblast-specific gene expression
To study whether the bone phenotype of HSD17B2TG at prepubertal age is associated with osteoblast differentiation, the primary osteoblasts were isolated from the calvaria of neonatal HSD17B2TG mice (2–3 days old), and RT-PCR was used to analyze the expression of osteoblast-specific gene expression. Three of the marker genes of osteoblast differentiation (Runx2, osteopontin, and osteocalcin) were expressed at a lower level, and one (collagen type 1a) was expressed normally in the osteoblasts isolated from the HSD17B2TG mice (Fig. 7). The human Hsd17b2 transgene expression was also identified in the osteoblasts obtained from TG mice.
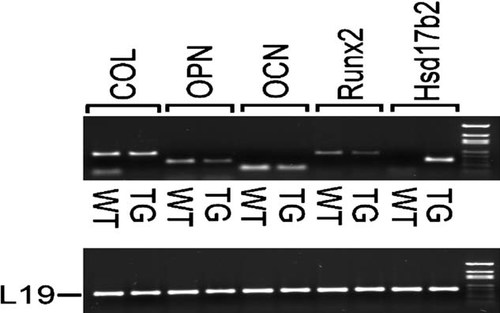
Expression of marker genes for osteoblast differentiation (Runx2, Cbfa1/AML3, core binding factor α/acute myelogenous leukemia; OPN, osteopontin; OCN, osteocalcin; COL, collagen type 1a) in HSD17B2TG (TG) and wildtype (WT) mice. The data showed lower expression of the Runx2, OPN, and OCN in TG mice compared with WT mice. Hsd17b2 indicates the transgene expression.
DISCUSSION
Various skeletal parameters were affected at prepubertal age in male mice universally expressing human HSD17B2. The mice presented with significantly shorter tibias and femurs, lower density of trabecular bone in proximal tibia and distal femur, and lower density of cortical bone of tibial and femoral diaphysis. Furthermore, the areas of cortical bone at both of the diaphyses were smaller. The data also indicated that the percentage of trabecular bone volume and thickness were markedly smaller, and the bone formation rate of femoral diaphysis was slower on the periosteum but higher on the endocortical surface.
The serum IGF-I levels correlated well with length, ash weight, BMD, cortical cross-sectional area, polar moment of inertia, and thickness of the growth plate. The GH/IGF-I axis has an important role in skeletal maturation, pubertal growth, and bone mass accrual.30 The effect of GH and IGF-I on bone mass is derived from their stimulatory action on osteoblast proliferation and differentiation and collagen synthesis, leading to increased bone formation.31, 32 Before puberty, GH and IGF-I are the primary hormones essential for maintaining slow, but continuous, bone growth. Once the onset of puberty is triggered by marked increase in gonadal sex steroid production, GH and IGF-I production also increases. This, in turn, stimulates the secretion of sex steroids by gonads.33 Thus, GH, IGF-I, and sex steroids act coordinately to support the pubertal growth spurt.34, 35 In the present TG mouse model with ubiquitous expression of HSD17B2, the coordinated action of IGF-I and sex steroids is disrupted, resulting in lower circulating IGF-I and testosterone concentrations at the prepubertal period, and thus, to delay in both the appearance of the typical measure of male puberty (balanopreputial separation) and in bone development. Furthermore, the increased conversion of the highly active 17-hydroxy steroids (testosterone, estradiol) to less potent 17-keto forms (androstenedione, estrone) by the transgene expressed in the bone is likely to further reduce the androgen and estrogen action in the bone. Thus, the local inactivation of sex steroids in the bone together with the reduced circulating concentration of IGF-I and testosterone is likely the cause of the phenotype observed.
GH and IGF-I are known to increase serum osteocalcin,31 and thus, the decreased serum osteocalcin levels may be caused by the observed decline in serum IGF-I levels in HSD17B2TG males at prepubertal age. The formation of IGFs, their receptors, and their binding proteins in skeletal tissues is regulated by many hormones, including GH and sex steroids. Knockout studies have confirmed that both IGF-I and IGF-II are essential for normal prenatal growth, because mice deficient in either IGF-I or IGF-II were only 60% of normal size at birth.36 Thereafter, the role of IGF-I becomes predominant, and both endocrine and paracrine action in the bone has been well documented for IGF-I. The endocrine production of IGF-I in the liver is highly dependent on GH action, and similar to HSD17B2TG mice, growth hormone receptor (GHR)-null mice exhibit severe postnatal growth retardation, associated with markedly reduced IGF-I concentrations. However, these mice have normal T concentrations.37 Interestingly, in GHR knockout mice, the femoral length and the growth plate width were not significantly shorter at the age of 2 wk, but significant differences were observed from 3 wk onward.38 Thus, the reduced bone growth in HSD17B2TG mice was initiated at a younger age compared with the GHR-null mice. However, similar to HSD17B2TG mice, trabecular bone remodeling was markedly reduced in GHR-null mice, and reduced osteoblast surface in GHR-null mice indicated impaired osteoblast proliferation or life span.39 Like GHR- and IGF-I–null mice, HSD17B2TG male mice showed a reduced width of the growth plate and abnormal skeletal metabolism at the prepubertal age.
IGF-I stimulates the differentiation and maturation of osteoblasts that mediate the bone-forming processes of the mammalian skeleton and are specialized for the production of extracellular matrix and for the mineralization process.31, 32 Accordingly, we observed reduced expression of Runx2, osteopontin, and osteocalcin in the osteoblasts of the HSD17B2TG mice compared with WT controls, thus indicating an inappropriate osteoblast differentiation affecting the skeletal development in HSD17B2TG mice. However, expression of the transgene in the osteoblasts also provides the possibility that local HSD17B2 activity has effects on bone function.
HSD17B2 inactivates both estrogens and androgens, but although several previous studies elucidated the dysfunction of skeletal development in Esr1-, Esr2-, Ar-, and Cyp19a1-deficient male mice, the bone phenotypes of these knockout models were different from that of the HSD17B2TG males. In the adult Esr1 knockout and Esr1 and 2 double knockout males,40, 41 there were minor skeletal abnormalities with reduced longitudinal bone growth and reduced BMD. However, no skeletal defects or altered bone growth or remodeling were detected at birth, postnatal age, or at puberty, whereas bone changes were observed in mice at 10 wk of age. In the Esr2 knockout male mice, no bone abnormalities were displayed at any age.42 The Cyp19a1-deficient mice presented osteopenia in the lumbar spine and were characterized by a significant decrease in trabecular bone volume and trabecular thickness. The low bone turnover in Cyp19a1 KO males indicated the presence of an age-related osteopenia. The Cyp19a1 KO males also showed significantly reduced longitudinal femur growth compared with WT littermates. However, all skeletal abnormalities were reported at 46- to 65-day-old mice, and no bone changes were noted in newborns or during pubertal age.43
The Ar KO male mice showed growth retardation compared with WT male littermates, and this was associated with reduced serum androgen levels.44 X-ray and histomorphometric analysis showed severe osteopenia of femora and tibias in 8-wk-old Ar KO males. The bone shape and length were, however, not affected by Ar inactivation, and the bone loss was caused by high bone turnover with increased bone resorption. No skeletal phenotypes have been reported at the prepubertal or pubertal age.44
The single knockout mouse models for sex steroid receptors (Esr1, Esr2, Ar) do not mimic well the phenotype observed in this study, indicating that that the local inactivation of both androgens and estrogens simultaneously by HSD17B2, together with the reduced IGF-I concentration, results in a more severe delay in bone development at the prepubertal period. Furthermore, in a previous study, we observed severely disrupted spermatogenesis in the HSD17B2TG males that was partially rescued by a synthetic retinoid analog (TTNPB),26 and the testis phenotype was similar to that observed in Rar-deficient models and vitamin A–deficient male mice.45-49 Because growth retardation has been also observed in various Rar-deficient mouse models,50-54 we can not exclude the possibility that the bone phenotype observed in HSD17B2TG mice would be caused by a metabolic role of the HSD17B2 that is independent of its ability to inactivate the sex steroids. Because of high sensitivity of skeleton to retinoids, skeletal abnormalities in the Rar-deficient mice have not been reported being rescued successfully. However, it has been suggested that the retinoid action on skeletal development could be mediated by changes of IGF binding protein (IGFBP) axis.55
Acknowledgements
The authors thank research assistants Nina Messner, Erja Mäntysalo, Heli Niittymäki, Taina Kirjonen, and Hannele Rekola for technical assistance and Katja Fagerlund for her help. This work was supported by The Academy of Finland, Hormos Medical.