Enhanced Chondrogenesis and Wnt Signaling in PTH-Treated Fractures†
The authors state that they have no conflicts of interest.
Abstract
Studies have shown that systemic PTH treatment enhanced the rate of bone repair in rodent models. However, the mechanisms through which PTH affects bone repair have not been elucidated. In these studies we show that PTH primarily enhanced the earliest stages of endochondral bone repair by increasing chondrocyte recruitment and rate of differentiation. In coordination with these cellular events, we observed an increased level of canonical Wnt-signaling in PTH-treated bones at multiple time-points across the time-course of fracture repair, supporting the conclusion that PTH responses are at least in part mediated through Wnt signaling.
Introduction: Since FDA approval of PTH [PTH(1–34); Forteo] as a treatment for osteoporosis, there has been interest in its use in other musculoskeletal conditions. Fracture repair is one area in which PTH may have a significant clinical impact. Multiple animal studies have shown that systemic PTH treatment of healing fractures increased both callus volume and return of mechanical competence in models of fracture healing. Whereas the potential for PTH has been established, the mechanism(s) by which PTH produces these effects remain elusive.
Materials and Methods: Closed femoral fractures were generated in 8-wk-old male C57Bl/6 mice followed by daily systemic injections of either saline (control) or 30 μg/kg PTH(1–34) for 14 days after fracture. Bones were harvested at days 2, 3, 5, 7, 10, 14, 21, and 28 after fracture and analyzed at the tissue level by radiography and histomorphometry and at the molecular and biochemical levels level by RNase protection assay (RPA), real-time PCR, and Western blot analysis.
Results: Quantitative μCT analysis showed that PTH treatment induced a larger callus cross-sectional area, length, and total volume compared with controls. Molecular analysis of the expression of extracellular matrix genes associated with chondrogenesis and osteogenesis showed that PTH treated fractures displayed a 3-fold greater increase in chondrogenesis relative to osteogenesis over the course of the repair process. In addition, chondrocyte hypertrophy occurred earlier in the PTH-treated callus tissues. Analysis of the expression of potential mediators of PTH actions showed that PTH treatment significantly induced the expression of Wnts 4, 5a, 5b, and 10b and increased levels of unphosphorylated, nuclear localized β-catenin protein, a central feature of canonical Wnt signaling.
Conclusions: These results showed that the PTH-mediated enhancement of fracture repair is primarily associated with an amplification of chondrocyte recruitment and maturation in the early fracture callus. Associated with these cellular effects, we observed an increase in canonical Wnt signaling supporting the conclusion that PTH effects on bone repair are mediated at least in part through the activation of Wnt-signaling pathways.
INTRODUCTION
Fracture healing is a biologically optimized repair process that leads to skeletal union through the regeneration of bone. Despite the efficiency of this repair process, patients still require several months before a healing fracture achieves sufficient mechanical competence to support normal physiological loads. Consequently, there is significant interest in treatments that could enhance the rate of repair providing for a more rapid return to an active lifestyle and work. In addition, it has been estimated that between 5% and 10% of the 7.9 million fractures occurring annually in the United States exhibit some degree of impaired healing.1 The identification of a compound that could effectively enhance repair under these less than optimal conditions would be of significant social and economic benefit.
The ability of daily systemic injections of PTH to enhance fracture healing has been shown in several rodent models. A number of reports over the last several years have examined PTH effects on fracture healing with doses ranging from 10 to 200 μg/kg of daily PTH(1–34) treatment in rat models. All showed significant increases in mechanical properties of the calluses, rates of callus formation, and callus tissue volume.2-4 In other studies using models of impaired bone metabolism, PTH analogs were shown to reverse the inhibition of bone healing observed in aged and ovariectomized rats5 and rabbits treated with corticosteroids.6 Although the ability of systemic PTH to enhance the rate of bone repair is well established in animal models, our understanding of the mechanism(s) by which PTH induces an anabolic response during skeletal repair remains limited. Although PTH acts on coupled remodeling in intact bone and this is believed to contribute to its primary actions in improving bone mass in osteoporosis,7-9 it is unclear if this is the primary mechanism by which it enhances healing fractures. It should be noted that the majority of the mechanical integrity of the fracture callus is regained before significant remodeling. The consistent reports of increased callus volumes in rats treated with systemic PTH suggest that a primary mechanism of PTH action on bone repair is to amplify the early phases of the endochondral process during which the callus template is laid down.2-4, 10-15
Fracture callus formation during endochondral bone repair has been shown to recapitulate many of the biological processes associated with embryonic and postnatal bone formation in the growth plate. The primary difference between the two processes is the condensed time-course of endochondral bone formation during fracture repair. Unlike endochondral bone formation of the growth plates that occurs in a multicycle process, fracture repair represents one coordinated condensed round of bone formation with a single chondrogenic phase followed by a single round of osteoblast-mediated bone formation. Despite the condensed time-course of fracture repair, many of the same regulatory mechanisms that coordinate bone formation in the growth plate seem to regulate the endochondral process during bone repair.
Given that PTH and the related peptide, PTHrP, signal through a common receptor PTHR1, it is reasonable to hypothesize that systemic PTH may regulate endochondral bone formation by altering the local PTHrP/Indian hedgehog (Ihh) regulatory loop shown to coordinate endochondral bone formation in the growth plate. Because, in these studies, PTH is administered as a single daily injection and the half-life of circulating PTH is on the order of minutes or less, we expect that the effect of systemic PTH will be intermittent. In the growth plate, Ihh coordinates chondrocyte and osteoblast proliferation and differentiation. Ihh is expressed primarily by the prehypertrophic chondrocytes of the growth plate and regulates adjacent chondrocyte differentiation indirectly through its control of the local levels of PTHrP.16-20 PTHrP maintains chondrocytes in a proliferative phase in part by antagonizing Runx2 activity, required for chondrocyte hypertrophy and osteoblast differentiation, and by activating Sox9 activity, a transcriptional regulator of chondrocyte differentiation expressed in prehypertrophic chondrocytes.21 In addition to regulating chondrocyte differentiation, Ihh signaling is also required for osteoblast differentiation during endochondral bone formation. Previous studies have shown that Ihh is essential for initial specification of osteoblast progenitors into Runx2-positive osteoblasts. Thus, the PTHrP/Ihh feedback loop plays an important role in regulating both chondrogenesis and osteogenesis and coordinates the two processes during endochondral bone growth in the growth plate. Based on the descriptive studies that have examined the expression and localization of PTHrP and Ihh in the fracture callus, endochondral bone repair seems to be regulated by PTHrP and Ihh in a manner consistent with their regulatory role in the growth plate.22, 23
A number of recent mechanistic studies analyzing the regulatory interactions in the growth plate have shown that Ihh signaling is coordinated with canonical Wnt signaling during endochondral bone formation. Wnt signaling has gained a great deal of attention in bone biology over the past few years, primarily because of the observations that gain and loss of function mutations in the Wnt co-receptor, lipoprotein-related peptide 5 (LRP5), are associated with gain or loss of bone mass and the disease osteoporosis pseudoglioma syndrome (OPPG).24-26 There are currently 18 identified murine Wnts divided into two classes (canonical and noncanonical) based on their functional ability to induce a secondary body axis in embryos.27 Whereas very little is known about noncanonical Wnt signaling, the canonical Wnt/β-catenin signaling pathway and its regulatory roles in endochondral bone formation have been extensively studied. Several recent papers28-31 provided compelling evidence that canonical Wnt signaling regulates mesenchymal progenitor lineage selection, promoting osteogenesis and suppressing chondrogenesis. During the later stages of chondrogenic differentiation, canonical Wnt/β-catenin signaling is upregulated and positively regulates chondrocyte hypertrophy. Taken together, these studies and others show a unique regulation of embryonic endochondral bone growth in which canonical Wnt/β-catenin signaling plays multiple, stage-specific regulatory roles in coordination with Ihh.
To understand how systemic PTH impacts the repair processes during endochondral bone repair, we analyzed the tissue responses to PTH after fracture. Specific attention was focused on defining the effects of systemic PTH on callus tissue volumes, compositions, and relative rates of differentiation. Molecular analyses were used to define the relative rate of cellular differentiation in treated and untreated callus tissues based on the expression of chondrogenic and osteogenic-associated transcriptional regulators and extracellular matrix genes. In addition, we examined the effects of systemic PTH treatment on the expression of regulators of endochondral bone formation including PTHRP, Ihh, and components of the Wnt signaling pathways over the course of fracture repair.
MATERIALS AND METHODS
Fracture procedure
Unilateral fractures were produced in the right femur of isoflurane-anesthetized 8-wk-old male C57BL/6 mice using the previously described protocol and a scaled-down version of the apparatus32 originally described by Bonnarens and Einhorn.33 Mice received daily injections beginning 1 day after fracture of either 30 μg/kg rhPTH(1–34) (Bachem, Bubendorf Switzerland) or vehicle alone (sterile saline) through subcutaneous injection daily over the first 14 days of the 21-day standard repair time-course. All bones were assessed at harvesting by X-ray (model MX-20; Faxitron, Wheeling, IL, USA). Fractures that did not meet standard criteria were not included in the subsequent analyses.
μCT
After removal of the intramedullary pin, bones were imaged using μCT at a resolution of 12 μm/voxel using a Scanco μCT 40 system (Scanco Medical, Basserdorf, Switzerland). Callus size (total volume and length) and density (volume fraction and mineral density) were quantified for each specimen. For each experimental group, five to seven bones were imaged. A two-factor ANOVA with time and treatment as the factors, followed by a Tukey posthoc test, was performed on each of the μCT outcome measures. As with the histological measurements, these μCT measurements were made on only the portions of the callus external to the periosteal surface of the cortex; that is, new bone formation in the medullary canal was not included. A semiautomated segmentation procedure was used to define the outer boundary of the callus and to exclude the original cortex. A global thresholding algorithm was applied with a fixed, constant threshold for all specimens. Voxels with intensities (gray values) higher than the threshold were considered to contain mineralized tissue. A constrained 3D Gaussian filter (filter width = 0.8, filter support = 1 voxel) was used to partially suppress image noise.
The callus total volume was defined as the volume of all tissues (bone, cartilage, void) in the callus, whereas the callus bone volume was defined as the volume of only the portion of the callus containing mineralized tissue. The callus bone volume fraction was defined as the ratio of bone volume to total volume. Callus length was determined by calculating the total distance along the diaphyseal axis that was spanned by the callus. The mineral density was computed for each voxel using a standard curve that relates linear attenuation to mineral density. This curve was determined from μCT scans performed weekly on a set of hydroxyapatite phantoms of five different mineral densities that was provided by the system manufacturer. The mean and SD of the mineral density of all voxels above the fixed threshold were computed to quantify the average callus mineral density and the intraspecimen heterogeneity in mineral density, respectively.
Histological analysis
Bones were removed by disarticulation of the forelimb through the knee joint. After removal of skin and superficial layers of soft tissues, the bones were fixed in 4% paraformaldehyde in PBS, decalcified in EDTA, and embedded for sectioning as previously described.34 Sections were subsequently stained with Safranin-o and fast green. Immunohistochemical localization of activated β-catenin protein was carried out using a commercially available antibody specific for the unphosphorylated form of β-catenin (Upstate, Charlottesville, VA, USA). Staining was visualized using the Vectastain ABC kit per the manufacturer's suggested protocol (Vector Laboratories, Burlingame, CA, USA).
Molecular analyses
RNA samples were analyzed in duplicate sets of pooled samples (n = 3–5; fracture calluses and control). Pooled specimen assays were run in triplicate. Total RNA was prepared from tissues as previously described.32, 34 RNase protection analysis was carried out using commercially purchased template sets (BD Biosciences) per the manufacturer's instructions as previously described. Individual band intensities were quantified by direct β-counting (Becton Dickinson) and normalized to an internal L32 control. Real-time RT-PCR assays were carried out as described in Wang et al.35 using commercially purchased Taqman primers sets (Applied Biosystems, Foster City, CA, USA).
Western blot analyses
Fracture callus tissue was harvested at the indicated time-points after fracture, and soft tissues were removed. Callus tissue was powdered in liquid nitrogen using a sterile mortar and pestle. Nuclear extracts were isolated using the Pierce NE-PER Nuclear extraction kit per the manufacturer's instructions (Pierce Biotechnology, Rockford, IL, USA). Fifty micrograms of protein was resolved on a 10% SDS-PAGE. Blots were sequentially probed with a monoclonal antibody against the unphosphorylated active β-catenin (Upstate) followed by an anti-lamin antibody for normalization of loading (Sigma-Aldrich). Staining was visualized using the Amersham ECL Western blotting Detection kit (GE Healthcare, Buckinghamshire, UK).
RESULTS
Faxitron and μCT imaging
Faxitron and standard histological analysis showed a visible increase in callus size and apparent bone density visible by day 14 and approaching bridging by day 21 in response to PTH (Figs. 1A and 1B). By histology, the majority of increased callus size was determined to be made up of chondrogenic cells. The quantitative analysis of bone healing from the μCT analysis showed that PTH treatment induced a larger callus, in terms of average cross-sectional area, length, and total volume (Figs. 1C and 1D). Observable differences in callus size were detected by day 10 after fracture, before new endochondral bone formation, supporting the conclusion that the callus at this time was predominantly composed of chondrogenic lineage cells. This overall increase in callus size translated into a higher total callus mineral content (bone volume; Fig. 1E) but not a higher average mineral density (Fig. 1F). These observations showed that PTH-treated fractures have a larger volume of mineralized callus but not a higher density of mineral in the callus.
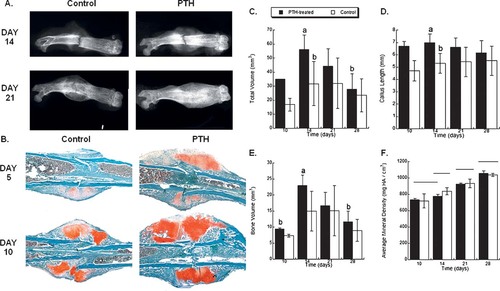
(A) Radiographic images of vehicle vs. PTH(1–34)–treated fractures at 14 and 21 days after fracture taken by Faxitron. (B) Histological images of vehicle vs. PTH(1–34)–treated fractures at 5 and 10 days after fracture taken at ×4. All sections stained with Safranin O/fast green, which stains chondrogenic cells red. Note the increased cartilage volume at day 5 and 10. (C–F) Graphic presentations of selected quantitative data from the μCT analysis of fracture healing. The results of a two-factor ANOVA with a Tukey posthoc assessment are given on each plot (if no differences were found, then there are no letter annotations or footnotes). (C) Total callus volume, effect of treatment (p = 0.0016) and time (14 > 28, p = 0.0043). (D) Callus length a not equal to b. Effect of treatment (p = 0.0001). (E) Bone volume, effect of treatment (p = 0.0318) and time (14, 21 > 10, p = 0.0001). (F) Average mineral density, groups not connected by the same horizontal line are significantly different. Effect over time (28 > 21 > 14 > 10, p < 0.0001). Each group contains n = 5 bones.
Analysis of chondrogenic and osteogenic lineage progression
The underlying cellular and molecular basis for the effect of PTH on fracture healing was next examined by quantifying the expression profiles of mRNA that define the progression of chondrocyte and osteoblast differentiation during the time-course of fracture repair. Gene expression was quantified using either RNase protection assay (RPA) and/or real-time PCR.
As seen in the RPA, the expression of collagen type II and type X collagen both showed a greater induction in their expression in response to PTH treatment (Fig. 2). The total cumulative expression of chondrogenic associated collagens over fracture repair (sum of expression values for days 5–21) increased by ∼19% in PTH-treated bones compared with controls, whereas the cumulative osteoblast-associated osteocalcin expression only increased by ∼6%. This 3-fold greater relative chondrogenic versus osteogenic response showed that the primary cellular response to PTH treatment was chondrogenic consistent with our interpretation of our radiographic and histological observations.

Analysis of the PTH effects on cellular differentiation during fracture healing. (A) Autoradiographic image of ribonuclease protection analysis of extracellular matrix genes associated with the progression of skeletal tissue differentiation over the time course of fracture repair. (B) Graphic representations of the relative expression levels of selected mRNAs seen in A (Col X and OC) and real-time PCR quantification of gene expression for selected transcriptional regulators of chondrogenic vs. osteogenic differentiation (Sox9, Sox5, Runx2, and Osterix). RPA values were obtained by direct β-counting from gel, and all values where normalized to an internal L32 probe. Real-time PCR data are normalized to β-actin (Ct value/Ct value for β-actin) and presented as the fold change in expression relative to uninjured bone (time 0; ▴, PTH; ○, control).
Several observations also support an increased rate of chondrocyte maturation in response to PTH. The earlier peak in collagen type X expression (day 7 in PTH-treated bones versus day 10 in controls) suggested that the rate of chondrocyte maturation was enhanced by systemic PTH. Consistent with this finding were the observations that Sox9 and Sox5 were highly induced by PTH and that Sox5 expression peaked 3 days earlier (day 7) than in vehicle-treated fractures (day 10) in correlation with the earlier collagen type X expression. These observations support the conclusion that systemic PTH enhanced the rate of chondrocyte maturation and mineralization in the fracture callus.
PTH treatment had a relatively small but statistically significant affect on osteogenesis during the course of bone repair (Fig. 2). However, only in the latest time-points analyzed (days 21 and 28) did osteocalcin levels begin to substantially exceed the control expression (day 28; data not shown). The induction of osteoblast regulatory factors Runx2 and Osterix was enhanced in PTH-treated fractures relative to controls on days 10 and 14, respectively, but the timing of peak induction did not change between vehicle and PTH-treated samples, suggesting that osteogenic differentiation is not accelerated in response to systemic PTH treatments (Fig. 2).
Analysis of PTH effects on PTHrP/Ihh expression
Real-time PCR was used to quantify the expression of PTHrP and Ihh across fracture repair in both control and PTH-treated bones. PTH treatment initially increased in PTHrP expression 3-fold relative to controls on days 2 and 3 after fracture. By day 5, Ihh expression levels were highly induced in both PTH and vehicle-treated samples (Fig. 3). PTH-treated bones displayed a 2-fold higher Ihh expression relative to controls by day 7 and continuing through day 10. Ihh is normally expressed in committed prehypertrophic chondrocytes, suggesting this increased expression reflects a large increase in prehypertrophic cells by day 5 in both control and PTH treated callus tissue.

Graphic representation of real-time PCR analysis of the PTH effects on PTHrP and IHH mRNA expression during fracture healing. The mRNA species that was assayed in each real-time PCR reaction is denoted in the figure. All data are normalized to β-actin (Ct value/Ct value for β-actin) and presented as the fold change in expression relative to uninjured bone (time 0; ▴, PTH; ○, control).
Analysis of PTH effects on canonical Wnt signaling
To determine whether systemic PTH treatment leads to changes in the net activation of canonical Wnt signaling in the fracture callus, we quantified the amount of β-catenin protein in nuclear extracts from fracture callus tissues at different time-points during repair by Western blotting (Fig. 4). Our results showed that systemic PTH treatment enhanced canonical Wnt signaling relative to the corresponding control samples at multiple time-points during fracture repair. Western blot quantification using an antibody specific for the unphosphorylated active β-catenin form showed that PTH treatment leads to increased nuclear localized β-catenin levels in callus tissue on days 3, 7, and 14 after fracture. However, on day 5, nuclear β-catenin levels were reduced in the callus tissues treated with PTH compared with controls (Fig. 4). Real-time PCR quantification of β-catenin gene expression across repair showed that the changes observed in β-catenin protein levels on day 5 after fracture in PTH-treated samples are not correlated with the expression of the β-catenin gene (transcription) because the mRNA levels increased through out repair (Fig. 4).
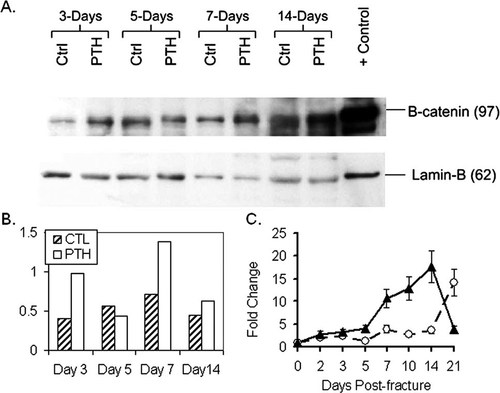
(A) Quantification of active nuclear localized β-catenin protein by Western blot. (B) Corresponding normalized value is graphically depicted. (C) Real-time PCR quantification of β-catenin expression across the time-course of bone repair. Data are normalized to β-actin (Ct value/Ct value for β-actin) and presented as the fold change in expression relative to uninjured bone (time 0; ▴, PTH; ○, control).
Immunohistochemical localization of β-catenin in the fracture callus showed that PTH treatment does not significantly alter the spatial profile of β-catenin protein expression in callus tissues. Analysis of protein localization in the fracture callus 5 days after fracture (Fig. 5A) showed that unphosphorylated β-catenin is localized in osteoblastic cells lining the trabecular bone formed along the periosteal layer adjacent to the fracture site. In addition, staining was observed in maturing chondrocytes of the callus and osteocytes located in the cortical bone. The central area of the callus did not seem to express significant levels of β-catenin protein. At later time-points (day 14 after fracture), the staining pattern was similar, but the differences in tissue composition become evident between control (Figs. 5B and 5D) and PTH-treated bones (Figs. 5C and 5E), with the PTH-treated calluses containing more mature chondrogenic cells and increased visible nuclear staining for unphosphorylated β-catenin. In addition, PTH-treated bones appeared to have increased trabecular bone formation along the periosteal surfaces and increased numbers of β-catenin–positive osteoblastic cells lining the trabecular surfaces.

Immunohistochemical localization of active β-catenin protein in the fracture callus. (A) Composite photomicrograph of a control day 5 fracture callus showing positive staining for activated β-catenin protein. Boxes 1 and 2 demarcate the areas depicted in B and C and D and E, respectively, representing the area of chondrogenesis (Box 1) and periosteal associated new trabecular bone formation (Box 2). (B–E) Day 14 fracture callus tissues in control (B and D) and PTH-treated fractures (C and E).
To identify specific members of the Wnt family associated with the PTH-induced increase in canonical Wnt signaling, we next analyzed the expression profiles across bone repair of 16 Wnt family members including Wnts1, 2, 3, 3a, 4, 5a, 5b, 6, 7a, 7b, 8d, 10a, 10b, 11, 13, and 15, as well as the Wnt co-receptors LRP5 and 6 and the Wnt inhibitors DKK1 and sclerostin. Sclerostin was specifically included based on the reports that it is a direct target of systemic PTH in intact bone. Initial RPA and real-time PCR analysis of the expression of these Wnt factors over the time-course of bone repair showed that only a limited number of these signaling molecules are induced to significant levels over intact bone during repair (data not shown), consistent with previous reports based on microarray analyses.36, 37 We observed only Wnts 4, 5a, 5b 10b, and 11 expressed at significant levels in fracture callus tissue. The first group affected by PTH treatment included Wnts 4, 5a, and 5b, each previously identified as a regulator of chondrogenesis.38-41 In vehicle-treated tissues, real-time PCR showed that all three of these Wnts increased during the chondrogenic phase of repair (days 5–10). PTH treatment led to an increased expression of Wnts 4, 5a, and 5b relative to control samples (Fig. 6). Wnt 5a in particular was induced in response to PTH treatment by ∼10-fold over controls on days 2 and 3 after fracture and remained elevated 2-fold over controls in day 5 callus tissues (Fig. 6).
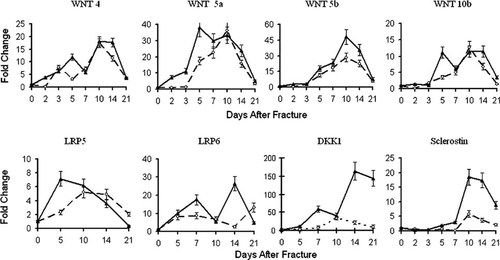
Graphic representation of real-time PCR analysis of the PTH effects on selected Wnt mRNA expression during fracture healing. The mRNA species that was assayed in each real-time PCR reaction is denoted in the figure. All data are normalized to β-actin (Ct value/Ct value for β-actin) and presented as the fold change in expression relative to uninjured bone (time 0; ▴, PTH; ○, control).
The second group of Wnts examined included Wnt10b and the Wnt co-receptors LRP5 and 6, each previously associated with osteogenesis.27 Wnt 10b has been specifically associated with osteoblast differentiation and the specification of osteogenic over adipogenic differentiation of mesenchymal stem cells.42 In the control specimens, Wnt10b expression peaked at day 10 after fracture, consistent with the induction of osteogenesis (Fig. 6). In PTH-treated fractures, Wnt10b displayed a biphasic expression profile with an early induction around day 5 and a subsequent peak over days 10–14 after fracture in correlation with the major osteogenic phase of endochondral repair. The expression profile of the Wnt co-receptor LRP6 was also biphasic. LRP5 expression, on the other hand, gradually rose until peaking on day 10 in control bones, whereas the PTH-treated callus peaked earlier on day 5 after fracture. In addition to increased expression of Wnt ligands and co-receptors, systemic PTH treatment was associated with the increased expression of several soluble Wnt inhibitors specifically Dkk1 and sclerostin.
DISCUSSION
Given the robust response of bone to systemic PTH treatment in patients with osteoporosis and in animals during fracture repair under both normal and impaired conditions, there is a significant interest in the clinical potential of PTH for the treatment of fractures in patients. Moreover, because of the potential use of this drug in other musculoskeletal conditions, such as in diseases affecting cartilage (e.g., osteoarthritis), there is a great need to understand the mechanism of action through which PTH enhances skeletal tissue repair processes.
In these studies, we specifically focused on the cellular responses to systemic PTH treatment during the anabolic phase of endochondral bone formation. Our results using both Faxitron and μCT imaging showed that PTH-treated fractures generated a larger total callus volume (Fig. 1). Although PTH enhanced the callus volume, it did not increase the average density of mineral in the callus tissue relative to controls. Quantitative comparison of chondrogenic versus osteogenic extracellular matrix gene expression in callus tissues across the anabolic phase of fracture repair showed that PTH preferentially enhanced chondrogenesis over osteogenesis (3-fold greater increase in chondrogenesis versus osteogenesis; Fig. 2). In addition to our observations showing that PTH increased the volume of cartilage in the callus, we also observed an increased rate in chondrocyte hypertrophy in fracture tissues treated with PTH. The earlier induction of chondrocyte hypertrophy in the callus was indicated by both an earlier peak in Sox5 expression and the corresponding earlier induction Collagen type X expression (Fig. 2). The combination of an increased callus volume and more rapid mineralization of the cartilaginous callus would presumably produce a more mechanically stable environment around fracture site.
Although these data support the conclusion that a primary mechanism of PTH action on fracture repair is to increase chondrogenic cell proliferation and differentiation, these data do not address the molecular mechanism(s) through which PTH achieved these cellular responses. Based on the fact that PTH signaling is mediated through the common PTHR1 receptor shared with PTHrP, it seemed highly likely that PTH would act in part by modifying the PTHrP/Ihh regulatory system. It has been previously shown that the targeted disruption of PTHR1 or PTHrP in mice results in dwarfism caused by a reduction in the zone of proliferating cells and an advanced onset of hypertrophic differentiation in the growth plate.16, 17, 19 In our studies, PTH-treated bones initially showed an increase in PTHrP expression relative to controls specifically during the earliest time-points in the repair process (days 2–3) concomitantly with a relative decreased expression of Ihh (Fig. 3). Given that PTHrP primarily maintains chondrocytes in a proliferative phase, this observation would be consistent with conditions that would favor the increased proliferation of chondroprogenitors and prehypertrophic chondrocytes in the callus at these early time-points. Consistent with these observations are the previous reports that systemic PTH treatment was associated with increased osteo- and chondro-progenitor proliferation between days 2 and 4 after fracture in a rat femoral fracture model.14, 15 However, by day 5 after fracture, the relative ratio of PTHrP to Ihh levels switched in both control and PTH-treated bones, with Ihh expression exceeding that of PTHrP, consistent with the initiation of chondrocyte maturation in the fracture callus.
The fact that systemic PTH treatment, presumably acting through PTHR1, could promote both chondrocyte proliferation and maturation may seem counterintuitive given the well-described role of PTHrP in the inhibition of chondrocyte hypertrophy. However, given the route of PTH administration in these studies (daily systemic injection), the half-life of circulating PTH (minutes or less), and the known divergence between the effects of intermittent and continuous PTH treatment on bone (anabolic versus catabolic, respectively), it is our interpretation that this divergent affect on chondrocyte maturation is related to the intermittent nature of the PTH exposure. Furthermore, our data suggest that the cellular responses to systemic PTH are related more to its affects on the local PTHrP/Ihh regulatory system than the direct effect of the transient PTH signaling through the PTHR1.
There has been a significant interest in what the secondary signals are that mediate the PTHrP/Ihh effects on chondrogenic cell differentiation. BMPs have previously been implicated as potential downstream signals to Ihh within this network. However, systematic analysis of this interaction during embryonic bone formation by both gain and loss-of-function studies on both BMP and Ihh showed that BMPs, while required, are not a secondary signal to Ihh in the regulation of chondrocyte differentiation.17, 18
There are a number of recent studies that have shown that the PTHrP/Ihh feedback loop and Ihh specifically interact with the canonical Wnt signaling pathway.20, 31 Our current data suggest that the Wnt signaling pathways may be important secondary mediators of PTH effects on fracture repair. Western blot analysis showed that the nuclear localized active β-catenin protein levels were increased relative to controls in response to PTH treatment on days 3, 7, and 14 after fracture, consistent with increased activation of the canonical Wnt signaling by PTH (Fig. 4). The increased canonical Wnt signaling on days 7 and 14 is consistent with the enhanced chondrocyte maturation observed in PTH-treated bones. However, on day 5, nuclear β-catenin levels were reduced compared with controls in PTH-treated bones (Fig. 4). This transient decrease in nuclear β-catenin levels in the PTH-treated bones on day 5 may be significant because it is correlated with the timing of chondrogenic cell recruitment into the callus. Increased canonical Wnt signaling is primarily associated with an inhibition of chondrogenic lineage recruitment.28-31 Spatially, we observed active β-catenin expression in the maturing chondrocytes and osteoblasts (Figs. 5B and 5E, day 14 fracture callus) with increased staining observable in PTH-treated bones. Visible staining was absent in the central region of the callus where progenitors and very early chondrocytes are located (Fig. 5A, day 5 callus) consistent with the corresponding chondrogenic cellular lineage recruitment.
Several Wnt ligands were highly induced by PTH treatment on day 5 after fracture. The expression of Wnt5a in particular was induced by ∼10-fold early in repair (days 2–3) peaking at day 5 in PTH-treated fractures, the window during which the differentiation fate of mesenchymal progenitors into chondrocytes is being determined (Fig. 6). The increased expression of Wnt5a is interesting in light of its role in promoting chondrogenesis by antagonizing canonical Wnt signaling and by targeting β-catenin for degradation.43, 44 These results suggest the possibility that systemic PTH treatment generated larger cartilaginous callus volumes during fracture repair through the preferential recruitment of progenitor cells into the chondrogenic lineage, potentially through a mechanism that involves the targeted inhibition of canonical Wnt signaling. Additional targeted studies will be needed to validate this hypothesis.
In summary, our data on the actions of systemic PTH treatment on fracture repair showed that PTH preferentially enhanced the early chondrogenic stages of endochondral bone formation. We found that systemic PTH treatment during fracture repair leads to increased chondrogenesis in the callus and an enhanced rate of chondrocyte maturation and mineralization. Our expression data and quantification of Wnt/β-catenin signaling showed that systemic PTH treatment affects the PTHrP/Ihh feedback loop and canonical Wnt signaling in a temporally specific manner that correlated with the enhanced chondrogenic cell recruitment and maturation in the fracture callus. These observations support the conclusion that the effects of systemic PTH treatment on fracture repair are mediated, at least in part, through Wnt signaling.
Acknowledgements
The authors acknowledge the assistance of Lee Silkman and Kevin Wang with the animal studies and Kelly Labrecque for administrative support. This work was supported by Orthopedic Research and Education Foundation and Orthopedic Trauma Association Grants to SK and by PO1-AR049920 from the National Institutes of Health to TAE.




