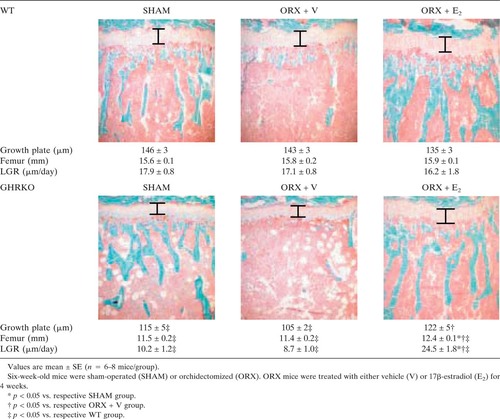
Growth Without Growth Hormone Receptor: Estradiol Is a Major Growth Hormone-Independent Regulator of Hepatic IGF-I Synthesis†‡
This study was presented in part at the 26th Annual Meeting of the American Society for Bone and Mineral Research, Seattle, WA, USA, October 1–5, 2004, and has been awarded with a Young Investigator Award.
All authors have no conflict of interest.
Abstract
The role of estrogens in the regulation of pubertal growth independently of GH and its receptor was studied in male mice with disrupted GHRKO. E2 rescued skeletal growth rates in GHRKO associated with an increase in hepatic and serum IGF-I. These data show that E2 rescues pubertal growth during GH resistance through a novel mechanism of GHR-independent stimulation of hepatic IGF-I production.
Introduction: Growth hormone (GH) and estrogen play a pivotal role in pubertal growth and bone mineral acquisition. Estrogens can affect GH secretion and thereby provide a GH-dependent mechanism for their effects on skeletal growth. It is presently unclear if or to what extent estrogens are able to regulate pubertal growth and bone mineral accrual independently of GH and its receptor.
Materials and Methods: Estradiol (E2; 0.03 μg/day by subcutaneous silastic implants) was administered to orchidectomized (ORX) male mice with disrupted GHR (GHRKO) and corresponding WTs during late puberty (6–10 weeks). Longitudinal and radial bone growth, IGF-I in serum and its expression in liver, muscle, and bone, and liver gene expression were studied by histomorphometry, RIA, RT-PCR, microarrays, and Western blotting, respectively.
Results: E2 stimulated not only longitudinal (femur length and growth plate thickness) and radial growth (cortical thickness and periosteal perimeter), but also rescued longitudinal and periosteal growth rates in ORX GHRKO, whereas no significant changes occurred in WT. E2 thereby upregulated serum IGF-I and liver IGF-I synthesis (+21% and +52%, respectively) in ORX GHRKO, whereas IGF-I synthesis in femur or muscle was unaffected. Study of the underlying mechanism of the stimulation of hepatic IGF-I expression showed that E2 restored downregulated receptor signaling systems, such as the estrogen receptor α and the prolactin receptor. E2 thereby recovered the Janus kinase (JAK)/signal transducers and activators of transcription (STAT) pathway as evidenced by a significantly increased activation of the transcription factor STAT5 in ORX GHRKO.
Conclusions: Our data show a stimulation of skeletal growth through upregulation of hepatic IGF-I by a hormone other than GH. E2 rescues pubertal skeletal growth during GH resistance through a novel mechanism of GHR-independent stimulation of IGF-I synthesis in the liver.
INTRODUCTION
LARON SYNDROME—A RARE defect in the gene encoding the growth hormone receptor (GHR) resulting in growth hormone (GH) resistance or insensitivity—is characterized by severe growth failure, high serum GH, and extremely low levels of circulating IGF-I.1, 2 The GHR gene disrupted or “knockout” (GHRKO) mouse model has a disruption of its receptor mRNA, as previously described by Zhou et al.,3 and is considered to be a model for human Laron-type dwarfism.4 Both in patients with Laron Syndrome1 and in GHRKO mice,5 administration of IGF-I rescues growth failure, supporting the concept that circulating IGF-I, which is principally derived from the liver, has the capacity to mediate the growth-promoting effects of pituitary GH.
GH action on the liver is transduced by the Janus kinase (JAK)/signal transducers and activators of transcription (STAT) signaling pathway. JAK2 phosphorylates the GHR on tyrosine residues, which form docking sites for other signaling molecules such as STAT5.6 In mice, there are two homologous STAT5 proteins, STAT5a and STAT5b, which are encoded by two different but highly related genes.7 After activation through phosphorylation, STAT5 can stimulate IGF-I gene transcription.8
In addition to the well-established role of the GH-IGF-I axis, estrogens are also known to play an essential role in the pubertal growth spurt and bone mineral accrual.9, 10 Reports of natural mutations in the estrogen receptor11 and the aromatase gene in men12-16—along with evidence from transgenic estrogen-resistant male mice17 and administration of aromatase inhibitors in male rats18—have called attention to the pivotal role of estrogens in male skeletal growth. Key findings in the context of estrogen resistance or deficiency include low BMD and failure to establish peak bone mass.11-18 Estrogen-deficient men experience no pubertal growth spurt and have a sustained linear growth without epiphyseal fusion. Estrogens induce closure of the epiphyseal growth plate during late puberty, thereby limiting longitudinal growth and ultimate bone size. During early puberty, however, estrogens stimulate longitudinal growth. This stimulatory effect is considered to primarily reflect interaction with the GH-IGF-I axis. Indeed, estrogens stimulate GH secretion and, in turn, GH-induced hepatic synthesis of IGF-I.19-21 Besides their effect on linear growth, estrogens are also involved in the regulation of radial bone expansion as recently described in an adolescent male.14 However, it is presently unclear if or to what extent estrogens may be able to regulate pubertal skeletal growth and bone mineral acquisition independently of GH or its receptor. To address this question, we examined the effects of estradiol (E2) therapy on pubertal growth in GHRKO male mice and wildtype (WT) controls. Longitudinal and radial skeletal growth and bone mineral acquisition were evaluated as well as changes serum IGF-I and hepatic gene expression after E2 treatment.
MATERIALS AND METHODS
Animals
Homozygous GHRKO mice were generated by disruption of the GHR/BP gene by homologous recombination, as previously described.3 Their genetic background was Sv129Ola/Balb/c. Genotyping was performed using PCR amplification.22 Mice lived in conventional conditions: 12-h light/dark cycle, standard diet (1% calcium, 0.76% phosphate), water ad libitum. At 6 weeks of age, male WT and homozygous mice were either sham-operated (Sham) or orchidectomized (ORX). ORX mice were treated for 4 weeks with either vehicle (V) or 17β-estradiol (E2; 0.03 μg/day) by subcutaneous silastic implants (Silclear Tubing; Degania Silicone, Jordan Valley, Israel) in the cervical region.23 Vehicle animals received empty implants. To assess its physiological action, 6-week-old female mice were ovariectomized and also replaced with the E2 implants. The weight of the uterus expressed per gram of body weight was 8.2 ± 1.2 mg/g compared with 6.0 ± 0.2 mg/g in age-matched female controls. Therefore, the E2 dose used is a physiological replacement dose in female mice. All mice were injected intraperitoneally with the fluorochrome calcein at a 5-day interval and were killed 1 day after the second injection. The ethical committee of the Katholieke Universiteit Leuven approved all experimental procedures.
Histomorphometric analysis
A femur and tibia were immersed in Burckhardt's fixative (24 h, 4°C), kept in 100% ethanol, and embedded in methylmethacrylate. Longitudinal sections of the tibia were cut at 4 μm thickness using a rotation microtome (RM 2155 Autocut; Leica, Heidelberg, Germany). Growth plate thickness was determined on sections, stained by a modified Goldner technique, at 30 points at a regular distance between points. Longitudinal growth rate (LGR, μm/day) was calculated by measuring, at 50 points across the distal edge of the growth plate, the distance between calcein bands. Cross-sections of the femur perpendicularly to the long axis were prepared at 200 μm thickness in the middiaphyseal region using the contact-point precision band saw (Exakt, Norderstedt, Germany). Sections were ground to a final thickness of 25 μm using a grinding system (Exakt) and were left unstained. Three sections in the mid-diaphyseal region were measured by fluorescence microscopy, and the bone formation rate (BFR/B.Pm., μm2/μm/day) was assessed at both the endocortical and periosteal bone surfaces. The BFR was obtained by the product of mineral apposition rate (MAR) and mineralizing perimeter per bone perimeter (Min.Pm./B.Pm., %). The mineralizing perimeter is calculated as follows: Min.Pm. = [dL + (sL/2)]/B.Pm., where dL represents the length of the double labels, and sL represents the length of single labels, along the entire endocortical or periosteal bone surfaces. The MAR (μm/day) was calculated as the mean width of double labels, divided by interlabel time (5 days). The Min.Pm. is a measure for osteoblast number and MAR for osteoblast activity. All measurements were performed with a Kontron Image Analyzing computer (KS400 3.00; Kontron Bildanalyze, Munich, Germany) and a Zeiss microscope with drawing attachment. Specific software was developed in collaboration with the manufacturer. Histomorphometric parameters are reported according to the recommended American Society for Bone and Mineral Research nomenclature.24
Analysis of serum IGF-I, IGFBP-3, and prolactin
After acid-ethanol extraction, serum IGF-I concentrations were measured by an in-house RIA in the presence of an excess of IGF-II (25 ng/tube).25, 26 Serum IGFBP-3 levels were measured using a mouse specific ELISA kit (R&D Systems, Minneapolis, MN, USA). Serum prolactin (Prl) was determined by RIA. Mouse prolactin immunoreactants were provided by Dr AF Parlow (NHPP, Torrance, CA, USA). All samples were measured in the same assay, and intra-assay CV was 7%.
RT-PCR analysis
Total RNA was prepared from liver, quadriceps muscle, and femur (n = 6–8/group), using TriZol Reagent (Life Technologies). RT-PCR analysis was performed using the ABI Prism 7000 Sequence Detection System (PE Applied Biosystems, Stockholm, Sweden), using probes labeled with the reporter fluorescent dye FAM. The sequence for the specific IGF-I probe is 5′-TTCAACAAGCCCACAGGCTAT-3′, and for the IGF-I primers is forward primer (FP), 5′-GCTCTTCAGTTCGTGTGTGGAC-3′ and reverse primer (RP), 5′-CATCTCCAGTCTCCTCAGATC-3′. Predesigned primers and a probe labeled with the reporter fluorescent dye VIC, specific for 18S rRNA, were included in the reactions as an internal standard. The oligonucleotide primers and probes were purchased from PE Applied Biosystems. The cDNA was amplified at the following conditions: 1 cycle at 50°C for 2 minutes and 95°C for 1 minute, followed by 40 cycles at 95°C for 15 s and 60°C for 1 minute. The mRNA amount of each gene was calculated using the “standard curve method” (multiplex reaction, following the instructions in User Bulletin 2; PE Applied Biosystems) and adjusted for the expression of 18 S rRNA.
Microarray analysis
For microarray analysis, liver RNA from four randomly chosen samples of ORX and ORX + E2 WT and GHRKO mice (n = 4/group) was further purified with the RNeasy Mini Kit (Qiagen/Benelux B.V.). The quality and quantity of total liver RNA was analyzed with the Agilent 2100 BioAnalyzer (Agilent, Waldbronn, Germany) and NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies), respectively. Each tested experimental condition was repeated as four independent biological replicates (N = 4). Total RNA (5 μg) was reverse-transcribed using the SuperScript Choice System (Invitrogen, Carlsbad, CA, USA) with oligo-dT primers containing a T7 RNA polymerase promotor site. cDNA was in vitro transcribed and labeled with biotin using the IVT labeling kit (Affymetrix, Santa Clara, CA, USA). Biotinylated cRNA was fragmented, and the quality of labeled and fragmented cRNA, respectively, was assessed with the Agilent 2100 BioAnalyzer. Quality of the hybridization mix from 7 of 16 samples was also confirmed by hybridization to an Affymetrix test chip (Test3-chip). Fragmented cRNA (15 μg) was hybridized overnight to the Affymetrix Mouse Genome 430 2.0 GeneChip Array, which contains 45,037 probe sets. The arrays were washed according to the protocol described in the Affymetrix Expression Analyses Technical Manual and scanned on a 2-μm Affymetrix 3000 GeneScanner. The resulting image files (.dat files) were analyzed using Affymetrix GCOS software. The fluorescence intensity of each chip was scaled to a target signal of 150, using the global scaling method. The scaling factors of the individual arrays did not differ >3-fold. Quality evaluation of the microarrays included Spike-In controls (BioB, BioC, BioD, and cre), a 3′-5′ ratio of GAPDH < 3.0, and average background signal per probe set < 100. Analysis of differentially expressed mRNA was performed for ORX + V and ORX + E2 groups of both WT and GHRKO mice and between ORX + V WT and KO groups, using GCOS. Transcripts were considered as differentially expressed when the following criteria were met: (1) present (P) calls in all four replicates of the control group, the experimental group, or in both; (2) consistent decreased (D) or increased (I) change in expression call for all 16 possible pairwise comparisons between the four control and four experimental samples; (3) a mean absolute value (16 comparisons) of the signal log ratio (SLR) of 1.0 or higher (≥1.0 and ≤ −1.0 for increased and decreased expression, respectively); and (4) significant change between means of control and experimental signal as assessed by a two-tailed unpaired Student's t-tests in which the acceptance level of significance was corrected for multiple comparisons by a Holm's test.27
Western immunoblotting for STAT5 and phosphorylated STAT5
Homogenates of the liver were prepared from the ORX + V and ORX + E2 group of both WT and GHRKO (n = 6–8/group). Liver homogenates were suspended in reducing SDS sample buffer (80 mM Tris [pH 6.9], 110 mM SDS, 10% glycerol, and 100 mM dithiothreitol). After boiling (95°C, 3 minutes), samples (100 μg) were separated on a 10% Tris-glycine gel and transferred to a Hybond enhanced chemiluminescence membrane (Amersham International). Equal protein loading was confirmed by Ponceau-S staining of membranes after transfer. Membranes were blocked in a Tris-buffered saline solution with 5% nonfatty milk and 0.05% Tween. The membranes were incubated with the primary antibodies overnight at 4°C. STAT5 antibody was obtained from Abcam, and phospho-STAT5A/B (Tyr694/699) antibody was obtained from Upstate Cell Signaling Solutions (Campro Scientific). Membranes were washed in Tris-buffered saline solution with 0.05% Tween, and immunoreactive signals were detected by a 1-h incubation at room temperature with goat-anti-rabbit secondary antibody, coated with horseradish peroxidase (DakoCytomation, Glostrup, Denmark), followed by chemiluminescent detection (Western Lightning; PerkinElmer Life Sciences). After exposing the blots to Hyperfilm MP (Amersham Biosciences), band intensities were determined using a Sharp JX-330 scanner and Imagemaster 1D software program (Pharmacia Biotech).
Statistical analysis
Statistical analysis of data (except microarray analysis) was performed using NCSS software (Kaysville, UT, USA). One-way ANOVA, followed by Fisher's least significant difference multiple comparison test and t-test, was performed to assess significance of difference between groups of the same genotype and between respective KO and WT groups. Correlation analysis was performed within the ORX + E2 GHRKO group. Spearman-Rank correlation coefficient (r) was calculated (pairwise). p < 0.05 was accepted as significant.
RESULTS
Longitudinal growth in GHRKO mice
E2 stimulated longitudinal growth in ORX GHRKO mice because it significantly increased femoral length (p < 0.01 versus ORX + V GHRKO), induced thickening of the growth plate (p < 0.05 versus ORX + V GRHKO), and fully rescued longitudinal growth rate (p < 0.001 versus ORX + V GHRKO; Table 1), whereas no changes were observed in WT mice.
Radial skeletal growth in GHRKO mice
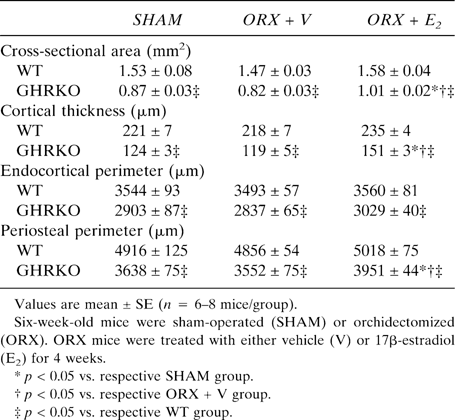
Cross-sectional area, cortical thickness, and periosteal perimeter were significantly reduced in sham-operated GHRKO mice compared with sham-operated WT mice (p < 0.001; Table 2). This resulted from a significant decrease in periosteal BFR in sham GHRKO mice compared with sham WT mice (p < 0.05; Fig. 1A). This decrease was associated with a decline in osteoblast number, as indicated by a reduced periosteal mineralizing perimeter (p = 0.01; Fig. 1B). Similarly, but to a lesser extent, periosteal osteoblast activity was also decreased in sham-operated GHRKO mice, as reflected by a reduced periosteal MAR (p < 0.05 versus sham WT; Fig. 1C). Interestingly, E2 expanded periosteal perimeters and thickened femoral cortices in ORX GHRKO mice (p < 0.001 versus ORX GHRKO; Table 2), as a result of a pronounced stimulation of periosteal BFR (Fig. 1A). In this process, E2 fully rescued the rate of periosteal bone formation to WT levels, mainly by a net increase of osteoblast number, as indicated by an elevated mineralizing perimeter (Fig. 1B) and as reflected by an enhanced labeling length at the periosteal bone surface (Fig. 1D). However, E2 suppressed the BFR at the endocortical bone surface (p < 0.05 versus ORX + V; Figs. 1D and 2A), as a result of a decrease in the mineralizing perimeter in ORX GHRKO mice (Fig. 2B) and in the MAR in both ORX WT and ORX GHRKO mice (Fig. 2C). In addition, E2 not only enhanced skeletal growth but also overall body growth, as reflected by a greater body weight gain during the experimental period in E2-treated GHRKO mice compared with V-treated GHRKO mice (data not shown).
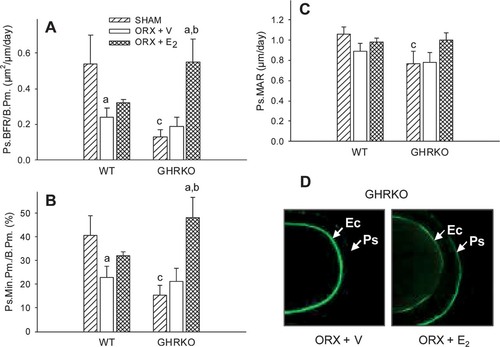
(A) Periosteal BFR per bone perimeter (Ps.BFR/B.Pm., μm2/μm/day), (B) periosteal mineralizing perimeter per bone perimeter (Ps.Min.Pm./B.Pm., %), and (C) periosteal MAR rate (Ps.MAR, μm/day) in WT and GHRKO male mice as measured by dynamic histomorphometry on cortical cross-sections, and (D) calcein labels at endocortical (Ec) and periosteal (Ps) bone surface in V- and E2-treated GHRKO. Six-week-old mice were sham-operated (SHAM) or orchidectomized (ORX). ORX mice were treated with either V or E2 for 4 weeks.ap < 0.05 vs. respective SHAM group;bp < 0.05 vs. respective ORX + V group;cp < 0.05 vs. respective WT group (n = 6–8 mice/group).
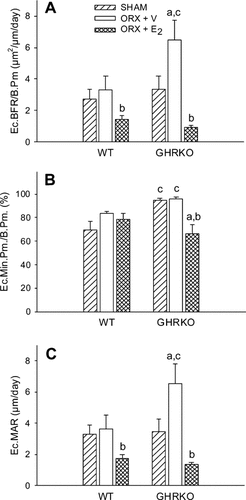
(A) Endocortical BFR per bone perimeter (Ec.BFR/B.Pm., μm2/μm/day), (B) endocortical mineralizing perimeter per bone perimeter (Ec.Min.Pm./B.Pm., %), and (C) endocortical MAR (Ec.MAR, μm/day) in WT and GHRKO male mice as measured by dynamic histomorphometry on cortical cross-sections. Six-week-old mice were sham-operated (SHAM) or orchidectomized (ORX). ORX mice were treated with either V or E2 for 4 weeks.ap < 0.05 vs. respective SHAM group;bp < 0.05 vs. respective ORX + V group;cp < 0.05 vs. respective WT group (n = 6–8 mice/group).
Serum IGF-I levels and liver IGF-I mRNA expression in GHRKO mice
As expected, serum IGF-I was severely reduced in GHRKO mice (>95%; Fig. 3). E2, however, partially restored serum IGF-I in ORX GHRKO mice to 23% of WT sham levels (Fig. 3). Moreover, this partial restoration seems relevant for cortical bone metabolism, as a significant correlation (r = 0.86, p = 0.01) between periosteal bone formation and serum IGF-I levels of individual animals in this group was shown (Fig. 3, inset). To look for a molecular mechanism of the partially restored serum IGF-I levels, we studied gene expression at the mRNA level in various tissues using real-time RT-PCR (Figs. 4A-4C). Hepatic IGF-I mRNA was dramatically decreased in GHRKO mice (>95%; Fig. 4A). Interestingly, treatment with E2 in the ORX GHRKO mice increased hepatic IGF-I mRNA synthesis from 3.8% to 53% of normal WT sham levels (Fig. 4A). The E2-induced increment in hepatic IGF-I mRNA was not specific for the GHRKO mutation because it was also observed in E2-treated WT mice (Fig. 4A). Muscle IGF-I was significantly lower in sham GHRKO mice compared with sham WT mice (Fig. 4B), whereas femur IGF-I was not different between experimental groups (Fig. 4C). In addition, serum IGF binding protein 3 (IGFBP-3) was drastically decreased in ORX GHRKO mice (>90%), but was restored to 26% of normal IGFBP-3 levels in E2-treated GHRKO mice (data not shown).

Serum IGF-I in WT and GHRKO male mice and correlation with the periosteal bone formation. Serum IGF-I, as measured by RIA, is expressed as percent from SHAM WT. Six-week-old mice were sham-operated (SHAM) or orchidectomized (ORX). ORX mice were treated with either V or E2 for 4 weeks.ap < 0.05 vs. respective SHAM group;bp < 0.05 vs. respective ORX + V group;cp < 0.05 vs. respective WT group (n = 6–8 mice/group).
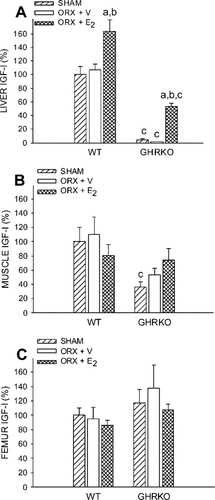
IGF-I mRNA expression in (A) liver, (B) quadriceps muscle, (C) and femur, as measured by real-time RT-PCR, in WT and GHRKO male mice. Values are expressed as percent from SHAM WT. Six-week-old mice were sham-operated (SHAM) or orchidectomized (ORX). ORX mice were treated with either V or E2 for 4 weeks.ap < 0.05 vs. respective SHAM group;bp < 0.05 vs. respective ORX + V group;cp < 0.05 vs. respective WT group (n = 6–8 mice/group).
E2 affects receptor expression in GHRKO mice
To further analyze the molecular basis of the E2-induced upregulation of hepatic IGF-I mRNA abundance in ORX GHRKO mice, genome-wide mRNA expression profiles in the liver from WT and GHRKO mice either with or without E2 treatment were studied.
As a positive control for the chosen model and method of RNA analysis, three probe sets on the array that recognize the truncated GHR mRNA reported significant loss of signal in the GHRKO mutation (decrease of up to 85% of the wildtype level; p < 0.000001) and no recovery in the knockout animals that were treated with E2 (data not shown). Further evidence for inactivation of the GHR-signaling pathway was shown by the significant downregulation of two negative regulators of the JAK-STAT signaling pathway in the liver of GHRKO mice: suppressor of cytokine signaling 2 (Socs2, represented by two probe sets: ProbeSetID 1449109_at and 1418507_s_at) and cytokine inducible SH2-containing protein (Cish, ProbeSetID 1448724_at) with a signal log ratio (SLR) of −4.0 and −2.7, respectively, compared with WT. Noticeable in the context of estrogen metabolism in the liver of ORX GHRKO mice was the very high expression of two transcripts encoding estrogen-inactivating sulfotransferases (estrogen-preferring Sult1e1, ProbeSetID 1420447_at and dehydroepiandrosterone-preferring Sult2a2, ProbeSet ID 1419528_at) with a SLR of 9.4 and 3.8, respectively, compared with WT.
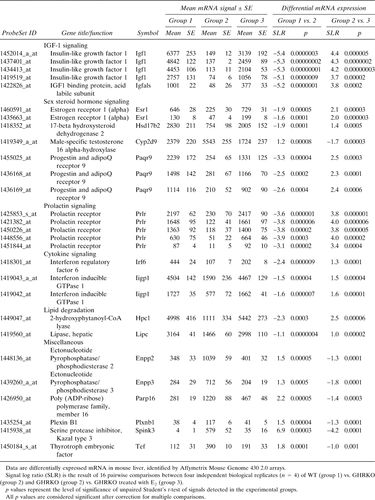
Because we were interested in the molecular mechanisms of estrogen-induced recovery of GHR-deficient livers, we based our filtering strategy on transcripts that were altered in the GHRKO and showed a compensatory (inverse) change after E2 treatment. As shown in Table 3, only a very limited amount of transcripts fulfilled these criteria (28 probe sets, representing 17 different mRNAs with known function), and the majority of these were related to hormonal signaling and IGF-I production. First, the array data confirmed the RT-PCR experiments as IGF-I mRNA expression (represented by four probe sets) was almost completely abolished in the GHRKO mutation as was IGFBP acid labile subunit (IGFBP, ALS) mRNA. Interestingly, both transcripts were significantly upregulated after E2 treatment (Table 3). Second, of particular interest, given the nature of the gene knockout, was the significant downregulation in the liver of GHRKO mice of mRNA encoding the estrogen receptor α (ERα; represented by two probe sets), the prolactin receptor (PrlR; represented by five concordant probe sets, 12-fold downregulation, individual p values <0005), and an orphan receptor involved in sex steroid hormone signaling, the progestin and adipoQ receptor 9 (PaqR9; represented by three probe sets), indicating that GHR signaling is important for the integrity of other major receptor signaling systems in the liver (Table 3). Remarkably, E2 therapy induced a full compensation for this multireceptor deficiency in GHRKO mice (Table 3). Third, of potential relevance for sex steroid metabolism is the compensatory E2-induced increase in the expression of 17β hydroxysteroid dehydrogenase 2, an enzyme predominantly responsible for the conversion of E2 to estrone in the liver (Table 3). E2 also suppressed the expression of the cytochrome P450 enzyme CYP2D9, a male-specific testosterone 16α-hydroxylase, in ORX GHRKO mice.28 Additionally, other transcripts that showed E2-induced compensatory changes included the transcription factor interferon regulatory factor 6 and interferon inducible GTPase I, both involved in the regulation of cytokine signaling,29, 30 as well as two lipolytic enzymes, 2-hydroxyphytanoyl-CoA lyase and hepatic lipase.31, 32 Last, six other transcripts, ectonucleotide pyrophosphatase/phosphodiesterase 2 and 3, poly (ADP-ribose) polymerase, plexin B1, Spink3, and thryrotroph embryonic factor were classified as miscellaneous and are involved in nucleotide metabolism, DNA repair, signal transduction, protein processing, and transcription, respectively.33-37
E2 induces increased STAT5 activation
The powerful and complete recovery of PrlR mRNA expression suggested a compensation of a putative mechanism in GHR-deficient livers. Because both GHR and PrlR activate STAT5, and as phosphorylated STAT5 (STAT5-P) is involved in the expression of the IGF-I gene,8 we quantified total STAT5 and STAT5-P in livers from the four experimental groups of mice by Western immunoblotting. Total STAT5 expression was not different between groups, except for a slight increase in ORX GHRKO mice (Fig. 5A). The STAT5-P protein level, both absolute (Fig. 5B) and relative (Fig. 5C), was significantly enhanced in both E2-treated WT and GHRKO mice (p < 0.0005 versus respective ORX + V groups). The induction of STAT5 phosphorylation through increased PrlR activation is further supported by a significant increase in serum prolactin levels in both E2-treated WT and GHRKO mice (p < 0.0005 versus respective ORX + V groups; Fig. 6).
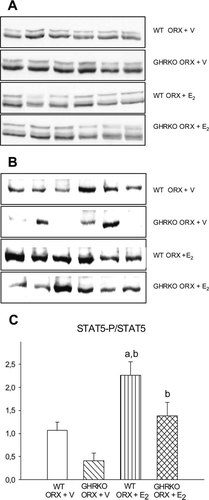
Representative Western blots of (A) total STAT5 and (B) STAT5-P. Six samples per group are shown. Total STAT5 is represented by two bands: the upper representing STAT5a and the lower representing STAT5b. (C) Phosporylated STAT5 (STAT5-P) protein expression, corrected for total STAT5 protein, in WT and GHRKO male mice. ORX mice were treated with either V or E2 for 4 weeks.ap < 0.05 vs. WT ORX + V;bp < 0.05 vs. GHRKO ORX + V (n = 6–8/group).
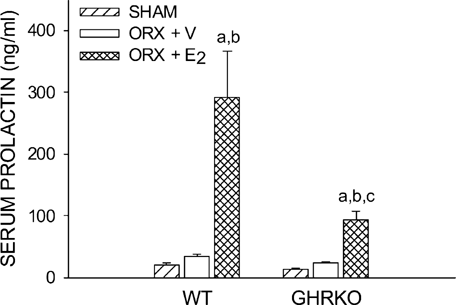
Serum prolactin levels (ng/ml), as measured by RIA, in WT and GHRKO male mice. Six-week-old mice were sham-operated (SHAM) or orchidectomized (ORX). ORX mice were treated with either V or E2 for 4 weeks.ap < 0.05 vs. respective SHAM group;bp < 0.05 vs. respective ORX + V group;cp < 0.05 vs. respective WT group (n = 6–8 mice/group).
DISCUSSION
The effects of GH on somatic growth have been inextricably linked with the actions of IGF-I since the somatomedin hypothesis was first formulated almost 50 years ago.38 Although some GH actions do not involve IGF-I, and IGF-I is not entirely regulated by GH,39 their interdependent roles in controlling growth have been widely established.40, 41 GH stimulates postnatal growth mainly by GH-induced hepatic IGF-I synthesis through GHR activation.42 Disruption of this receptor decreases IGF-I synthesis not only in the liver but also in muscle, and thereby causes a severe growth failure.
In GHRKO mice, the extremely low hepatic expression of IGF-I in untreated animals was associated with a severely decreased periosteal bone formation. E2 strongly upregulated hepatic IGF-I gene expression in ORX GHRKO mice. This upregulation not only increased serum IGF-I but was also associated with an increased periosteal bone formation, supporting the notion that the E2-induced IGF-I expression caused the radial bone expansion in GHRKO mice. Recently, periosteal bone expansion was also reported in an E2-treated aromatase-deficient boy.14 Although a potential interaction of E2 with GH and/or IGF-I was not assessed in this case report, it is not excluded that, also in men, the E2-induced periosteal bone apposition is indirectly mediated by IGF-I. Our study shows that circulating IGF-I is the major determinant of radial cortical bone growth. Skeletal IGF-I, on the other hand, was not affected in GHRKO mice, indicating that, in line with one previous study,39 skeletal IGF-I expression is GH independent and therefore less important for postnatal bone growth.
Furthermore, the E2-induced increase in serum IGF-I stimulated longitudinal bone growth. In the GHRKO mouse, a premature reduction in chondrocyte proliferation and a subsequent early decline in growth plate thickness were previously observed.5 However, either systemic administration of IGF-I5 or an E2-induced increase in serum IGF-I, as observed in this study, are able to restore longitudinal growth, supporting a direct effect of IGF-I on chondrocyte proliferation and growth plate thickness.
Furthermore, serum IGF-I levels in E2-treated GHRKO mice, corresponding with ∼25% of those observed in normal animals, were sufficient to restore bone growth. This finding supports the concept that bone growth can be maintained at low thresholds of serum IGF-I. In line with this, selective disruption of hepatic IGF-I synthesis or the gene encoding IGFBP ALS—both resulting in an over 70% decrease in serum IGF-I—have little effect on skeletal growth in young mice and cause only a mild phenotype at a later age.41, 43, 44 Any further decline in serum IGF-I below this threshold, as observed in GHRKO mice and in mice with a double gene disruption of both hepatic IGF-I and IGFBP ALS, induces severe growth retardation.3, 39, 41 In GHRKO mice, the low IGF-I levels are accompanied by low serum IGFBP-3 and low IGFBP ALS mRNA, two proteins essential for the regulation of the half-life and biological activity of IGF-I. Treatment with E2 increased not only serum IGF-I, but also IGFBP-3 and IGFBP ALS, indicating that the upregulation of all these proteins is crucial for the endocrine function of IGF-I. A physiological role of estrogens acting through ERα in the regulation of serum IGF-I is further supported by the finding that ERαKO, but not ERβKO, male mice have reduced serum IGF-I levels, associated with reduced longitudinal and radial bone growth.17
An interesting and potentially significant novel finding, given the nature of the gene knockout, is the strong downregulation of ERα and PrlR (both long and short form). The reduced ERα expression is accompanied by the significantly enhanced expression of two sulfotransferases, both capable of inactivating E2 by sulfoconjugation and thereby preventing receptor binding.45 As a result, the liver of GHRKO mice is less responsive to estrogenic stimulation not only because of the estrogen receptor deficiency but also because of E2 deprivation. In line with the notion that estrogens can regulate the expression of their receptor,46, 47 E2 treatment fully restored the ERα deficit. Furthermore, the changes in ERα expression are paralleled by similar changes in PrlR expression. In this respect, ovariectomized female rats show a decreased hepatic PrlR mRNA expression (both long and short form).48 Additionally, treatment of castrated male and female rats with E2 increased hepatic PrlR mRNA,48, 49 consistent with the presence of an estrogen responsive element in the promoter region of the PrlR gene.50 In line with these findings, the full recovery of PrlR expression in E2-treated GHRKO mice supports the concept that ERα regulates the expression of the PrlR in the liver.
The full recovery of PrlR expression as well as the E2-induced increase in serum prolactin suggested a compensation of a putative mechanism involving the JAK-STAT pathway.51 The participation of STAT5 in IGF-I gene expression has been shown both in vitro52 and in vivo.53 Moreover, a STAT5 binding site (TTCNNNGAA) has been identified within the second intron of the mouse, rat, and human IGF-I gene and has been shown to bind STAT5 to activate IGF-I gene expression in vivo.54 In our study, the assessment of STAT5 activation through phosphorylation revealed that phosphorylated STAT5 was increased in E2-treated GHRKO mice, supporting the notion that STAT5 activation significantly contributed to the enhanced IGF-I gene expression. Further evidence that the JAK-STAT pathway is functionally recovered in E2-treated GHRKO livers is suggested by the observed upregulation of suppressor of cytokine signaling 2 mRNA (SLR of 2.7; p = 0.0045). Therefore, we hypothesize that the rise in serum prolactin and subsequent enhanced PrlR transduction induces STAT5 activation, followed by the increased hepatic IGF-I synthesis in E2-treated GHRKO mice. However, as previously shown in vitro, we cannot exclude a direct effect of E2 on STAT5 phosphorylation,55 and further study is needed to define the possibility of a direct effect of E2 on STAT5 in vivo.
Liver-derived IGF-I is not only important for bone metabolism but also for carbohydrate and lipid metabolism,56, 57 as well as blood pressure regulation.58 Future research should address to what extent treatment with estrogen or estrogen-like compounds (e.g., selective estrogen receptor modulators) might be clinically useful for the restoration of hepatic IGF-I synthesis in patients with disease-related or age-dependent GH/IGF-I deficiency and/or GH-resistant conditions.
We conclude that, in the absence of a functional GHR and in the context of very low serum IGF-I levels, E2 stimulates liver IGF-I production. The anabolic effects of the subsequent E2-induced rise in serum IGF-I on longitudinal and radial bone growth provide evidence for the importance of endocrine IGF-I in mediating growth. Skeletal expression of IGF-I, on the other hand, is not regulated by GH or E2 and may be less important for skeletal growth. These findings show that E2 rescues pubertal growth during GH resistance through a novel mechanism of GHR-independent stimulation of IGF-I production in the liver.
Acknowledgements
The authors thank E Van Herck, F Vanderhoydonc, W Coopmans, and A Hansevi for technical assistance. This study was supported by Grant OT/01/39 from the Katholieke Universiteit Leuven, Grant G.0417.03 from the Fund for Scientific Research-Flanders, Belgium (F.W.O.-Vlaanderen), the Swedish Research Council, the Swedish Foundation for Strategic Research, the Lundberg Foundation, the Torsten and Ragnar Söderberg's Foundation, and Petrus and Augusta Hedlunds Foundation. DV and SB are Senior Clinical Investigators and FS is Research Professor (BOF) of the Fund for Scientific Research-Flanders, Belgium (F.W.O.-Vlaanderen). JJK was supported, in part, by the state of Ohio's Eminent Scholars Program that includes a gift from Milton and Lawrence Goll and by DiAthegen LLC.