Dominant Negative N-Cadherin Inhibits Osteoclast Differentiation by Interfering With β-Catenin Regulation of RANKL, Independent of Cell-Cell Adhesion†
The authors have no conflict of interest.
Abstract
We studied the effects of dominant negative N-cadherin (NCadΔC) expression in ST2 cells on their ability to support osteoclastogenesis. Expression of NCadΔC in ST2 cells did not decrease cell-to-cell adhesion but significantly reduced osteoclast formation when co-cultured with BMMs. NCadΔC inhibited β-catenin/TCF signaling, resulting in decreased RANKL expression, which could contribute to the reduced osteoclast formation.
Introduction: Cadherin is a calcium-dependent cell adhesion molecule that plays major roles during embryonic development and morphogenesis. Classic cadherins interact with β-catenin, which is also involved in the Wnt signaling pathway. We tested whether disruption of N-cadherin function in stromal cells by dominant negative N-cadherin affects their ability to support osteoclastogenesis by altering heterotypic interaction with osteoclast precursors.
Materials and Methods: ST2 cells were transduced with retrovirus encoding extracellular domain-truncated, dominant negative N-cadherin (NCadΔC) and co-cultured with bone marrow macrophages (BMMs) to study the ability to support osteoclastogenesis. As a downstream target of NCadΔC, β-catenin/T-cell factor (TCF) transcriptional activity was analyzed using TOPflash reporter construct. Real-time RT-PCR analysis and RANKL-luciferase reporter assays were performed to study the effects of NCadΔC on the osteoprotegerin (OPG)/RANKL system.
Results: Immunoblotting analysis showed that primary bone marrow stromal cells, ST2 cells, and BMMs expressed N-cadherin. Retroviral expression of NCadΔC in ST2 cells did not significantly inhibit cell adhesion but markedly impaired the formation of TRACP+ osteoclasts (>40%) when co-cultured with BMMs. However, the inhibition of osteoclastogenesis was not reproduced by neutralizing antibody against N-cadherin. Expression of NCadΔC, however, strongly suppressed β-catenin/TCF transcriptional activity in ST2 cells, which was rescued by constitutively active β-catenin adenovirus (Ad ΔN46 β-catenin) or constitutively active TCF mutant (pCS2-VP16ΔβXTCF-3). As a potential downstream target of Wnt signaling, we found that the expression of RANKL was reduced in ST2 cells expressing NCadΔC. Moreover, Wnt-3A, Ad ΔN46 β-catenin, and VP16ΔβXTCF-3 increased the expression of RANKL and enhanced the transcriptional activity of mouse RANKL promoter in ST2 cells.
Conclusions: Our data suggest that expression of dominant negative N-cadherin in ST2 cells suppressed osteoclastogenesis by interfering with β-catenin regulation of RANKL independent of cell-cell adhesion.
INTRODUCTION
THE CADHERINS AREa family of cell surface glycoproteins that mediate calcium-dependent cell-to-cell adhesion in a wide variety of tissues and species.1, 2 The classical cadherins, E-, P-, and N-cadherin, possess an extracellular N-terminal sequence, a single transmembrane domain, and a short cytoplasmic tail.3, 4 Cadherin-mediated cell-cell adhesion typically requires homotypic homophilic binding of the extracellular domain of cadherins on adjacent cells, which is highly variable among the cadherin isotypes. However, recent studies have shown an involvement of cadherins in heterophilic and even heterotypic interactions.5-7 The well-established role of cadherins in mediating cell interactions, coupled with the complicated spatio-temporal patterns of their expressions during development, suggest that cadherins play critical roles in the morphogenesis, acquisition, and maintenance of cell polarity and in the regulation of cell proliferation and differentiation.1, 8, 9 The cytoplasmic domain interacts with several proteins, including catenins and plakoglobin, which link cadherins to the actin cytoskeleton.8 In addition to its function as a structural adaptor protein linking cadherins to the cytoskeleton, β-catenin plays a central role in the Wnt signaling pathway.10, 11 In the canonical Wnt/β-catenin pathway, the binding of Wnt proteins to frizzled receptors activates intracellular Dishevelled, which inhibits the kinase activity of a complex containing glycogen synthetase kinase-3, Axin, β-catenin, and other proteins. Because this complex targets β-catenin for rapid degradation through phosphorylation, Wnt signaling allows the stabilization and nuclear translocation of β-catenin, which binds to the lymphoid enhancer binding factor (LEF)/T cell transcription factor (TCF) family of transcription factors to regulate the expression of Wnt target genes.12-14
We previously reported that human trabecular osteoblasts and bone marrow stromal cells (BMSCs) express a repertoire of cadherins, including, N-cadherin, cadherin-11, and cadherin-4.15 This was not unexpected because the osteoblast cell layers that cover most of the bone surface have an epithelium-like polarized structure, and active osteoblasts are interconnected by numerous junctional structures. Moreover, using mouse mesenchymal cell lines, we identified a critical role of N-cadherin during the early stage of osteoblast differentiation from the mesenchymal precursors, whereas cadherin-11 is involved in the late period of differentiation.16 The role of N-cadherin in mature osteoblasts has been shown using a dominant negative N-cadherin construct (NCadΔC), which lacks most of the extracellular domain. Using this construct, we and others have shown that interference of N-cadherin-dependent cell-cell adhesion results in altered bone matrix protein expression and reduced matrix mineralization in mouse and rat osteoblasts both in vitro17, 18 and in vivo.19
In contrast to this evidence on the role of cadherin in osteoblastogenesis, it is not well established whether cadherins play a role in the formation of osteoclasts. Osteoclasts are multinucleated bone-resorbing cells that differentiate from hematopoietic mononuclear precursor cells through a series of sequential steps of proliferation, fusion, and maturation.20 During the development of osteoclasts, osteoclast precursor cells are considered to interact closely with osteoblasts and/or stromal cells.21 Two cytokines derived from stromal cells, RANKL and macrophage-colony-stimulating factor (M-CSF), have been implicated to play essential roles in osteoclastogenesis.22, 23 However, the critical role of fusion in the formation of osteoclasts and the close contact between stromal and mononuclear precursors suggests that direct cell-cell interaction should play a role in this highly complicated process. Indeed, using a co-culture system, it has been shown that vascular cell adhesion molecule 1 (VCAM-1)24 or cadherin-625 plays a role in the heterotypic interaction between osteoblast/stromal cells and osteoclast precursors.
In this study, we investigated whether N-cadherin-mediated cell-cell interaction plays a role in the formation of osteoclasts. Toward this end, we overexpressed a dominant negative N-cadherin construct (NCadΔC) in ST2 cells in a co-culture system and evaluated the ability to support osteoclastogenesis. We have also addressed the molecular events associated with NCadΔC overexpression in ST2 cells.
MATERIALS AND METHODS
Reagents
Neutralizing monoclonal antibody to chicken N-cadherin (anti-A-CAM; Sigma, St Louis, MO, USA) recognizes an extracellular domain, whereas mouse anti-N-cadherin antibody (Transduction Laboratories, Lexington, KY, USA) is directed against the cytoplasmic tail of N-cadherin. Monoclonal mouse anti-HA antibody was obtained from Covance (Princeton, NJ, USA), polyclonal rabbit anti-β-catenin antibody from Cell Signaling Technology (Beverly, MA, USA), polyclonal anti-USF1 antibody from Santa Cruz Biotechnology (Santa Cruz, CA, USA), mouse monoclonal anti-PLCγ antibody from Upstate Biotechnology (Lake Placid, NY, USA), and Cy3-conjugated goat anti-mouse IgG was from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). All the other chemicals, including the tissue culture media were from Sigma.
To prepare Wnt-3A conditioned medium (CM), Wnt-3A-expressing L cells (American Type Culture Collection, Manassas, VA, USA) were grown in serum-free DMEM, and Wnt-3A CM was harvested after 24 h. Wnt-3A CM was concentrated 30 times using a Centricon (Millipore, Billerica, MA, USA).
Plasmids
The NCadΔC construct, cloned in pSP72 (pSP72NCadΔC) vector, was obtained from Dr Jeffery Gordon (Washington University, St Louis, MO, USA) with the permission of Dr Chris Kintner (Salk Institute, San Diego, CA, USA). A hemagglutinin (HA) epitope was incorporated at the C-terminal end of NCadΔC by PCR and ligated into the BglII/EcoRI site of pMSCV-IRES-GFP retroviral vector upstream of IRES, giving pMSCV-NCadΔC-IRES-GFP. pEF-BOS-NCadΔC, used in the transient transfection experiment, was generated by PCR amplification of NCadΔC with flanking XbaI sites at both 5′ and 3′ ends and cloned into pEF-BOS plasmid.26
TOPflash reporter construct containing three copies of the LEF1/TCF-4 binding sequences or its mutant, FOPflash, containing three copies of mutant binding site, both cloned upstream of the thymidine kinase minimal promoter, and a luciferase encoding gene were purchased from Upstate Biotechnology (Waltham, MA, USA). Mouse RANKL promoter-luciferase reporter construct, pRANKL-Luc, containing a 1-kb segment (position −1005 to +13) has been previously described.27
Retrovirus generation and transduction
For the transient generation of VSV-G pseudo-typed retrovirus, 293T cells were transfected with pMD-gag-pol, pMD-VSVG, and the retroviral vectors pMSCV-NCadΔC-IRES-GFP or pMSCV-IRES-GFP using LipofectAMINE Plus reagents (Invitrogen, Carlsbad, CA, USA). Viral supernatant was collected 48 h after DNA addition, filtered through a 0.45-μm syringe filter (Nalgene, Rochester, NY, USA), and stored at −80°C. ST2 cells were transduced with virus-containing supernatants in the presence of 5 μg/ml polybrene for 5 h. Cells were collected 48–72 h later, and green fluorescent protein (GFP)+ fractions were FACS-sorted using a BD FACS Vantage Cell Sorter (Franklin Lakes, NJ, USA) and used for co-culture study.
Generation of adenovirus expressing constitutively active β-catenin
To generate constitutively active β-catenin expression, mutant β-catenin construct lacking the 46 amino-terminal amino acids (ΔN46 β-catenin) was generated by PCR using a wildtype human β-catenin plasmid (kindly provided by Dr Bert Vogelstein, The Johns Hopkins University, Baltimore, MD, USA) as a template. The removal of these 46 amino acids resulted in loss of all four phosphorylation sites and conferred constitutively active expression. The resulting products were subcloned into a shuttle vector (pAdTrack-CMV; Qbiogene, Carlsbad, CA, USA). The recombinant shuttle vector was co-transfected with adenoviral genome (pAdEasy-1; Qbiogene) into E. coli (BJ5183), where homologous recombination occurred. The recombinant adenovirus construct was transfected into 293 cells to obtain viral particles (Ad ΔN46 β-catenin), which were purified by CsCl ultracentrifugation and dialysis.
Isolation of bone marrow macrophages and stromal cells
Mouse bone marrow cells containing both mononuclear hemopoietic cells and stromal cells were collected from the femora and tibias of 4-week-old C57BL male mice (Korea Biolink, Eumsung, Korea). Cells were washed twice with serum-free αMEM (Sigma) and suspended in αMEM containing 10% FBS. To isolate bone marrow macrophages (BMMs), we used a well-characterized technique essentially as described previously.28 Briefly, bone marrow cells were cultured for 24 h in αMEM supplemented with 10% FBS in the presence of M-CSF (100 ng/ml). Nonadherent cells were collected and layered on a Ficoll-Hypaque gradient, and cells at the gradient interface were collected and cultured for 3–4 days at 5 × 106 cells per 100-mm plate in αMEM containing 10% FBS supplemented daily with 100 ng/ml M-CSF. It has been established that almost all of the adherent cells at this stage show the characteristics of mononuclear macrophages,29 and in this study, we refer to these cells as BMMs. BMMs were used for co-culture with ST2 cells (described below).
For the isolation of stromal cells, 10–50 × 106 nucleated cells from the mouse bone marrow, as described above, were suspended in αMEM containing 10% FBS and plated in 75-cm2 culture flask. After 3 days, approximately one-half of the nonadherent cells was removed by replacing one-half of the medium. Similarly one-half of the medium was replaced with fresh medium on days 10 and 16. The cells were harvested after they reached confluence for the N-cadherin expression study.
Co-culture of ST2 cells with BMMs
ST2 cells transduced with either MSCV-NCadΔC-IRES-GFP or MSCV-IRES-GFP virus were propagated and mixed with BMMs at a ratio of 1:10 and cultured in a 24-well plate in αMEM supplemented with 10% FBS, 10 nM 1,25(OH)2 vitamin D3, and 100 nM dexamethasone. After 7 days of culture, the cells were fixed and stained for TRACP, and the number of multinucleated osteoclast-like cells formed was counted. To evaluate the effect of neutralizing N-cadherin antibody, 10 μg/ml of A-CAM antibody or control rat IgG was added to the co-culture every other day.
TRACP staining
After co-culture, adherent cells were fixed in 60% (vol/vol) acetone solution prepared in sodium citrate buffer (pH 5.4) for 30 s. Fixed cells were washed twice with distilled water and incubated for 10 minutes at room temperature in acetate buffer (0.1 M sodium acetate, pH 5.0) containing 0.01% naphthol AS-MX phosphate (Sigma) and 0.03% fast red violet LB salt (Sigma) in the presence of 50 mM sodium tartrate. TRACP+ multinucleated (three or more nuclei) cells (osteoclast-like cells) in each well were scored by manually counting the cells across the entire well under a microscope.
Adhesion assay
A cell adhesion assay was performed using a modification of a previously described method.17, 30 Briefly, ST2 cells (5 × 104 cells/well) were placed onto 48-well culture plates (Costar, Cambridge, MA, USA) and cultured to confluence in αMEM containing 10% FBS. The plates were washed three times with PBS before the addition of BMMs. The 2 × 105 BMMs were labeled with51Cr (Dupont NEN, Wilmington, DE, USA) in αMEM with 1% BSA, and the labeled cells were mobilized from the culture dish by incubation with 2 mg/ml trypsin and 0.5 mM EDTA in Ca2+- and Mg2+-free Hank's balanced salt solution (low calcium solution [LCS]) for ∼15–20 minutes at 37°C. After addition of 100 mg/ml soybean trypsin inhibitor, cells were washed and resuspended (2.5 × 105 cells/ml) in LCS. One milliliter of this cell suspension was added on top of the ST2 cells expressing NCadΔC or empty vector and washed three times in LCS at room temperature. Immediately before the transfer over the ST2 cell layers, 1 M CaCl2 was added to the51Cr-labeled cell suspensions to raise the extracellular Ca2+ concentration to >1 mM.17, 30 The dishes were returned to the tissue culture incubator and left at 37°C for 1 h to allow the51Cr-labeled BMMs to adhere onto the ST2 cell substratum. Thereafter, each dish was carefully washed five times with LCS + 1 mM CaCl2 to remove nonadherent cells. The contents of each well containing adherent BMMs were lysed with 250 μl of 1% Triton X-100, and the emission of the contents of each well was measured using a gamma counter. Data were expressed as mean percentage of the binding of indicated cells from a representative experiment.
RT-PCR
First-strand cDNA was synthesized using AMV reverse transcriptase (Roche, Basel, Switzerland). RT reaction was carried out using 1 μg total RNA using the following conditions: 42°C for 60 minutes, 95°C for 5 minutes, and 4°C for 5 minutes. PCR was performed using 2 μl of cDNA, 20 pmol of each primer (synthesized by Bioneer Corp., Chungwon, Korea), 200 μM of dNTPs, 1 mM of MgCl2, and 1 U of Taq polymerase in a 50-μl reaction volume containing 1× Taq polymerase buffer using a Perkin-Elmer Gene Amp PCR System 2400. The sense and antisense primers 5′-ATTCAGCACCCACCTCAGTC-3′ and 5′-TCCGCCTCTTGAGGTAACAC-3′; 5′-GGGTGTGAACCACGAGAAAT-3′ and 5′-TTACTCCTTGGAGGCCATGT-3′ were used to amplify N-cadherin and GAPDH-producing bands of 413 and 610 bp, respectively.
Quantitative real-time RT-PCR measurements of gene expression
We used a previously reported set of optimal oligonucleotide primers and TaqMan probes.31 The sequences of the primers and probes used were as follows: RANKL sense 5′-TGGAAGGCTCATGGTTGGAT-3′, antisense 5′-CATTGATGGTGAGGTGTGCAA-3′, and probe 5′-AGGCTTGCCTCGCTGGGCCAC-3′; osteoprotegerin (OPG) sense 5′-AGCTGCTGAAGCTGTGGAA-3′, antisense 5′-TGTTCGAGTGGCCGAGAT-3′, and probe 5′-CCAAGACATTGACCTCTGTGAAAGCA-3′. Probes were labeled with a reporter fluorescent dye, FAM, at the 5′ end and a quencher fluorescent dye, TAMRA (6-carboxy-tetramethyl rodamine), at the 3′ end (Bioneer Corp.). Commercially available primers and probes were used to quantify GAPDH expression for normalization in RT-PCR (TaqMan Rodent GAPDH Control Reagent; Perkin Elmer Biosytems, Palo Alto, CA, USA). Cycling conditions used were 95°C for 15 s and 60°C for 1 minute for 40 cycles. Real-time TaqMan PCR was performed in an ABI PRISM 7700 Sequence detector (Perkin-Elmer Biosystems). All PCR reactions were performed in duplicate, and the RANKL and OPG signals were normalized to GAPDH signal in the same reaction.
Northern blot analysis
Total cellular RNA (20 μg/lane) was separated on 1% formaldehyde agarose gels by electrophoresis, blotted onto nylon membranes, and UV cross-linked. The membranes were hybridized using [32P]labeled probes in ULTRAhyb solution (Ambion, Austin, TX, USA) at 42°C overnight and washed twice in 2× SSC and 0.1% SDS at 42°C, followed by one high-stringency wash in 0.2× SSC and 0.1% SDS at 42°C for 15 minutes. The following cDNA probes were used: a 0.5-kb BglI-KpnI fragment of mouse β-catenin and a 1.9-kb BamHI fragment of rat β-actin.
Western blotting
Cell lysates were prepared and processed from cells plated in a 100-mm dish as described previously.16 SDS-PAGE was performed on 10% polyacrylamide gels, and the resolved proteins were transferred onto nitrocellulose membranes. Membranes were blocked with 0.1% Tween-20 TBS containing 2% BSA and 3% dry milk, at pH 7.4, for 1 h. Relevant primary antibodies were added, and incubation was continued for another hour, and after washing in 0.1% Tween-20 TBS, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h. After extensive washing, bands were visualized by chemiluminescence using an ECL kit (Amersham Pharmacia Biotech) according to the manufacturer's instructions.
Immunoprecipitations
ST2 cells were lysed in 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40, and 0.5% sodium deoxycholate, containing a cocktail of protease inhibitors (Sigma). Lysates were precleared for 3 h at 4°C with protein G-Sepharose (Roche, Mannheim, Germany). For immunoprecipitation of endogenous N-cadherin and dominant negative cadherin from ST2 cells, we used anti-N-cadherin antibody and anti-HA antibody, respectively. Lysates were incubated for 3 h at 4°C before being incubated with protein G-Sepharose. After extensive washing, the immunoprecipitates were electrophoresed in SDS-polyacrylamide gels, and the expression levels of the proteins of interest were verified by Western analyses using anti-β-catenin antibody.
Immunofluorescence
Cells grown on 12-mm round glass coverslips were fixed in 3% paraformaldehyde, permeabilized in 0.5% Triton X-100 buffer on ice, and incubated in PBS containing 2% heat-inactivated goat serum and 1% Triton X-100 for 10 minutes. The coverslips were incubated with the appropriate dilutions of anti-HA antibody (1:400) for 45 minutes at room temperature. After three washes in PBS, they were incubated with Cy3-conjugated goat anti-mouse IgG (1:200) for 30–60 minutes. After washing, the coverslips were mounted on glass slides with Anti-fade solution (Molecular Probes, Eugene, OR, USA). Immunofluorescence images were visualized by confocal microscopy (Model TCS SP2; Leica Microsystems, Wetzlar, Germany).
Reporter gene assays
ST2 cells were plated at high density (3 × 105 cells/well) onto 12-well plates. Appropriate plasmids were transfected into each well using LipofectAMINE Plus reagent per the manufacturer's instructions. Cell lysates (0.25 ml/well) were prepared using the Promega Luciferase Assay System, and reporter activity was measured using a luminometer (Lumat LB 9507). All luciferase values were normalized against the β-galactosidase or luciferase activities from the co-transfected pCMV-β-gal or pRL-TK Renila luciferase plasmid, respectively.
Statistical analysis
All data are presented as mean ± SD. The data were analyzed by one-way ANOVA or Student's t-test. When the ANOVA performed over all groups indicated a significant difference among the groups, statistical difference between two groups was subsequently evaluated by Student-Newman-Keuls multiple comparison test. A p value <0.05 was considered significant for all statistical analyses.
RESULTS
Expression of N-cadherin in BMSCs and osteoclast precursors
We initially assessed the expression of N-cadherin in the cells involved in osteoclast generation (i.e., BMSCs and mononuclear precursors). RT-PCR analysis with specific primers for mouse N-cadherin was performed. Figure 1 shows that the expected 413-bp cDNA fragment could be amplified by reverse transcription from the RNA of freshly isolated BMSCs. A positive signal was also obtained using the two mouse bone marrow-derived stromal cell lines, ST2 and MC3T3-G2/PA6 (Fig. 1A).

Expression of N-cadherin in mouse bone marrow-derived cells. (A) RT-PCR analysis of N-cadherin expression. mRNA coding for mouse N-cadherin was detected by RT-PCR analysis from total RNA prepared from mouse BMSCs (lane 1), ST2 cells (lane 2), MC3T3-G2/PA6 cells (lane 3), RAW 264.7 macrophage cell line (lane 4), and BMMs (lane 5). No amplification was observed from 3T3-L1 cells, which were used as a negative control (lane 6). (B) Western blot analysis of N-cadherin expression. Whole cell lysates from BMSC (lane 1), ST2 cells (lane 2), MC3T3-G2/PA6 cells (lane 3), RAW 264.7 macrophage cell line (lane 4), BMM (lane 5), and 3T3-L1 cells (lane 6) were separated by SDS-PAGE and blotted with an N-cadherin antibody. A band of ∼130 kDa, corresponding to N-cadherin, was detected in the lysates from BMSC, ST2, MC3T3-G2/PA6, and RAW 264.7 cells, and a very weak expression was observed in lysates from BMMs.
We analyzed the expression of N-cadherin in osteoclast precursors. As shown in Fig. 1, relatively weak N-cadherin expression was observed in BMMs isolated from long bones. However, we could identify a clear N-cadherin signal in the mouse macrophage cell line, RAW264.7, that has been recently characterized for its ability to differentiate to form osteoclast and also their bone resorption capacity.32
Western blot analysis of protein extracts from primary BMSCs and two stromal cell lines revealed prominent signals of N-cadherin expression at 130 kDa (Fig. 1B). However, similar to the RT-PCR analysis result, we could barely detect N-cadherin expression in protein extracts of BMMs, which is a heterogeneous cell population. In contrast, cell extracts of RAW264.7 cells, a homogenous pre-osteoclastic macrophage cell line, showed clear N-cadherin expression, suggesting that the subpopulation of BMMs that can differentiate to osteoclasts expresses N-cadherin (Fig. 1B). These results indicate that the two cell populations involved in osteoclastogenesis express N-cadherin at the mRNA and protein level and suggest a role for N-cadherin in the interaction between these two populations.
Establishment of a stable cell line expressing dominant negative N-cadherin
To study the role of N-cadherin in the heterotypic interaction between mononuclear cells and stromal cells, we examined the consequences of expressing dominant negative N-cadherins in ST2 cells on their ability to support osteoclast differentiation. For this work, we used the NCadΔC construct, derived from an in-frame deletion of most of the extracellular domain of Xenopus N-cadherin, which has been shown to function as a dominant negative cadherin in intestinal epithelial cells33 and mouse osteoblastic MC3T3-E1 cells.17 To facilitate efficient gene transfer, the NCadΔC cDNA with a 3′ HA tag was introduced into the pMSCV-GFP retroviral vector under the transcriptional control of the MSCV-long terminal repeat and upstream of an IRES-GFP expression cassette, thereby allowing the expression of both NCadΔC and GFP from a single bicistronic mRNA (Fig. 2A).
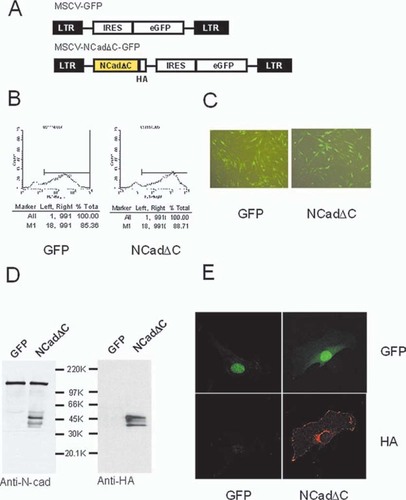
Retroviral transduction of NCadΔC into ST2 cells. (A) Schematic diagram of the retroviral construct used in this study. The solid rectangles represent Moloney murine leukemia virus LTRs. NCadΔC, truncated N-cadherin; HA, hemagglutinin epitope; IRES, internal ribosomal entry site of the encephalomyocarditis virus; GFP, green fluorescent protein gene. (B) FACS analysis of GFP expression of ST2 cells transduced with MSCV-GFP (GFP) or MSCV-NCadΔC-GFP (NCadΔC) virus to monitor the expression of GFP. GFP+ cells were FACS sorted 48 h after retrovirus infection. (C) Fluorescent microscopy of representative fields of ST2 cells that were FACS sorted and collected. Cells were examined under a fluorescent microscope to visualize GFP in both cell populations. More than 85% of the cells were stably transduced by either MSCV-GFP (GFP) or MSCV-NCadΔC-GFP (NCadΔC) virus. (D) Western blot analysis of total cell lysates from mouse ST2 cells. Cell lysates from confluent cultures were analyzed by SDS-PAGE and Western blotting with anti-N-cadherin antibody (left) or anti-HA antibody (right). A band of ∼45 kDa, corresponding to the truncated NCadΔC, was detected in cells carrying the dominant negative construct (NCadΔC) but not in cells harboring GFP only (GFP). (E) Immunofluorescence analysis of truncated cadherins expression in ST2 cells. ST2 cells transduced with control virus (GFP) or NCadΔC (NCadΔC) virus were seeded onto coverslips and allowed to recover overnight. Cells were fixed and incubated with anti-HA antibody (1:400) followed by incubation with Cy3-conjugated goat anti-mouse antibody (1:200). After washing, the coverslips were observed under a confocal microscope.
Using this vector, we could routinely generate high-titer retrovirus capable of stably transducing >85% of ST2 cells (Figs. 2B and 2C). The expression of NCadΔC protein in ST2 cells was confirmed by Western blot analysis using an antibody that recognizes the intracellular portion of N-cadherin and therefore reacts with both endogenous N-cadherin and the truncated NCadΔC mutant (Fig. 2D, left) and also anti-HA antibody directed against only the truncated NCadΔC (Fig. 2D, right). A band of ∼45 kDa, corresponding to the truncated NCadΔC, was detected in cells carrying the dominant negative construct but not in cells harboring GFP only. The abundance of endogenous N-cadherin protein (∼130 kDa) was not altered by the expression of NCadΔC (Fig. 2D, left). Using indirect immunofluorescence with anti-HA antibody, NCadΔC specific fluorescence was found to be localized at the cytoplasmic membrane and some in perinuclear region (Fig. 2E).
Inhibition of osteoclastogenesis by dominant negative N-cadherin expression in ST2 cells
We next examined whether the expression of NCadΔC in these cells inhibits calcium-dependent cell-cell adhesion as observed in our previous study.17 As shown in Fig. 3, the quantitation of the51Cr-labeled BMMs adherent to an ST2 cells stably transduced with NCadΔC was not significantly different from the number of adherent cells to vector-transduced ST2 cells (Fig. 3A). To clarify the functional role of N-cadherin in the cell-cell adhesion between ST2 cells and BMMs, we also tested the effects of neutralizing N-cadherin antibody, anti-A-CAM. As expected, treatment with neutralizing N-cadherin antibody significantly reduced the number of adherent cells (Fig. 3B), suggesting that N-cadherin plays a role in the heterotypic cell adhesion between ST2 cells and BMMs, but expression of NCadΔC did not significantly affect cell adhesion between these cells.
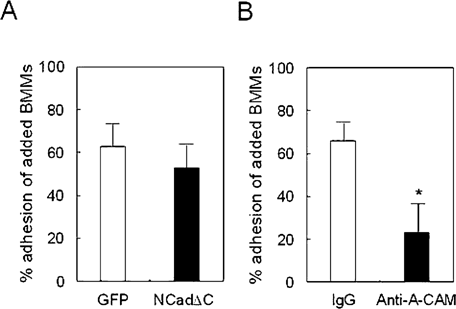
Adhesion of BMMs to ST2 cells. (A) BMMs were labeled with51Cr and laid on top of confluent, unlabeled ST2 cells transduced with pMSCV-GFP (GFP) or pMSCV-NCadΔC-GFP (NCadΔC) virus. After 1 h of incubation in a calcium-containing medium, nonadherent cells were removed by gentle washing. Cell-cell adhesion in NCadΔC-transduced cells (NCadΔC) was not significantly different from that in vector-transduced cells (GFP). (B) Labeled BMMs were incubated with ST2 cells in the absence or presence of anti-A-CAM antibody, a neutralizing N-cadherin antibody. Treatment with anti-A-CAM significantly reduced the number of adherent cells. The percentage of binding of added BMMs is expressed as mean ± SD. *p < 0.05 vs. IgG.
We assessed the consequences of expressing NCadΔC on the ability of ST2 cells to support osteoclast differentiation. When ST2 cells overexpressing NCadΔC were co-cultured with mouse BMMs for 7 days in the presence of 1,25(OH)2 vitamin D3 and dexamethasone, multinucleated osteoclast-like cell formation was reduced by 46% compared with the co-culture between vector-transduced ST2 cells and BMMs (Figs. 4A and 4B). We next determined whether the reduced formation of osteoclasts by NCadΔC was reproduced by anti-A-CAM antibody, which neutralizes N-cadherin. However, as shown in Fig. 4C, the addition of anti-A-CAM antibody to a co-culture of BMMs and ST2 cells did not affect the formation of multinucleated TRACP+ cells compared with the control rat IgG-treated cells.
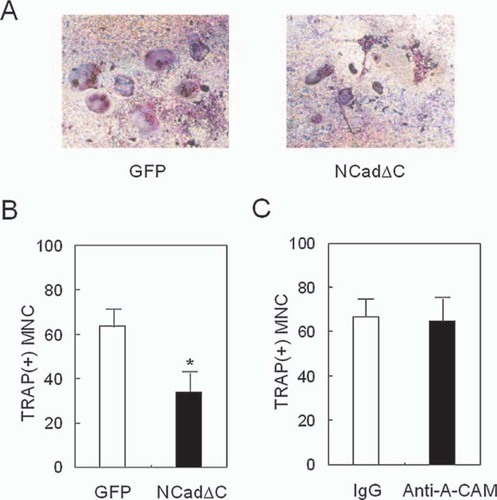
Effects of dominant negative N-cadherins on the formation of TRACP+ MNCs. ST2 cells transduced with pMSCV-GFP (GFP) or pMSCV-NCad-C-GFP (NCadΔC) virus were co-cultured with mouse BMMs in the presence of 10 nM 1,25(OH)2 vitamin D3 and 100 nM dexamethasone. (A) Photomicrographs showing reduced osteoclast formation by expressing NCadΔC in ST2 cells. (B) The number of TRACP+ MNCs in co-culture of BMMs with ST2 cells. ST2 cells expressing NCadΔC (closed bar) showed decreased ability to support multinucleated osteoclast-like cell formation compared with empty vector-transduced control cells (open bar). Data shown are mean ± SD. *p < 0.05 vs. GFP. (C) The number of TRACP+ MNCs in co-culture of BMMs with ST2 cells in the presence of anti-A-CAM antibody or control IgG. The addition of anti-A-CAM antibody to a coculture of BMMs and ST2 cells did not affect the formation of TRACP+ MNCs cells compared with the control rat IgG-treated cells. Data shown are mean ± SD.
In summary, the overexpression of NCadΔC in ST2 cells did not significantly alter cell adhesion but markedly inhibited the ability of ST2 cells to support osteoclastogenesis. The inhibition of osteoclastogenesis was not reproduced by neutralizing antibody against N-cadherin. Therefore, it is less likely that alteration of cellular adhesion is a major mechanism of reduced formation of osteoclasts by dominant negative cadherin.
Effects of NCadΔC on the level and cellular distribution of β-catenin
Given the aforementioned results, we explored the possible disruption of the cadherin-mediated downstream cellular signal by NCadΔC expression, which could explain the reduced ability of ST2 cells to support osteoclastogenesis. The expression of dominant negative cadherin has been suggested to sequester β-catenin in the plasma membrane, thereby reducing the nuclear availability of β-catenin for its transcriptional activity.19, 34 Therefore, we first studied the level of β-catenin expression in NCadΔC-expressing cells. As shown in Fig. 5A, the expression of NCadΔC induced robust increase in the β-catenin level by Western blot analysis. Nevertheless, Northern blot analysis showed that the mRNA level of β-catenin was not affected by NCadΔC expression (Fig. 5B), suggesting that NCadΔC may increase the stability of β-catenin by complexing with and protecting β-catenin from degradation. This notion was supported by a co-immunoprecipitation experiment. As shown in Fig. 5C, immunoprecipitations of cell lysates from ST2 cells expressing NCadΔC with anti-HA pulled down β-catenin (Fig. 5C, left), showing an direct association between NCadΔC and β-catenin. Moreover, the abundance of β-catenin associated with anti-N-cadherin antibody, which recognizes both endogenous N-cadherin and NCadΔC, was much higher in ST2 cells expressing NCadΔC than in cells expressing vector only, indicating that NCadΔC may stabilize β-catenin by direct interaction (Fig. 5C, right). Cell fractionation study showed that the level of cytosol/membrane fractions of β-catenin was higher in ST2 cells expressing NCadΔC compared with cells with empty vector, whereas the ratio was reversed in the nuclear fraction (Fig. 5D). These results indicate that expression of NCadΔC has almost completely sequestered β-catenin at the cytosol/membrane fraction.
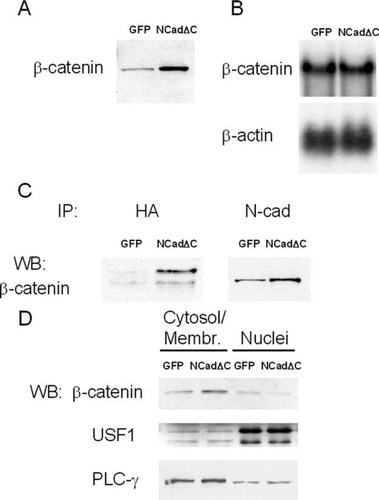
Stabilization of β-catenin by dominant negative N-cadherin. (A) Western blot of proteins from ST2 cells transduced with pMSCV-IRES-GFP virus (GFP) or pMSCV-NCadΔC-IRES-GFP virus (NCadΔC) with anti-β-catenin antibody. Expression of NCadΔC induced robust increase in the total cellular β-catenin level. (B) Northern blot analysis of β-catenin mRNA using equivalent amounts of total RNA with [32P]labeled β-catenin probe. The mRNA level of β-catenin was not affected by NCadΔC expression. (C) Co-immunoprecipitation with anti-HA antibody (left) or anti-N-cadherin antibody (right) from ST2 cell extracts. Proteins bound to the protein A Sepharose beads were resolved by SDS-PAGE and immunoblotted with anti-β-catenin antibody. Stably transduced NCadΔC is directly associated with and stabilizes β-catenin. (D) The cytosolic/membrane and nuclear fractions of ST2 cells were separated and blotted using an anti-β-catenin antibody. The level of cytosol/membrane fractions of β-catenin was higher in ST2 cells expressing NCadΔC compared with cells with empty vector, whereas the ratio was reversed in nuclear fraction. Western blots for USF1 or PLCγ were performed to verify the separation of nuclear and cytosolic/membrane fractions, respectively.
Inhibition of β-catenin-driven transactivation by NCadΔC
We next studied the effects of the expression of NCadΔC on the transcriptional activation of a β-catenin-TCF-responsive luciferase reporter construct (TOPflash). As shown in Fig. 6A, transient transfection of pEF-BOS-NCadΔC in ST2 cells resulted in a significant decrease (42%) in luciferase activity relative to pEF-BOS-transfected cells (compare lanes 4 and 5). As a control, the same experiments were performed using the FOPflash luciferase reporter gene that lacks the β-catenin binding sites. FOPflash luciferase activity was not changed regardless of the expression of NCadΔC (lanes 1 and 2). Although TOPflash responded to treatment with lithium in basal condition as expected (compare lanes 4 and 6), lithium did not significantly increase the suppressed luciferase activity by NCadΔC (compare lanes 5 and 7). However, TOPflash luciferase activity was reversed by transduction of the Ad ΔN46 β-catenin, a constitutively active β-catenin mutant (lane 8), or transfection of pCS2-VP16ΔβXTCF-3, a constitutively active Xenopus TCF-3 (lane 9). These results suggest that, although the cellular level of β-catenin is increased by NCadΔC expression, its transcriptional activity is reduced compared with vector transduced cells.
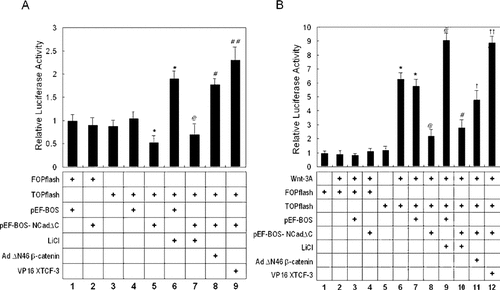
Regulation of β-catenin-TCF promoter activity by NCadΔC. ST cells transfected with pEF-BOS-NCadΔC-or pEF-BOS plasmid were assayed for Wnt signal-mediated β-catenin-TCF-regulated transcription using luciferase reporter plasmids containing intact (TOPflash) or mutated (FOPflash) multimeric TCF-binding sites. (A) Effects of NCadΔC on TOPflash activity in the basal condition. Transfection of pEF-BOS-NCadΔC significantly inhibited TOPflash activity by 42% (lane 5), which was recovered by Ad ΔN46 β-catenin, constitutively active β-catenin mutant (lane 8), or transfection of pCS2-VP16ΔβXTCF-3, a constitutively active Xenopus TCF-3 (lane 9). *p < 0.05 vs. lane 4;@p < 0.05 vs. lane 6;#p < 0.05 and##p < 0.01 vs. lane 5. (B) Effects of NCadΔC on Wnt-3A-stimulated TOPflash activity. Treatment of Wnt-3A significantly increased TOPflash activity (lane 6), which was suppressed by transfection of pEF-BOS-NCadΔC by 62% (compare lanes 7 and 8) and recovered by Ad ΔN46 β-catenin (lane 11) or pCS2-VP16ΔβXTCF-3 (lane 12). Luciferase activity values were normalized against the β-galactosidase or luciferase activities from the co-transfected pCMV-β-gal or pRL-TK Renila luciferase plasmid, respectively. All values are expressed relative to basal promoter activity as fold induction. *p < 0.01 vs. lane 5;@p < 0.05 vs. lane 7;#p < 0.05 vs. lane 9;†p < 0.05 and††p < 0.01 vs. lane 8. Values are presented as means ± SD, and results are representative of three experiments each performed in triplicate.
To further clarify the inhibition of Wnt/β-catenin signaling by NCadΔC, we studies TOPflash activity in the presence of Wnt-3A, one of the well-characterized activators of the canonical Wnt pathway. As shown in Fig. 6B, the basal TOPflash reporter activity in ST2 cells (lane 5) was 6-fold or more increased by treatment with Wnt-3A CM as expected (lane 6). However, transfection of pEF-BOS-NCadΔC in this setting significantly inhibited the expression of luciferase activity by 62%, whereas transfection of pEF-BOS empty vector did not affect the activity (compare lanes 8 and 7). The same experiments performed using the FOPflash luciferase reporter gene did not reveal any changes regardless of treatment with Wnt-3A or the expression of NCadΔC (lanes 1–4). The reduced TOPflash reporter activity by NCadΔC expression was reversed by transduction of the Ad ΔN46 β-catenin (lane 11) or transfection of pCS2-VP16ΔβXTCF-3 (lane 12). Again, treatment with lithium did not significantly increase the luciferase activity (compare lanes 8 and 10), whereas in the absence of NCadΔC, lithium enhanced the TOPflash activity in response to Wnt-3A (compare lanes 7 and 9). The rescue of TOPflash activity by Ad ΔN46 β-catenin or pCS2-VP16ΔβXTCF-3 but not by lithium, an inhibitor of GSK-3β, also favors the notion that expression of NCadΔC expression inhibits Wnt/β-catenin transcriptional activity by sequestering β-catenin at the cytosol/membrane fraction.
NCadΔC inhibits RANKL expression through β-catenin signaling
The inhibition of osteoclastogenesis by NCadΔC expression in ST2 cells and the suppression of β-catenin driven transactivation at the same time prompted us to study the possibility that NCadΔC affects the OPG/RANKL balance, the key regulators in osteoclastogenesis, through altering the Wnt/β-catenin signal. In support of this notion, quantitative real-time RT-PCR analysis showed that the basal and 1,25(OH)2 vitamin D3-stimulated RANKL expression in ST2 cells was suppressed by 53% and 47%, respectively, by expression of NCadΔC (Fig. 7A, left). However, the level of OPG expression was unchanged by expression of NCadΔC in these cells both in basal conditions and after treatment with 1,25(OH)2 vitamin D3, which resulted in suppression of OPG expression (Fig. 7A, right).
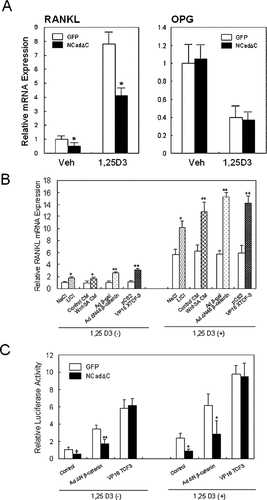
Regulation of RANKL expression by Wnt signaling. (A) Inhibition of RANKL expression by NCadΔC. ST2 cells transduced with pMSCV-GFP (GFP) or pMSCV-NCadΔC-GFP (NCadΔC) virus were cultured in αMEM containing 10% FBS in the absence or presence of 10 nM of 1,25(OH)2 vitamin D3 and harvested. Expression of RANKL and OPG was analyzed by real-time quantitative RT-PCR analysis. Expression of RANKL in ST2 cells expressing NCadΔC was 53% or 47% lower in the absence or presence of 1,25(OH)2 vitamin D3, respectively, than that of ST2 cells expressing empty vector, whereas the level of OPG expression was unchanged. The expression levels were normalized on the basis of GAPDH expression. Results (means ± SD) are representative of two independent experiments, both performed in triplicate. Veh, vehicle. 1,25D3, 1,25(OH)2 vitamin D3. *p < 0.05 vs. GFP. (B) Stimulation of RANKL expression by Wnt activators. ST2 cells were cultured in αMEM with 10% FBS in the absence or presence of 10 nM of 1,25(OH)2 vitamin D3 and harvested after treatment with 40 mM lithium chloride, 40 mM sodium chloride, Wnt-3A conditioned medium (CM), or control CM, or transduction with Ad ΔN46 β-catenin or Ad β-gal, or transfection with pCS2 or pCS2-VP16ΔβXTCF-3 plasmids 48 h before harvest. Real-time quantitative RT-PCR analysis was performed. Different Wnt signaling activators significantly stimulated the mRNA expression of RANKL compared with the respective controls. Results (means ± SD) are representative of two independent experiments, both performed in triplicate. *p < 0.05 and **p < 0.01 vs. respective controls. (C) Regulation of RANKL promoter activity by Wnt signaling. ST2 cells were cultured in αMEM with 10% FBS in the absence or presence of 10 nM of 1,25(OH)2 vitamin D3 and transfected with pRANKL-Luc reporter plasmid and transduced with adenovirus or co-transfected with expression plasmids, as indicated. Forty-eight hours after transfection, the cells were harvested, and luciferase activity was measured as in Fig. 6. Transduction of Ad ΔN46 β-catenin or transfection of pCS2-VP16ΔβXTCF-3 significantly increased the transcriptional activity of RANKL promoter. Transfection of NCadΔC significantly reduced the reporter activity induced by Ad ΔN46 β-catenin but not the activity induced by pCS2-VP16ΔβXTCF-3. All values are expressed relative to basal promoter activity as fold induction. Values are presented as means ± SD, and results are representative of three experiments each performed in triplicate. *p < 0.05 and **p < 0.01 vs. respective controls.
We then asked whether the Wnt/β-catenin signal regulates the expression of RANKL by using different Wnt signaling activators. As shown in Fig. 7B, the treatment with lithium or Wnt-3A CM, transduction of Ad ΔN46 β-catenin, or transfection of pCS2-VP16ΔβXTCF-3 significantly stimulated the mRNA expression of RANKL compared with the respective controls. The induction of RANKL mRNA expression was also observed in the presence of 1,25(OH)2 vitamin D3 (Fig. 7B). To further confirm the role of Wnt-β/catenin signaling in the regulation of RANKL expression, we analyzed luciferase activity in cells transfected with pRANKL-Luc, which encompassed the −1005/+13 segment of the mouse RANKL promoter, harboring most of the regulatory elements.27 Consistent with RT-PCR results, the transduction of Ad ΔN46 β-catenin or transfection of pCS2-VP16ΔβXTCF-3 significantly increased the transcriptional activity of RANKL promoter, both in the absence or presence of 1,25(OH)2 vitamin D3 (Fig. 7C). Transfection of NCadΔC, however, significantly reduced the basal and induced reporter activity by Ad ΔN46 β-catenin. NCadΔC did not affect the reporter activity induced by pCS2-VP16ΔβXTCF-3, in which the β-catenin-binding domain of TCF has been replaced by activation domain of the viral transcription factor VP-16. Taken together, these results suggest that Wnt/β-catenin signaling regulates RANKL expression at the transcriptional level, and dominant negative N-cadherin reduces its expression by binding with and keeping β-catenin from interacting with TCF.
DISCUSSION
Osteoclastogenesis is a complicated process regulated by finely orchestrated interactions between osteoclast precursors and osteoblasts/stromal cells in the bone marrow environment. A co-culture system of mouse osteoblast/stromal cells and hematopoietic cells has established the concept that cell-to-cell contact between cells of the osteoblast lineage and hematopoietic cells is necessary for inducing differentiation of osteoclasts. Whereas numerous studies have been performed on the roles of growth factors and cytokines, the role of the cell adhesion molecule cadherin has not received a great deal of attention in this field. In this study, we showed that both mouse BMSCs and mononuclear osteoclast precursors express N-cadherin and that dominant negative N-cadherin expression in a stromal cell line, ST2, resulted in reduction in its ability to support osteoclastogenesis.
Of the bone marrow cells, only a subpopulation of mononuclear cells seems to express N-cadherin. This was shown by the barely detectable expression of N-cadherin in BMMs by immunoblotting, whereas the more sensitive RT-PCR analysis produced a more clear signal. However, strong expression of N-cadherin was observed in RAW 264.7 cells, a well-characterized macrophage cell line that can differentiate into osteoclasts, indicating that the subsets of BMMs that express N-cadherins correspond to the cells that can differentiate into osteoclasts. These findings initially suggested that N-cadherin-mediated cellular adhesion between ST2 cells and BMMs may contribute to the formation of osteoclasts in a co-culture system. However, we found that the expression of dominant negative cadherins in BMSC-derived ST2 cells did not significantly affect cell adhesion but strongly inhibited the formation of osteoclasts. Moreover, our finding that the addition of neutralizing antibody against N-cadherin did not affect the osteoclastogenic potential of ST2 cells suggest that alterations in cell adhesion per se may not be the critical element in the regulation of osteoclastogenesis in the co-culture system. Alternatively, the relatively low level of N-cadherin expression in BMMs may not be sufficient for mediating hetrotypic interaction with ST2 cells and the subsequent regulation of osteoclastogenesis.
Several lines of evidence show that the expression of truncated cadherin disrupts not only cell-to-cell adhesion but also cadherin-mediated cellular signaling and function. Cadherin-mediated cellular signaling involves Wnt signaling, interaction with receptor tyrosine kinases, and signaling through Rho GTPases.35 Of these, signaling through Wnt pathway is of particular interest in the context of this study, because previous studies have suggested that the cytoplasmic domain of NCadΔC, where catenin binding is thought to occur, is responsible for dominant negative phenotypes.36 β-catenin, in addition to its role as an anchorage link between N-cadherin and the actin cytoskeleton, is also involved in the canonical Wnt signal transduction pathways.37 In this study, the expression of NCadΔC resulted in increased cytoplasmic levels of β-catenin possibly by complexing with and protecting β-catenin from degradation by the ubiquitin-proteasome system. Interestingly, however, the elevated level of β-catenin in NCadΔC-expressing cells did not enhance β-catenin/TCF transcriptional activity assessed by TOPflash reporter assay. The most likely explanation for this effect is that NCadΔC blocked β-catenin-mediated transactivation by sequestering β-catenin away from the nucleus. Indeed, we have found that the nuclear level of β-catenin in NCadΔC-expressing ST2 cells were lower than that of ST2 cells with empty vector. Another possibility is that expression of NCadΔC might compete with other transcription factors that interact with β-catenin. Support for this hypothesis comes from the studies of Sadot et al.,38 who observed that the expression of membrane-bound or free cadherin tail in CHO cells suppressed β-catenin-LEF-1 complex formation and inhibited its transactivation potential.
As a potential downstream target of NCadΔC expression and reduced activation of the β-catenin/TCF system, we showed that the expression of RANKL, a key regulator of osteoclastogenesis, is decreased. This is in agreement with our previous study in which calvaria cells from transgenic mice overexpressing NCadΔC showed reduced expression of RANKL compared with wildtype cells.19 In this in vivo study, however, the number of osteoclasts in transgenic mice was not significantly reduced compared with wildtype animals, possibly a result of the high variability inherent to the estimate. In contrast to the significant change in RANKL expression, the mRNA level of OPG was unchanged by the expression of NCadΔC. However, during the preparation of this manuscript, Glass et al.39 reported that β-catenin, together with TCF proteins, regulates OPG expression from osteoblasts using Tcf−/− mouse model. The reason for this apparent discrepancy is not clear at this time, but it can be speculated that the expression of NCadΔC can modulate the expression of OPG by other yet identified mechanisms in addition to the β-catenin/TCF system.
The regulation of RANKL expression by Wnt/β-catenin signaling is further supported by our findings that treatment of ST2 cells with Wnt-3A CM, lithium, constitutively active β-catenin adenovirus, or constitutively active XTCF-3 construct increased the expression of RANKL. This result is analogous to the recent findings of Sen et al.,40 who observed that blocking antibody against Frizzled 5, a receptor of Wnt-5A, resulted in the reduced expression of RANKL in synoviocytes. Increased abundance of RANKL mRNA by Wnt signaling seems to occur at the transcriptional level, as we have shown the transcriptional activity of murine RANKL-Luc by a series of Wnt signaling activation. Notably, NCadΔC has suppressed the reporter activity induced by constitutively active β-catenin (Ad ΔN46 β-catenin), whereas it failed to inhibit VP16ΔβXTCF-3-mediated induction of luciferase activity. Given that VP16ΔβXTCF-3 lacks the N-terminal β-catenin binding domain, these results further support that sequestering β-catenin is the main mechanism of NCadΔC-mediated repression of RANKL promoter activity. However, whether RANKL is a direct target of the β-catenin/TCF pathway is not clear. We failed to identify a consensus TCF-binding motif within 1 kb of promoter sequence we used in our study. Whether β-catenin directly binds and regulates RANKL promoter activity will require further work.
In summary, the results presented herein show that both mouse BMSCs and mononuclear osteoclast precursors express N-cadherin and that the expression of extracellular domain-deleted N-cadherin in stromal cell line ST2 cells inhibited its ability to support osteoclastogenesis. This inhibition seems to be mediated by the disruption of β-catenin/TCF transcriptional activity and a subsequent reduction in RANKL expression rather than altered cell-to-cell adhesion. Together with our previous report, which found that N-cadherin-mediated cell-cell interaction is critical in osteoblast maturation and function,17, 19 these results indicate that cadherin-mediated signaling is involved in both arms of bone remodeling: bone formation and resorption.
Acknowledgements
We thank Drs Yousef Abu-Amer (Washington University School of Medicine, St Louis, MO), Kwi-Ok Oh (Oscotec), and Ja-Hyun Baik (Yonsei University, Seoul, Korea) for providing the ST2, MC3T3-G2PA6 and Raw 264.7, and 293T cells, respectively; Dr Jeffery Gordon (Washington University, St Louis, MO) for the NCadΔC construct; Dr Shigekazu Nagata for the pEF-BOS plasmid; Neil A. Clipstone (Northwestern University, Chicago, IL) for the MSCV-IRES-GFP retroviral vector; Dr Bert Vogelstein (The Johns Hopkins University, Baltimore, MD) for human β-catenin plasmid; Dr Alin Vonica (The Rockefeller University, New York, NY) for pCS2-VP16ΔβXTCF; and Dr Yong Jin Lee (Asan Medical Center, Seoul, Korea) for taking confocal microscopy pictures. This work was supported by Korean Science and Engineering Foundation Grant R01–2000–000–00092–0.




