Regeneration of Defects in Articular Cartilage in Rat Knee Joints by CCN2 (Connective Tissue Growth Factor)†
The authors have no conflict of interest
Abstract
CTGF/CCN2, a hypertrophic chondrocyte-specific gene product, possessed the ability to repair damaged articular cartilage in two animal models, which were experimental osteoarthritis and full-thickness defects of articular cartilage. These findings suggest that CTGF/CCN2 may be useful in regeneration of articular cartilage.
Introduction: Connective tissue growth factor (CTGF)/CCN2 is a unique growth factor that stimulates the proliferation and differentiation, but not hypertrophy, of articular chondrocytes in vitro. The objective of this study was to investigate the therapeutic use of CTGF/CCN2.
Materials and Methods: The effects of recombinant CTGF/CCN2 (rCTGF/CCN2) on repair of damaged cartilage were evaluated by using both the monoiodoacetic acid (MIA)-induced experimental rat osteoarthritis (OA) model and full-thickness defects of rat articular cartilage in vivo.
Results: In the MIA-induced OA model, quantitative real-time RT-PCR assays showed a significant increase in the level of CTGF/CCN2 mRNA, and immunohistochemical analysis and in situ hybridization revealed that the clustered chondrocytes, in which clustering indicates an attempt to repair the damaged cartilage, produced CTGF/CCN2. Therefore, CTGF/CCN2 was suspected to play critical roles in cartilage repair. In fact, a single injection of rCTGF/CCN2 incorporated in gelatin hydrogel (rCTGF/CCN2-hydrogel) into the joint cavity of MIA-induced OA model rats repaired their articular cartilage to the extent that it became histologically similar to normal articular cartilage. Next, to examine the effect of rCTGF/CCN2 on the repair of articular cartilage, we created defects (2 mm in diameter) on the surface of articular cartilage in situ and implanted rCTGF/CCN2-hydrogel or PBS-hydrogel therein with collagen sponge. In the group implanted with rCTGF/CCN2-hydrogel collagen, new cartilage filled the defect 4 weeks postoperatively. In contrast, only soft tissue repair occurred when the PBS-hydrogel collagen was implanted. Consistent with these in vivo effects, rCTGF/CCN2 enhanced type II collagen and aggrecan mRNA expression in mouse bone marrow-derived stromal cells and induced chondrogenesis in vitro.
Conclusion: These findings suggest the utility of CTGF/CCN2 in the regeneration of articular cartilage.
INTRODUCTION
ARTICULAR CARTILAGE IS a hyaline cartilage that covers the heads of bones and enables frictionless and pain-free movement of the joint throughout life.1 However, it is a well-known fact that the capacity of articular cartilage to repair itself is limited because articular chondrocytes are highly differentiated cells.1 Osteoarthritis (OA) is one of the most common noninflammatory diseases of cartilage.2 Its characteristic features are initial superficial fissuring, which extends into the depth of the tissue, clustering of chondrocytes, and loss of chondrocytes and proteoglycans.2, 3 Eventually, the tissue degenerates, thus exposing the underlying bone.2, 3 Present treatments for the disease provide only transient relief from pain and do not repair the damaged articular cartilage. Therefore, novel regenerative medical strategies for the restoration of damaged articular cartilage are desired for this disease. Recently, it was reported that bone marrow stromal cell transplantation was clinically useful because these cells could proliferate in culture without losing their ability to form bone or cartilage.4, 5 However, although recent biochemical studies have shown that bone marrow stromal cells may be used as chondrocyte progenitor cells for cartilage healing, more information is needed regarding the variety of growth factors produced by differentiated chondrocytes and how these factors regulate chondrogenic differentiation to move such research toward clinical application.
Previously, we had cloned a gene fragment predominantly expressed in hypertrophic chondrocytes6, 7 from the human chondrosarcoma-derived cell line HCS-2/88-11 by differential display-PCR. Its gene, named hcs24 (hypertrophic chondrocyte-specific gene 24) was the human homolog of connective tissue growth factor (CTGF). CTGF/Hcs24 is a cysteine-rich, extracellular matrix (ECM)-associated, heparin-binding protein, and its structure is characterized by the presence of an N-terminal secretory signal, followed by four modular domains. Six distinct proteins with similar structure have now been described, and their genes comprise a family that is known as the CCN family (i.e., ctgf/fisp12/hcs24/ccn2, cyr61/cef10/ccn1, nov/ccn3, elm-1/wisp-1/ccn4, rcop-1/wisp-2/ctgf-3/ccn5, and wisp-3/ccn6).12-18 A consensus was recently reached to propose a unifying nomenclature for the CCN family, numbering them CCN1-CCN6 in the order in which they were described in the literature,18 but the name “CTGF” has been widely used, thus we use CTGF/CCN2 as an abbreviated form of CTGF/Fisp12/Hcs24/CCN2 in this article.
Although their exact functions are still unknown, these proteins are involved in a number of biological processes such as embryonic development, tissue repair, and tumor suppression.12-17 Previously, we reported that recombinant CTGF/CCN2 (rCTGF/CCN2) promoted the proliferation and differentiation of chondrocytes and osteoblasts in vitro19, 20 and stimulated the proliferation, migration, and tube formation of vascular endothelial cells in vitro and angiogenesis in vivo.21, 22 These findings suggest that CTGF/CCN2 produced by hypertrophic chondrocytes promotes endochondral ossification by enhancing the proliferation and maturation of chondrocytes in growth cartilage and of endothelial cells in bone by acting as a paracrine growth and differentiation factor.19, 21, 22 Interestingly, we recently found that rCTGF/CCN2 promoted not only the proliferation and differentiation of, but also matrix calcification by growth cartilage cells, whereas it did not induce terminal hypertrophy or calcification of articular cartilage cells.23 Therefore, this growth factor guides growth cartilage cells toward endochondral ossification and prompts articular cartilage cells to express and maintain their specific phenotypes, thus suggesting that the therapeutic application of rCTGF/CCN2 may provide a breakthrough in the treatment of joint diseases such as OA.
In this study, we used two strategies to investigate the reparative effect of rCTGF/CCN2 on articular cartilage in vivo. First, we produced monoiodoacetic acid (MIA)-induced experimental OA model in rats and investigated whether or not the injection of rCTGF/CCN2 into their joint cavities would stimulate articular cartilage repair in this model. Second, to clarify the direct effect of rCTGF/CCN2 on the repair of articular cartilage, we investigated whether full-thickness defects made in rat articular cartilage could be re-filled with hyaline cartilage-like tissue after the implantation of rCTGF/CCN2.
MATERIALS AND METHODS
Materials
Recombinant CTGF/CCN2 (rCTGF/CCN2) was purified as previously described.24, 25 Anti-CTGF/CCN2 serum was raised in rabbits by immunization with synthetic peptide of CTGF/CCN2 composed of 20 amino acids (240-259: RPCEADLEENIKKGKKCIRT).26 The specificity of the antiserum was confirmed using Western blot analysis, which showed that the synthetic peptide of CTGF/CCN2, but not of the corresponding region of Cyr61/CCN1, another member of CCN family, inhibited the binding of the antiserum to rCTGF/CCN2.22 Gelatin with a molecular weight of 99,000 and an isoelectrical point (pI) of 5.0, prepared by alkalinization followed by thermal denaturation of bovine bone, was kindly supplied by Nitta Gelatin (Osaka, Japan). It was named “acidic gelatin” based on its electrical mobility property. rCTGF/CCN2 with a molecular weight of 38,000 and a pI of 7.77 was also used in this study. The collagen sponge was purchased from Koken Co. (Tokyo, Japan).
Preparation of rCTGF/CCN2-incorporated gelatin hydrogels
Gelatin hydrogel microspheres were prepared by glutaraldehyde cross-linking of acidic gelatin as reported previously.27, 28 To incorporate rCTGF/CCN2 into these microspheres, we dropped 10 μl of PBS containing 0.5, 1.0, or 1.5 μg of rCTGF/CCN2 onto 1 mg of freeze-dried gelatin hydrogel microspheres and left them at 25°C for 1 h. Similarly, rCTGF/CCN2-free, control gelatin microspheres were prepared by using 10 μl of PBS without rCTGF/CCN2.
Animals and tissue preparation
A total of 72 male Wistar rats and 10 male ICR mice were used in these experiments. The Wistar rats were anesthetized and perfused with 10% formalin. The whole knee joints were dissected and further fixed in 10% formalin overnight at 4°C. Next, the tissues were decalcified with 10% EDTA for 2 weeks and embedded in paraffin. Sagittal sections were cut at a thickness of 5 μm and mounted on silane-coated slides. The mice were killed to obtain bone marrow stromal cells. The Animal Committee of Okayama University Dental School approved all of the procedures.
Animal experiments
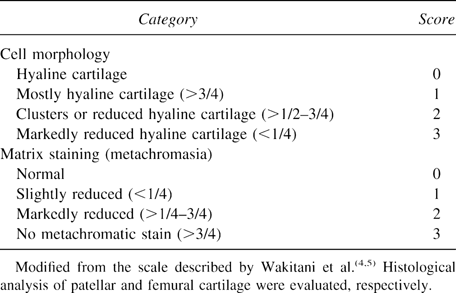
For induction of experimental OA, MIA (Sigma, St Louis, MO, USA) was prepared in PBS at a concentration of 60 mg/ml.29, 30 On day 0, the right knee joints of 35 Wistar rats (8 weeks old) were injected with MIA at a dose of 6 mg once, intraarticularly through the patellar ligament with MIA. The left knee joint (control) was injected with PBS. Before injection, the solution was filtered through a 0.22-μm filter to remove bacteria. Fourteen days after the injection with MIA, the rats were anesthetized and injected with rCTGF/CCN2- or PBS-incorporated gelatin hydrogel. Twenty-one days after the injection with MIA, the animals were killed and examined histologically. The sections were stained with toluidine blue. The sections from each animal were examined and scored under a histological scale, which is a modification of that described by Wakitani et al.4 The scale is composed of cell morphology and matrix staining and assigned a score ranging from 0 to 6 points (Table 1). The patellar and femoral cartilages were scored, respectively, and the sample score was added for each score. The cell morphology was graded from 0 (for tissues equivalent to the normal cartilage) to 3 points (for those lacking cartilaginous tissue). Matrix staining, or the degree of metachromatic staining with toluidine blue, was graded from 0 (for tissues comparable with the normal cartilage tissue) to 3 points (for those without metachromatic staining).
For the other experiment, 27 Wistar rats weighing ∼380 g were anesthetized with an intraperitoneal injection of sodium pentobarbital given at standard dose of 2.7 mg/kg. A 2-cm-long scalp skin cut was made at the midline of the parapatellar skin. The soft tissue was dissected to expose the capsule, the capsule was incised, and the patella was dislocated laterally to expose the patella groove of the femur. A defect, 2 mm in diameter and penetrating the subchondral bone plate, was prepared on the patellar groove in femur with a microdrill. One microgram of rCTGF/CCN2 that had been incorporated into gelatin hydrogels with collagen sponge (Kouken) was lyophilized and implanted in to the defect. The patella was repositioned, and the medial aspect of the capsule was closed with a nylon suture. The animals were allowed to feed freely in their cages immediately after the operation. Up to 4 weeks postoperatively, animals were processed, and the sites of defects were histologically examined by light microscopy.
Immunohistochemistry
Serial formalin-fixed paraffin sections were deparaffinized with xylene, rehydrated, and washed with PBS. The sections were incubated with 10% BSA (Sigma) for 30 minutes at room temperature to eliminate nonspecific binding, and subsequently overnight at 4°C with anti-CTGF/CCN2 serum diluted 1:100 PBS. After the sections had been washed with PBS, incubation was performed with a biotinylated secondary antibody and avidin peroxidase complex for 30 minutes at room temperature. Color was developed using 3,3′-diaminobenzidine tetrachloride (DAB; Sigma). Finally, the sections were counterstained with methyl green.
In situ hybridization
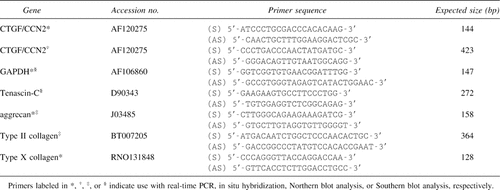
In situ hybridization was carried out as described previously.6 ctgf/ccn2 probe was labeled with digoxigenin, using a DIG RNA Labeling Kit (Roche, Mannheim, Germany) according to the manufacturer's instructions. A 423-bp fragment of a ctgf/ccn2 cDNA cloned in pGEM-T-easy (Promega) was linearized with NcoI and transcribed by SP6 RNA polymerase to generate an antisense riboprobe or linearized with SalI and transcribed by T7 RNA polymerase to generate a sense riboprobe. Hybridization was performed at 42°C overnight in a humidified chamber. Detection of hybridized probes was done with anti-digoxigenin antibody conjugated with 5[6]-carboxy-fluorescein-N-hydroxy-succinimide ester (Roche). The control hybridization incubated with the sense probe showed no detectable signals.
RNA isolation and real-time RT-PCR analysis
Quantitative real-time PCR was performed by using a LightCycler (Roche). The sequences of primers for ctgf/ccn2, gapdh, tenascin-C, aggrecan, type II collagen [α1(II)] and type X collagen [α1(X)] genes are given in Table 2. Total RNA was directly extracted from articular cartilage tissue of the femur and tibias of rats (n = 10) 3 weeks after the MIA injection (7 days after administration of rCTGF/CCN2) by use of ISOGEN-LS (Nippon Gene, Tokyo, Japan). Total RNA was reverse-transcribed to cDNA for 60 minutes at 37°C using oligo dT16 and avian myeloblastosis virus (AMV)-derived RT (Invitrogen, La Jolla, CA, USA). Amplification reactions were performed with a LightCycler-FastStart DNA Master SYBR Green I kit (Roche). Two microliters of diluted RT samples were used for quantitative two-step PCR (a 10-minute step at 95°C, followed by 45 cycles of 15 s at 95°C, 10 s at 63°C, and 4 s at 72°C) in the presence of 500 nM specific forward and reverse primers and 2 mM MgCl2. Each sample was analyzed in duplicate.
Southern blot analysis of RT-PCR products
RT-PCR analysis was performed as described previously.19 The primer sequences for PCR are displayed in Table 2. The amplification conditions were as follows: 95°C (1 minute)-60°C (2 minutes) for 20 cycles for tenascin-C and gapdh. The PCR products (10 μl) were electrophoresed in an agarose gel, and Southern blotting of the PCR products was performed as previously described.31 Membrane-transferred DNA was hybridized with random-primed DNA probes labeled with α[32P]-dCTP (Random Primer DNA Labeling Kit; Takara Shuzo Co. Tokyo, Japan). Hybridized membranes were washed and exposed to X-ray films at −70°C.
Western blot analysis
Proteins separated by SDS-PAGE were transferred to a polyvinylidene diflouride (PVDF) membrane by using a semi-dry blotting apparatus (AE-6677; ATTO Co. Tokyo, Japan). Western blot analysis was carried out essentially as described previously.11, 21, 23
Cell cultures
Mouse bone marrow cultures were prepared as described earlier32 with minor modifications. In brief, 10 ICR mice (6-week-old) were killed. Femora were aseptically removed and dissected free of adherent tissue. After the ends of the bones had been cut-off, the marrow was flushed out with serum-free αMEM. The cells were pelleted, resuspended, and counted with a hemocytometer to determine the total number of marrow cells obtained from femurs. Appropriate cell suspensions were made for subsequent cell plating.
Northern blot analysis
By use of ISOGEN reagent (Nippon Gene), total RNA was prepared from marrow stromal cells (MSCs) stimulated with 30 or 50 ng/ml rCTGF/CCN2. Then, 10 μg of total RNA was subjected to electrophoresis on a 1% formaldehyde-agarose gel and transferred onto a Hybond-N filter (Amersham Pharmacia Biotech). Northern blot analysis was performed as described previously.17, 23 Specific PCR products were used as probes (Table 2). Each probe was radiolabeled with α[32P]dCTP as described for Southern blot analysis.
Statistical analysis
Experimental results were represented as the mean ± SD. Unless otherwise specified, all experiments were repeated at least twice, and similar results were obtained. Statistical analysis was performed by using Student's t-test. For the statistical analysis of the histological scoring, mean values of the scores obtained from three independent investigators were computed. Statistical analysis was performed by using Mann-Whitney test.
RESULTS
Interaction of rCTGF/CCN2 with biodegradable gelatin hydrogel
For adsorption experiments, 1.0 μg rCTGF/CCN2 was first adsorbed into freeze-dried acidic gelatin hydrogels by dropping the rCTGF/CCN2 solution (10 μl) onto the dried hydrogels, which were then left at 25°C for 1 h for rCTGF/CCN2 impregnation. The hydrogels with the adsorbed rCTGF/CCN2 were placed in 50 μl of PBS and incubated at 37°C for 24 h. The amount of rCTGF/CCN2 adsorbed or nonadsorbed from each sample solution was determined by Western blotting of supernatant and pellet with an anti-CTGF/CCN2 serum, and the densitometrical values of the signals were obtained. As shown in Figs. 1A and 1B, ∼70% of the rCTGF/CCN2 applied was found to be in the hydrogel 24 h after immersion in the PBS (lane 3, column 3). On the other hand, the amount of rCTGF/CCN2 in the supernatant (desorbed from hydrogel) was <10% of the total rCTGF/CCN2 (lane 2, column 2). These findings indicate that rCTGF/CCN2 clearly interacted with the gelatin hydrogel. Interestingly, in addition to the expected 38-kDa protein, a larger molecule, with an apparent molecular weight of 60 kDa, was detected in the rCTGF/CCN2-adsorbed hydrogel. After immersion, CTGF/CCN2 molecules might have formed a homodimer or heterocomplex with the gelatin hydrogel, thus giving rise to the 60 kDa-specific signals. In addition, another specific signal, with an apparent molecular weight of ∼20 kDa, appeared. This 20-kDa molecule may be a cleaved form of the 38-kDa CTGF/CCN2 molecule.

In vitro interaction of rCTGF/CCN2 with biodegradable gelatin hydrogel. (A) For investigation of the in vitro profile of rCTGF/CCN2 adsorption by the gelatin hydrogels, gelatin hydrogel was soaked in the 1.0 μg rCTGF/CCN2 solution at 25°C. After incubation for 1 h, the hydrogels with the adsorbed rCTGF/CCN2 were placed in 50 μl of PBS at 37°C for 24 h. Then, the concentration of rCTGF/CCN2 desorbed from the gelatin hydrogel was determined by Western blot analysis. Lane 1 contained 1.0 μg of rCTGF/CCN2; lane 2, the supernatant of the gelatin hydrogel and rCTGF/CCN2 mixture 24 h after immersion; and lane 3, pelleted gelatin hydrogel and rCTGF/CCN2 mixture. Positions of molecular weight markers are shown at the left in kilodaltons. Solid arrowhead indicates the band corresponding to CTGF/CCN2 protein. Upper and lower open arrowheads indicate the bands corresponding to 60 and 20 kDa CTGF/CCN2-related signals, respectively. (B) The bands of the 38-kDa CTGF/CCN2 in A were quantified using Quantity One (pdi, Grand Island, NY, USA), and the ordinate shows the percentage of rCTGF/CCN2 adsorbed into gelatin hydrogel relative to that of initial solution (initial solution = 100%). The data are presented as the mean values and SD of two separate Western blot analyses by different experiments.
Induction of OA by MIA and expression of CTGF/CCN2 in OA cartilage
MIA was injected intraarticularly in the right knee joint of rats at a concentration of 6.0 mg/100 μl. Fourteen days after the injection, large chondrophytes were present in the periphery of the femoral condyles, as observed macroscopically (Fig. 2A). By histological analysis, cell death was observed in the central part of the femorotibial joint, clusters of chondrocytes had formed at the joint margins (Fig. 2B, a and c), and evidence of chondrophyte formation was found at the margins of the femoropatellar joint (Fig. 2B, a and b). These data show that this strategy can easily and quickly produce OA-like lesions and functional impairment in rats similar to those observed in the human disease. First, using this model, we performed quantitative real-time RT-PCR by using specific primer sets for ctgf/ccn2 and gapdh, to clarify the roles of CTGF/CCN2 in joint maintenance and recovery. The expression of ctgf/ccn2 was at a very low level in normal articular cartilage. On the other hand, the mRNA levels of ctgf/ccn2 in OA-induced cartilage were significantly higher than those in the normal cartilage (3.5 ± 0.07-fold; Fig. 2C).
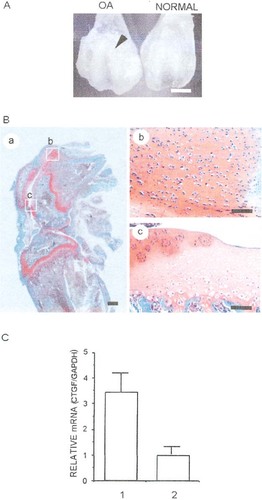
Induction of OA-like lesion by MIA and expression of ctgf/ccn2 in normal or OA-like articular cartilage. (A) Macroscopic features of femoral groove injected or not with 6 mg MIA. Fourteen days after intraarticular injection with the MIA, chondrophytosis was apparent in the femoral groove (arrowhead). (B) Histological sagittal section of rat knee joint 14 days after intraarticular injection with MIA. These sections were stained with safranin-O fast green. (Ba) A low-power magnification view of the rat OA joint. The areas surrounded by the boxes are enlarged in Bb and Bc. (Bb) Chondrophytes seen around the femoropatellar joint. (Bc) Evidence of loss of chondrocytes in weight-bearing areas and cluster formation at the joint margins is obvious. (C) Real-time RT-PCR analysis of ctgf/ccn2 and gapdh mRNAs in normal and OA cartilage isolated from rat knee joints. Column 1 represents results obtained with OA articular cartilage; and column 2 with normal articular cartilage. Scale bars = 3 mm (A), 1 mm (Ba), and 100 μm (Bb and Bc).
Expression and localization of CTGF/CCN2 in clusters of chondrocytes formed in the OA lesion
To dissect the role of CTGF/CCN2 in joint disorders in vivo, we investigated the expression and localization of CTGF/CCN2 in the MIA-induced OA model by using immunohistochemistry and in situ hybridization. Figures 3A and 3B show photomicrographs of sagittal sections of knee joint stained with toluidine blue. Chondrocyte clusters were seen in the femoropatellar cartilage, and areas strongly stained with metachromasia were observed around the formation (Fig. 3A). No similar abnormalities were seen in control sections (Fig. 3B). As shown in Figs. 3C and 3I, clusters of chondrocytes were distinctly stained with anti-CTGF/CCN2 antibodies and expressed ctgf/ccn2 mRNA (arrows). Control sections from untreated rats showed slight immunoreactivity and gene expression of ctgf/ccn2, respectively (Figs. 3D and 3J). Serial sections from the MIA-induced OA rats treated with preimmune serum and those hybridized with the sense probe showed no detectable immunoreactivity and expression signal (Figs. 3E, 3F, 3K, and 3L).

Immunohistochemical detection and in situ hybridization of CTGF/CCN2 in rat knee joint. Sections from a rat treated with MIA are shown in A, C, E, G, I, and K and those from a PBS-treated rat are shown in B, D, F, H, J, and L. A and B were stained with toluidine blue. After injection with MIA, reduced toluidine blue staining was observed in articular cartilage, and clusters were evident at the joint (A). C and D were stained with anti-CTGF/CCN2 serum. CTGF/CCN2 was detected in the clusters (C; arrows), but not in the PBS-treated cartilage (D). E and F were stained with preimmune serum as a negative control. G and H were phase-contrast microscopic views. I and J were hybridized with antisense probe for ctgf/ccn2 mRNA. The expression of ctgf/ccn2 mRNA was detected in the clusters (I; arrows), but not in the PBS-treated cartilage (J). The sections hybridized with sense probe (K and L) show no ctgf/ccn2 signal. Scale bar = 100 μm.
Effect of rCTGF/CCN2-hydrogel on MIA-induced experimental rat OA model
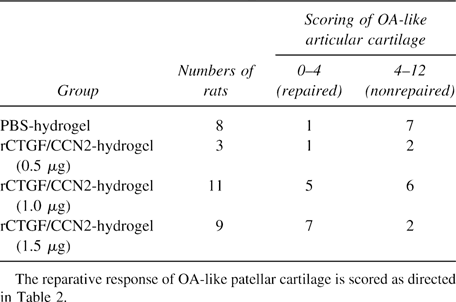
To investigate the effect of rCTGF/CCN2 as a therapeutic agent for OA, we injected rCTGF/CCN2-incorporated gelatin hydrogel into the knee joint of rats with MIA-induced OA at doses of 0.5, 1.0, or 1.5 μg/100 g of body weight. Then, we performed the histological study in the femoropatellar joint on day 7 after injection of rCTGF/CCN2-incorporated gelatin hydrogel. The resultant histological score of the OA-like articular cartilage is summarized in Table 3. Scores from 0 to 4 and those from 4 to 12 were defined as repaired tissue and OA-like tissue, respectively. As shown in Table 3 and Fig. 4B, injection of rCTGF/CCN2-incorporated gelatin hydrogel into the joint cavity improved the score at a dose of 1.5 μg (a total of nine animals, seven of which showed “repaired” response). A significant difference was observed between the PBS-hydrogel and 1.5 μg rCTGF/CCN2-hydrogel groups (p < 0.05). In the 0.5 μg rCTGF/CCN2-hydrogel-injected group, the histological scores indicated no difference compared with those of the PBS-hydrogel-injected group. Although 1.0 μg rCTGF/CCN2-hydrogel-injected groups resulted in “repaired” response in 5 of 11 animals, 6 animals showed no significant response in the OA-like articular cartilage. These results suggest that the effective dose of rCTGF/CCN2 is not <1.5 μg. Figure 4A shows histological sections of MIA-induced OA, which were obtained 7 days after the injection of gelatin hydrogel into the joint cavity. In the PBS-hydrogel-injected group, cell death was evidently observed in the articular cartilage, and metachromatic toluidine blue staining of the cartilage was greatly reduced (Fig. 4Ab). Although some histological differences were observed between PBS-hydrogel- and 1.0 μg rCTGF/CCN2-hydrogel-injected groups, such differences were not supported by statistical significance (Fig. 4Ac; Table 3). In contrast, articular cartilage was repaired after treatment with the hydrogel containing 1.5 μg of rCTGF/CCN2, and the histological findings were equivalent to those of normal cartilage (Fig. 4A, a and d). Interestingly, the synovial membrane appeared normal and contained no inflammatory cells either in PBS- or rCTGF/CCN2-hydrogel-treated samples. In fact, no significant signal for proliferating cell nuclear antigen (PCNA) was detected in synovial cells treated with PBS- or rCTGF/CCN2-hydrogel (data not shown).
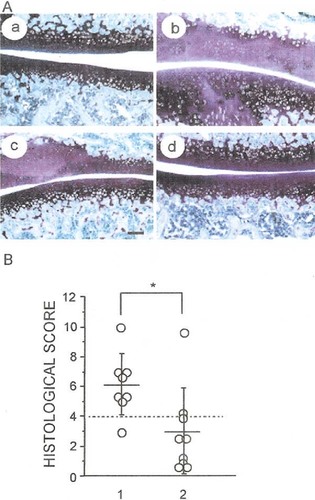
(A) Histological features of rat OA joints showing restoration of articular cartilage after intraarticular injection with rCTGF/CCN2-hydrogel. OA was induced by injection with 6 mg MIA into the right knee joints. Intraarticular injections with PBS- or rCTGF/CCN2-hydrogel were given on day 14, and the rats were killed 7 days later (21 days after the MIA injection). (a) Normal articular cartilage. (b) OA knee joint after the injection with PBS-hydrogel. (c and d) OA knee joint after injection with (c) 1.0 or (d) 1.5 μg rCTGF/CCN2-hydrogel. Toluidine blue staining was performed, and stimulation of chondrocyte-formation was observed after injection with rCTGF/CCN2-hydrogel. Scale bar = 250 μm. (B) Graph showing the histological scoring of both PBS-hydrogel (n = 8; lane 1)- and 1.5 μg rCTGF/CCN2-hydrogel (n = 9; lane 2)-injected groups. *Significant difference between columns 1 and 2 (p < 0.05).
Expression of tenascin-C, aggrecan, and type X collagen mRNA in normal, OA, and OA cartilage treated with rCTGF/CCN2
We injected 1.5 μg rCTGF/CCN2-hydrogel into the joint cavity pretreated with MIA. At day 7 after the injection, we examined the expression of tenascin-C and aggrecan, which are markers of articular cartilage cells, and type X collagen, which is a marker of hypertrophic chondrocytes, using PCR-Southern blot analysis and real-time-PCR analysis of RT products. The expression levels of tenascin-C and aggrecan mRNA were downregulated in the tissue from MIA-induced OA cartilage, whereas their expression levels were recovered to the normal level in the sample isolated from rCTGF/CCN2-injected cartilage (Figs. 5A and 5B). In contrast, the expression of type X collagen mRNA was upregulated in the MIA-induced OA cartilage, and the expression of this mRNA was recovered to the normal level in rCTGF/CCN2-treated cartilage (Fig. 5B).
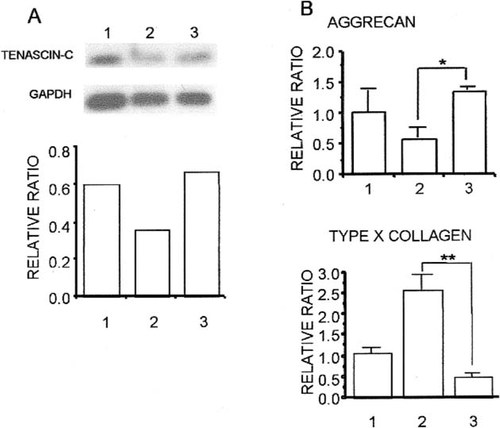
(A) Southern blotting analysis of RT-PCR products of tenascin-C and gapdh in the rat articular cartilage tissues. The RT-PCR products (10 μl) from normal articular cartilage (lane 1), MIA-induced OA cartilage (lane 2), and OA cartilage injected with 1.5 μg rCTGF/CCN2-hydrogel (lane 3) were electrophoresed on an agarose gel, blotted onto nylon membranes, and hybridized with probes specific for tenascin-C and gapdh. (Top) Autoradiograms. (Bottom) Expression of tenascin-C and gapdh was determined densitometrically, and the amount of tenascin-C mRNA was normalized to the amount of gapdh mRNA. The ordinate shows the relative ratio (tenascin-C/gapdh). (B) Real-time RT-PCR analysis of aggrecan, type X collagen, and gapdh mRNAs in the rat articular cartilage tissues. Column 1 represents results obtained with normal articular cartilage; column 2 with OA articular cartilage; and column 3 with OA articular cartilage treated with 1.5 μg rCTGF/CCN2-hydrogels. Significant differences between columns 2 and 3 (*p < 0.05, **p < 0.01).
Effect of rCTGF/CCN2-incorporated gelatin hydrogel on the repair of full thickness defects made in articular cartilage
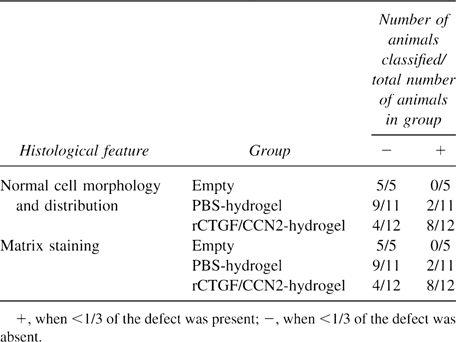
To investigate the direct effect of rCTGF/CCN2 on the repair of damaged articular cartilage, we implanted 1.0 μg of lyophilized rCTGF/CCN2-hydrogel with collagen sponge as the scaffold in the full thickness defects created on the surface of rat articular cartilage and performed histological analysis up to 4 weeks after the implantation. No discernible differences were observed between the PBS-hydrogel collagen and the empty-defect control groups. At 1 week after implantation, there seemed to be no difference in the defects between those that had been treated with rCTGF/CCN2-incorporated gelatin hydrogel and those treated with PBS-incorporated gelatin hydrogel (Figs. 6A and 6B). After 2 and 3 weeks, the reparative tissue treated with rCTGF/CCN2-hydrogel collagen that filled the deeper one-half of the defect showed slight staining with alcian blue. In the control, in which the defect was filled with PBS-hydrogel collagen or left empty, the defect was covered by fibrous soft tissue (Figs. 6C–6F). At 4 weeks after implantation, the histological appearance of the rCTGF/CCN2-hydrogel collagen graft indicated markedly improved repair of the defect compared with that obtained with the PBS-hydrogel collagen (Figs. 6G and 6H), and the cells therein resembled well-differentiated chondrocytes and were surrounded by an alcian blue-positive cartilage matrix. In contrast, there was no cartilage formation 4 weeks postoperatively, and the defect remained filled with fibrous soft tissue in the PBS-hydrogel collagen group. Table 4 summarizes the histological features of the defects at 4 weeks after implantation. Normal cell morphology and distribution were judged and classified either “+” (when more than one-third of the defects were chondrocyte-like cells) or “−” (when less than one-third or cartilaginous tissue was absent). Similarly, the matrix-staining finding was classified “+” (when more than one-third of the defects were alcian blue-positive) or “−” (when less than one-third or no staining). As shown in Table 4, the scores were improved with implantation of rCTGF/CCN2-hydrogel collagen (among 12 animals, 8 of which showed “+” findings in all categories). By contrast, the PBS-hydrogel collagen groups resulted in only two animals with all “+” scores, whereas nine animals showed no repair response to the defects.
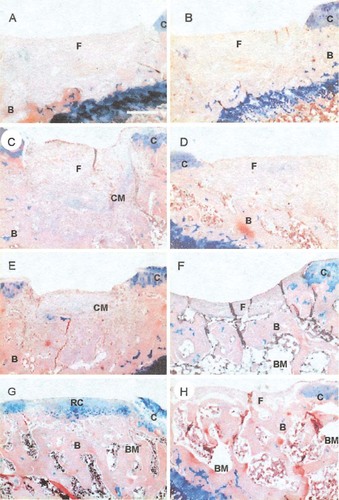
Microscopic appearance of the (A, C, E, and G) rCTGF/CCN2- and (B, D, F, and H) PBS-hydrogel collagen-treated tissue at (A and B) 1, (C and D) 2, (E and F) 3, and (G and H) 4 weeks after the operation. These sections were stained with alcian blue and hematoxylin-eosin. (A and B) Both PBS- and rCTGF/CCN2-treated articular defects were almost filled with soft tissue at 1 week after the operation. (C and E) The rCTGF/CCN2-treated tissue produced a cartilage matrix, whereas (D and F) the PBS-treated defect was still filled mostly with soft tissue at 2 and 3 weeks after the operation. At 4 weeks after the operation, the rCTGF/CCN2-treated defect was filled with cartilage, (G) the reparative tissue consisted of hyaline cartilage-like cartilage, and (H) the PBS-treated area of the defect remained filled with soft tissue. Scale bars = 250 μm. C, articular cartilage; CM, cartilage matrix; RC, regenerative articular cartilage; F, fibrous soft tissue; B, bone; BM, bone marrow.
Inducing effect of rCTGF/CCN2 on chondrogenic differentiation of MSCs
To investigate whether rCTGF/CCN2 could stimulate chondrogenic cell differentiation of MSCs or not, we cultured confluent MSCs for 14 days in αMEM with or without 30 or 50 ng/ml of rCTGF/CCN2. The cultures were thereafter processed for histochemical detection of cartilage matrix using toluidine blue staining (Fig. 7A). Some cartilage matrix was present in control cultures and in 30 ng/ml rCTGF/CCN2-treated cultures of MSCs, but a much larger amount of it was detected in 50 ng/ml rCTGF/CCN2-treated ones. In addition, to clarify the role of CTGF/CCN2 in the chondrocytic differentiation of MSCs, we used Northern blot analysis to investigate the effect of rCTGF/CCN2 on the mRNA expression of type II collagen and aggrecan in cultured MSCs (Fig. 7B). Chondrogenesis, as represented by the expression of type II collagen and aggrecan mRNAs, was clearly induced in MSCs treated with 50 ng/ml rCTGF/CCN2 (Fig. 7B).
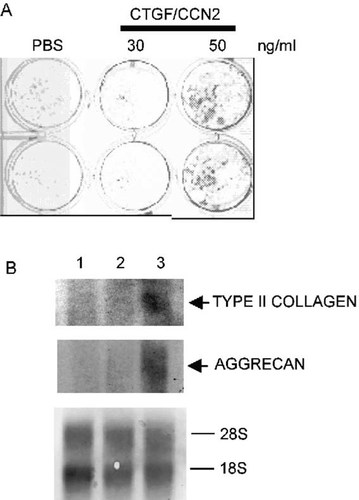
Induction of chondrogenic differentiation of MSCs by rCTGF/CCN2. (A) Duplicate cultures of MSCs were treated with 30 or 50 ng/ml of rCTGF/CCN2 for 14 days and were stained with toluidine blue. The PBS-treated or 30 ng/ml rCTGF/CCN2-treated MSCs revealed a faintly metachromatic matrix. The 50 ng/ml rCTGF/CCN2-treated MSCs secreted more metachromatic matrix than the PBS-treated or 30 ng/ml rCTGF/CCN2-treated MSCs. (B) Northern blot assays showing the levels of type II collagen and aggrecan mRNAs in MSCs treated with rCTGF/CCN2. Filters containing 10 μg of total RNA extracted from MSC cultures incubated in the absence (PBS-treated culture; lane 1) or presence of rCTGF/CCN2 (30 and 50 ng/ml; lanes 2 and 3, respectively) were hybridized with type II collagen and aggrecan probes, and ribosomal RNA was stained with methylene blue.
DISCUSSION
In this study, using two animal models, we have shown that CTGF/CCN2 possesses the ability to repair damaged articular cartilage, such as OA lesions. In one of the animal experiments, we investigated whether or not local administration of rCTGF/CCN2-gelatin hydrogel into the MIA-induced OA joints of rats could stimulate repair of articular cartilage. It was reported that a single injection of MIA into the knee joint cavity interfered with cartilage metabolism and led to OA-like lesions.29, 30 In this study, we confirmed by histological examination that articular damage was induced by MIA (Figs. 2A and 2B). At day 14 after administration of 6.0 mg of MIA, chondrocytes were depleted in the central part of the cartilage, and they proliferated and formed clusters at the margins of the femorotibial and femoropatellar joints. These findings indicate that this model can reproduce OA-like lesions similar to those observed in human clinical cases. Interestingly, CTGF/CCN2 was expressed at low levels in normal articular cartilage, whereas in OA articular cartilage, CTGF/CCN2 was strongly expressed in the clustered chondrocytes (Fig. 3). Because cluster formation represents a high proliferative response of chondrocytes, indicating an attempt to repair the damaged cartilage,2 the presence of CTGF/CCN2 in this region suggests that this factor plays an important role in the repair of articular cartilage. Therefore, we investigated the effect of treatment with rCTGF/CCN2.
Initially, we applied rCTGF/CCN2 in a simple aqueous solution for the repair of articular cartilage, using the MIA-induced OA model. Although cluster formation of chondrocytes was increased by treatment with rCTGF/CCN2 solution, histological features of lesions treated with rCTGF/CCN2 solution did not show significant improvement (data not shown). Successful tissue repair by rCTGF/CCN2 solution was not achieved for several probable reasons. One of them may be a short half-life of rCTGF/CCN2 in the joint cavity to exert biological activities. Thus, modification of the drug delivery system would be required for enhancing the in vivo efficacy of the applied rCTGF/CCN2. Recently, it was reported that a new hydrogel carrier system composed of gelatin succeeded in the sustained release of basic fibroblast growth factor (bFGF) and transforming growth factor (TGF)-β.27, 28 Therefore, we applied this sustained release strategy to rCTGF/CCN2. As shown in Fig. 1, we confirmed that rCTGF/CCN2 was immobilized into the gelatin hydrogel.
Next, we evaluated the effects of rCTGF/CCN2 incorporated into the gelatin hydrogel on articular cartilage repair, using the MIA-induced OA model. As shown in Fig. 4 and Table 3, incorporation into the hydrogel enabled 1.5 μg rCTGF/CCN2 to stimulate the articular cartilage repair, and the histological features of the repaired cartilage seemed similar to those of the normal cartilage. In addition, the OA-downregulated expression of tenascin-C and aggrecan, which are markers of articular chondrocytes, was rescued by treatment with rCTGF/CCN2 incorporated into gelatin hydrogel in the MIA-induced OA model. Interestingly, although CTGF/CCN2 was shown to be constitutively expressed in numerous fibrotic disorders in both skin and internal organs, such as atherosclerosis and pulmonary/renal fibrosis,33-37 the synovial membrane showed no response (data not shown). Furthermore, PCNA+ cells were not detected immunohistochemically in the synovial membrane after injection of either PBS-hydrogel or rCTGF/CCN2-hydrogel (data not shown). One possible mechanism is that CTGF/CCN2 may be efficiently trapped by cartilaginous ECM and is able to exert its functions in a matricrine manner specifically on chondrocytes. Further investigations are needed to verify this hypothesis regarding the differential action of CTGF/CCN2 in the joints.
In the other type of animal experiment, we prepared full thickness defects in the articular cartilage in adult rat knee joints, filled the defects with lyophilized collagen sponge containing rCTGF/CCN2-hydrogel, and found that rCTGF/CCN2-hydrogel collagen, but not the PBS-containing one, induced healing of the joint surface with regenerated cartilage, after 4 weeks (Fig. 6). Furthermore, even 6 weeks after implantation of PBS-hydrogel collagen, the defects still had not been replaced by new articular cartilage (data not shown), suggesting that these defects in adult rats would not be repaired spontaneously. One possible reason for this lack of repair may be the difference in the ability of regeneration between young and adult animals.38 Table 4 summarizes the histological features at 4 weeks after implantation of rCTGF/CCN2- or PBS-hydrogel collagen. The repaired tissue induced by rCTGF/CCN2 was histologically closer to normal hyaline cartilage than the PBS-hydrogel collagen-treated tissue (control). This is the first report describing the direct effect of CTGF/CCN2 on the repair of articular cartilage by means of rCTGF/CCN2-hydrogel collagen implanted into full thickness defects; however, further extensive investigation is still required to draw a final conclusion regarding the quality and stability of repaired tissue, because full functional recovery of the articular cartilage should be examined over a longer time course.
Cartilage regeneration is an attractive research field in tissue engineering because of its high clinical importance. It is known that regeneration of cartilage tissue is regulated by various types of growth factors, such as bFGF, TGF-β, and bone morphogenetic protein-2 (BMP-2).39-41 These factors initiate the immediate and rapid entrance of the bone marrow-derived stromal cells into the chondrogenic pathway, which results in rapid fabrication of cartilage tissue. Recently, it was reported that CTGF/CCN2 was expressed in the perichondrium at initial stages and in maturing chondrocytes at later stages of developing cartilage.42 These results indicated that CTGF/CCN2 was important for multiple aspects of chondrogenesis. In this study, rCTGF/CCN2 treatment of the MSCs presumably resulted in an increased level of chondrocyte-specific factors, such as type II collagen and aggrecan, as suggested by their heightened mRNA expression of these molecules (Fig. 7). Although it remains unclear how CTGF/CCN2 regulates the development of MSCs, one possible mechanism may involve stimulating the condensation of mesenchymal stem cells, which is the initiation of chondrogenesis. Interestingly, despite high levels of expression in perichondrium at the initial stages, no defect in chondrogenesis could be observed in ctgf/ccn2-null mice at this stage.42 These findings suggest that Cyr61/CCN1, which also promotes chondrogenesis in vitro,43 may rescue chondrocyte differentiation at initial stage in ctgf/ccn2 mutants. Moreover, we recently reported that CTGF/CCN2 directly stimulated the proliferation and differentiation, but not the terminal hypertrophic differentiation, of chondrocytes in articular cartilage in vitro.23 Taken together, our data suggest that CTGF/CCN2 might restore a defect in the articular cartilage not only by stimulating proliferation and differentiation of chondrocytes, but also by converting MSCs into chondrocytes.
In summary, for the first time, we showed that local administration of CTGF/CCN2-hydrogel into an arthritic joint cavity or full thickness articular cartilage defect stimulated the repair of articular cartilage in vivo. Although further studies using longer time courses are required before the clinical application of rCTGF/CCN2-hydrogel to joint diseases, such as OA, this study suggests that CTGF/CCN2 may be a novel and promising tool in OA therapeutics and tissue engineering for articular cartilage reconstruction.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research of the Ministry of Education, Science, Sports, and Culture of Japan (TN, SK, TK, and MT), Grants-in-Aid for Specific Diseases of the Ministry of Public Health and Welfare of Japan (MT), and grants from the Nakatomi Health Science Foundation (SK and MT), the Foundation for Growth Science in Japan (MT), the Sumitomo Foundation (MT), a Research Promotion Award for the Study of Bone and Joint Diseases from the Japan Rheumatism Foundation (MT), and Research for the Future Programme of The Japan Society for the Promotion of Science (JSPS; Project: Biological Tissue Engineering, JSPS-RFTF98I00201). We thank Tohru Nakanishi, Yoshiki Mukudai, Gen Yosimichi, Seiji Kondo, Takanori Eguchi, and Norifumi H Moritani for helpful suggestions and Yuki Nonami for technical and secretarial assistance.