Outcome of concomitant treatment with thiopurines and allopurinol in patients with inflammatory bowel disease: A nationwide Danish cohort study
Abstract
Background
Thiopurine and allopurinol in combination are associated with clinical remission in inflammatory bowel diseases but their influence on subsequent outcomes is unclear. We compared outcomes during exposure to both thiopurines and allopurinol versus thiopurines alone.
Methods
We established a nationwide cohort of patients with inflammatory bowel diseases exposed to thiopurines ± allopurinol during 1999–2014, using registry data. Patients were followed until hospitalization, surgery, anti-TNFα, or death (as a primary composite outcome). We used Poisson regression analyses to calculate incidence rate ratios overall and stratified by calendar period (assuming the combined exposure was unintended before 2009).
Results
A total of 10,367 patients with inflammatory bowel diseases (Crohn’s disease, n = 5484; ulcerative colitis, n = 4883) received thiopurines. Of these, 217 (2.1%) also received allopurinol. During 24,714 person years of follow-up, we observed 40 outcomes among thiopurine-allopurinol-exposed patients, and 4745 outcomes among those who were thiopurine exposed; incidence rate ratio, 1.26 (95% confidence interval, 0.92–1.73). The incidence rate ratios decreased over time: 4.88 (95% confidence interval 2.53–9.45) for 1999–2003, 2.19 (95% confidence interval, 1.17–4.09) for 2004–2008 and 0.80 (95% confidence interval, 0.52–1.23) for 2009–2014.
Conclusion
Our nationwide inflammatory bowel disease cohort study shows that concomitant thiopurine-allopurinol is as safe to use as thiopurines alone, with a tendency towards a positive effect on clinical outcomes in recent calendar periods when combined use was intended.
Key summary
- Summary of established knowledge on this subject
- Concomitant thiopurine and allopurinol therapy is associated with clinical and mucosal remission.
- The impact of concomitant exposure on subsequent outcomes (hospitalization, treatment escalation and surgery) remains unknown.
- What are the significant and/or new findings of this study?
- In this nationwide population-based inflammatory bowel disease cohort study, we found that concomitant treatment with thiopurines and allopurinol, compared to thiopurines alone, may have a positive impact on risk of surgery, hospitalizations and need for biologics, when thiopurines and allopurinol are intentionally used in combination.
- Clinically, this study indicates that adding allopurinol to thiopurine monotherapy is safe and may improve efficacy when used intentionally.
Introduction
The inflammatory bowel diseases (IBD) Crohn’s disease (CD) and ulcerative colitis (UC) are chronic diseases of the gastrointestinal tract that may influence both physical, psychological and social aspects of patients’ lives.1 Despite advances in medical therapies, surgery and hospitalization remain serious complications of the diseases affecting up to 50% of patients 10 years from diagnosis2 and causing high treatment costs.2,3 The newer anti-TNFαs are also costly3 and inefficient in up to 50–75% of patients.4–6 Hence, IBD poses a serious burden to both patients and healthcare systems and with increasing incidence worldwide, the need for efficient treatments is pronounced.2,7
Thiopurines (azathioprine and mercaptopurine) are immunomodulators prescribed for IBD when first-line therapy fails. Thiopurines lead to adverse effects in one-third of patients4,8 and their efficacy has been questioned after two randomized controlled trials showed low mucosal healing rates.4,5 The gout treatment allopurinol reverses some of the adverse effects induced by thiopurines, when combined with a reduced dose of thiopurines.9–11 Allopurinol increases the active thiopurine-metabolite 6-thioguanine (6-TGN) that is associated with disease remission and reduces the metabolite methylmercaptopurine (MeMP), which is associated with adverse effects of thiopurines.8,12 A recent observational study found mucosal healing in up to 46 % of patients using allopurinol co-therapy13 and a randomized trial found co-therapy to be more efficient than thiopurine alone in achieving steroid-free clinical remission.8
Allopurinol co-therapy has been prescribed in Denmark since 2008–2009 to reverse adverse effects of thiopurines or to optimize efficacy by optimizing metabolite levels (increased 6-TGN and reduced MeMP levels) in thiopurine users. Before 2008, patients with IBD may have been exposed unintentionally to concomitant thiopurines and allopurinol due to concomitant occurrence of gout or kidney stones.
The impact of concomitant exposure to thiopurines and allopurinol on treatment escalation and hospitalization remains unknown.
The aim of the present study was to assess the impact of concomitant exposure to allopurinol and thiopurines versus thiopurines alone on later risk of IBD-related hospitalization, IBD-related surgery, anti-TNFα initiation or death.
Methods
Study cohort
In the Danish National Patient Register (DNPR) we identified all persons above 18 years of age with a diagnosis of IBD in the period 1999–2014. The DNPR contains individual-level information on all inpatient contacts in Denmark since 1977 and outpatient contacts since 1995.14 As IBD patients, we included those with at least one diagnosis of CD or UC in the register based on the International Classification of Disease (ICD) 8 codes 563.00–563.09, 563.19 or 569.04 and ICD-10 codes K50 or K51. Diagnoses of IBD in the DNPR have been shown to be valid (90–97%) and almost complete (94%) when compared with pathology register information.15
Exposure
Information on thiopurine exposure was obtained from two different registers to obtain full coverage. From the DNPR, we extracted information about thiopurine prescriptions filled at hospitals, using procedure code BWHB83. Pharmacy-redeemed prescriptions were identified in the Registry of Medicinal Product Statistics that contains information on all prescriptions redeemed from Danish pharmacies since 1995.16 We extracted information on thiopurine exposure using ATC codes (Anatomical Therapeutic Chemical Classification) L04AX01, L01BB02 and M04AA51. Our study cohort was then defined by all patients with an IBD diagnosis exposed to thiopurines. Information on co-exposure to allopurinol was obtained from the Registry of Medicinal Product Statistics using ATC code M04AA01. We then defined two exposure groups: a monotherapy group exposed to thiopurines only and a concomitant exposure group exposed to both thiopurines and allopurinol. Patients were considered thiopurine exposed from the date of their second filled prescription with thiopurines. If allopurinol was initiated before or simultaneously to thiopurines, patients were considered co-exposed to both medications from the date of the second-filled thiopurine prescription. If patients were already exposed to thiopurines when allopurinol was initiated, patients were considered co-exposed from the date when they filled the second allopurinol prescription. Hence, the same patient could contribute person years (PY) in both exposure groups. Patients were considered in consecutive monotherapy if they filled a thiopurine prescription at least every 12 months, or co-exposed if they filled a prescription of both medications within 12 months. Patients with a registration of thiopurine exposure before 1999, or before their date of IBD diagnosis after 1999, were excluded from analyses (Figure 1).
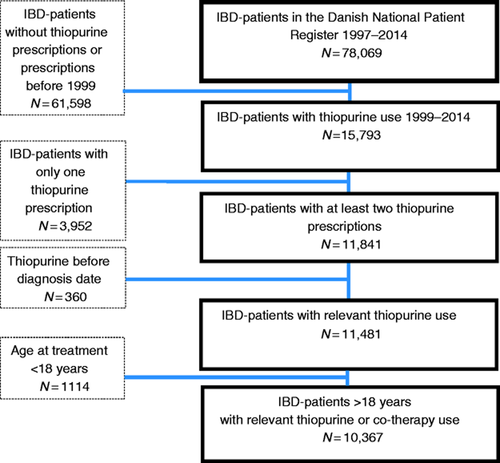
Flowchart of study cohort selection.
Outcomes
Our primary outcome was a composite of need for anti-TNFα treatment or any of the following adverse outcomes: IBD-related hospitalization, IBD-related major surgery or death, whichever came first. IBD-related hospitalizations were identified via the DNPR as hospitalization for either a primary diagnosis of IBD or a secondary diagnosis of IBD combined with a primary diagnosis of gastrointestinal related symptoms including abdominal pain, diarrhea, nausea, vomiting, rectal bleeding, stenosis, fistula, abscess, sub-ileus or ileus (Supplementary Table 1). Only admissions lasting more than 24 hours were included. Information on surgeries were found in the DNPR using surgical procedure codes based on the Nordic Medico-Statistical Committee Classification for Surgical Procedures from 1996 and onwards. Major surgery comprised colectomy, intestinal resections and other major surgical procedures (Supplementary Table 1). Anti-TNFα therapy was defined as initiation of infliximab or adalimumab. Procedure codes were extracted from the DNPR and ATC codes from the Registry of Medicinal Product Statistics (Supplementary Table 1). Death was identified in the Civil Registration System that contains dates of death and migrations among all Danish citizens.17
Covariates
Information on age and sex was extracted from the Civil Registration System. Age at diagnosis was extracted from the DNPR as the first registered diagnosis of IBD. Calendar time at medication initiation was divided into three eras: 1999–2003, 2004–2008 and 2009–2014. As a measure of IBD severity 1 year before inclusion, we assessed the number of IBD-related hospitalizations (0, 1–2, >2), per oral corticosteroid treatments (0, 1–2, >2), IBD-related major surgery (yes, no) and minor IBD-related surgery during the past year before inclusion (0, 1–2, >2). Minor surgery comprised intra-abdominal or perianal abscess or fistula surgery (Supplementary Table 1).
Statistical analyses
Patients were followed from medication exposure (thiopurine/allopurinol) until discontinuation of treatment, emigration, death, or end of follow-up on 31 December 2014, whichever came first. Thiopurine/allopurinol-thiopurine discontinuation was defined as not filing a prescription for more than 12 months. Exposure was considered a time-varying variable with IBD patients contributing person-time to the relevant exposure group.
We used SAS V.9.4 (SAS Institute, Cary, NC) for statistical analyses. We summarized quantitative data using means and standard deviations (SD) and binary data using proportions. We calculated incidence rate ratios (IRR) using Poisson regression models with 95% confidence intervals (CI). The analyses were adjusted for IBD-subtype, sex, age at treatment initiation, calendar year and age at IBD diagnosis. Because the risk of the outcomes and the efficacy of thiopurines may differ according to IBD subtype, calendar year, age at IBD-diagnosis and sex, stratified sub-analyses were also performed. Results were considered statistically significant when the two-sided p value was <0.05.
Ethical considerations
The study was approved by the Danish Data Protection Agency. Ethical approval is not required for registry-based research in Denmark. The study adheres to The Strengthening the Reporting of Observational Studies in Epidemiology statement. 18
Results
We identified 10,367 IBD patients (CD, n = 5484; UC, n = 4883) exposed to thiopurine therapy from 1999 to 2014, contributing 36,998 PY of follow-up. Of these patients, 217 were additionally exposed to allopurinol with 245 PY of follow-up (Figure 1).
Compared with patients treated with monotherapy, allopurinol co-exposed patients were older at IBD diagnosis (41.5 years vs 36.2 years, p < 0.001), older at medication initiation (47.2 years vs 40.7 years, p < 0.001) and their disease duration was longer (5.7 years vs 4.5 years, p < 0.001). Patients co-exposed to allopurinol more often had a UC diagnosis rather than a CD diagnosis (UC, 59.5% vs 46.8 %, p < 0.002) and more were men (62.7% vs 49.1%, p < 0.001). The majority of the allopurinol co-exposed patients started treatment in the latest part of the study (2009–2014, 73.3%) and of these patients, more started thiopurines prior to allopurinol (72.3%) or simultaneously (8.8%) (Table 2). Only 12.0% and 14.8% initiated co-treatment during 1999–2003 and 2004–2008 and of these patients, 69.2% and 75% received allopurinol prior to thiopurines (Table 2), which supports that it was more likely prescribed for other indications than IBD. Thiopurine exposure also increased, although less rapidly, over time (1999–2003, 32.0%; 2004–2008, 27.8%; 2009–2014, 40.3%). Severity of IBD in the year before thiopurine exposure was similar in the two groups, except for major surgeries that were less frequent in the co-exposed group (p = 0.02) (Table 1).
| Baseline description | Allopurinol | Monotherapy | p-value |
|---|---|---|---|
| n (%) | 217 (100.00) | 10150 (100.00) | |
| Monotherapy | – | – | |
| Allopurinol | – | – | |
| IBD-type | 0.002† | ||
| CD | 88 (40.55) | 5396 (53.16) | |
| UC | 129 (59.45) | 4754 (46.84) | |
| Sex | <0.001† | ||
| Women | 81 (37.33) | 5171 (50.95) | |
| Men | 136 (62.67) | 4979 (49.05) | |
| Age at IBD-diagnosis | <0.001‡ | ||
| Mean (SD), years | 41.47 (18.79) | 36.18 (16.10) | |
| < 17 years | 7 (3.2) | 286 (2.8) | |
| 17–40 years | 102 (47.0) | 6350 (62.3) | |
| >=40years | 108 (49.8) | 3514 (34.6) | |
| Age at index | <0.001† | ||
| Mean (SD), years | 47.19 (18.95) | 40.74 (16.49) | |
| <= 40 years | 91 (41.9) | 5735 (56.5) | |
| >40 years | 126 (58.1) | 4415 (43.5) | |
| Duration of IBD at index | <0.001‡ | ||
| Mean (SD), years | 5.68 (6.61) | 4.51 (6.42) | |
| <1 year | 18 (8.29) | 2535 (24.98) | |
| 1–5 years | 112 (51.61) | 4589 (45.21) | |
| >5 years | 87 (40.09) | 3026 (29.81) | |
| Calendar year at medication initiation | <0.001‡ | ||
| 1999–2003 | 26 (11.98) | 3241 (32.01) | |
| 2004–2008 | 32 (14.75) | 2824 (27.82) | |
| 2009–2014 | 159 (73.27) | 4085 (40.25) | |
| IBD-severity | |||
| Minor surgery past year | 0.51† | ||
| No | 210 (96.77) | 9732 (95.88) | |
| Yes | 7 (3.23) | 418 (4.12) | |
| 0 | 114 (52.53) | 5718 (56.33) | |
| 1–2 | 94 (43.32) | 4043 (39.83) | |
| > 2 | 9 (4.15) | 389 (3.83) | |
| Major surgery past year | 0.02† | ||
| No | >210 | 9601 (94.59) | |
| Yes | <5 | 549 (5.41) | |
| Steroid treatment past year | 0.98‡ | ||
| 0 | 56 (25.81) | 2615 (25.76) | |
| 1–2 | 38 (17.51) | 1830 (18.03) | |
| t>2 | 123 (56.68) | 5705 (56.21) |
- UC; Ulcerative Colitis, CD; Crohn’s Disease, IBD; Inflammatory bowel disease CI; confidence interval, SD; Standard deviation, ‡ P-value for the categorical variable † P-value for the binary variable
| Allopurinol prescribed first | Thiopurine prescribed first | Allopurinol- thiopurine prescribed simultaneously | |
|---|---|---|---|
| Calendar year | |||
| 1999–2003 | 18 | 8 | <5 |
| 2004–2008 | 24 | 8 | <5 |
| 2009–2014 | 30 | 115 | 10-15 |
- Simultaneous prescription: ± 30 days.
Impact of co-exposure on hospitalization, surgery, anti-TNFα and death
For the composite outcome (hospitalization, major surgery, anti-TNFα initiation or death), we observed 40 incidents in patients exposed to both allopurinol and thiopurines (IR, 310.1 per 1000 PY) versus 4745 in the thiopurine exposed group (IR, 193.0 per 1000 PY), resulting in a crude IRR of 1.61 (95% CI, 1.18–2.20). After adjustment for IBD-subtype, sex, age at treatment initiation, calendar year, and age at IBD diagnosis, the increase was no longer statistically significant (IRR, 1.26; 95% CI, 0.92–1.73) (Table 3). This applied to both genders and IBD subtype (Table 7).
| Outcomes | Allopurinol | Monotherapy | IRR in allopurinol vs. monotherapy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Person years | IR per 1000 person years | cases | Person years | IR per 1000 person years | Crude | Adjusted | |||||
| IRR | 95%CI | IRR | 95%CI | |||||||||
| Composite outcome | 40 | 129 | 310.1 | 4745 | 24585 | 193.0 | 1.61 | 1.18 | 2.20 | 1.26 | 0.92 | 1.73 |
| Separate outcomes | ||||||||||||
| Major surgery | 13 | 233 | 55.8 | 1447 | 34185 | 42.3 | 1.32 | 0.76 | 2.28 | 1.31 | 0.76 | 2.27 |
| Hospitalization | 32 | 167 | 191.1 | 3270 | 28087 | 116.4 | 1.64 | 1.16 | 2.33 | 1.61 | 1.14 | 2.29 |
| Anti-TNFα | 19 | 179 | 105.9 | 2415 | 31708 | 76.2 | 1.39 | 0.88 | 2.18 | 0.74 | 0.47 | 1.16 |
| Death | – | – | – | – | – | – | – | – | – | – | – | – |
- Adjusted for IBD-subtype, sex, age at treatment, age at IBD diagnosis and calendar year. IR; incidence rate, IRR; incidence rate ratio, CI; confidence interval, IBD; Inflammatory Bowel Disease, Composite outcome: major surgery, IBD-related hospitalization, anti-TNFα, or death. Separate data on deaths are excluded due to few cases.
| Hospitalizations | Allopurinol | Monotherapy | ||||
|---|---|---|---|---|---|---|
| Calendar year | Cases | Person years | IR per 1000 person years | Cases | Personyears | IR per 1000 person years |
| 1999– 2003 | 9 | 20 | 459.9 | 1295 | 11564 | 112.1 |
| 2004– 2008 | 9 | 31 | 292.1 | 1008 | 9302 | 108.4 |
| 2009– 2014 | 14 | 117 | 119.6 | 967 | 7246 | 133.4 |
- IR; incidence rate
For all calendar periods, patients co-exposed to allopurinol had a significantly increased risk of IBD-related hospitalization (adjusted IRR, 1.61; 95% CI, 1.14–2.29), whereas no associations with surgery, anti-TNFα initiation, or death were observed (Table 3).
Due to the assumption that concomitant treatment with thiopurines and allopurinol before 2009 was unintentional, we found it important to further stratify analyses by calendar-year periods. We observed that during years 1999–2003 (adjusted IRR, 4.88; 95% CI, 2.53–9.45) and years 2004–2008 (adjusted IRR, 2.19; 95% CI, 1.17–4.09), the risk of the composite outcome was significantly increased. However, during years 2009–2014, when allopurinol was more likely prescribed for IBD, the risk of the composite outcome tended to be decreased, although not statistically significantly (adjusted IRR, 0.80; 95% CI, 0.52–1.23) (Table 4). Crude IRs of hospitalizations seemed to decrease over time in the allopurinol group (IR 459.9 per 1000 PY, 1999–2003, IR 292.1 per 1000 PY, 2004–2008, IR 119.6 per 1000 PY, 2009–2014), whereas they did not change in the thiopurine group (Table 4). Anti-TNFα use increased over time (Table 5), whereas major surgeries stayed unchanged (Table 6).
| Initiation of anti-TNFα | Allopurinol | Monotherapy | ||||
|---|---|---|---|---|---|---|
| Calendar year | Cases | Person years | IR per 1000 person years | Cases | Personyears | IR per 1000 person years |
| 1999–2003 | – | – | – | 205 | 15648 | 13.1 |
| 2004–2008 | – | – | – | 708 | 9764 | 72.51 |
| 2009–2014 | 17 | 105 | 162.0 | 1502 | 6321 | 237.6 |
- IR; incidence rate. Separate data on anti-TNFα in the allopurinol group 1999–2003 and 2004–2009 are excluded due to few cases.
| Major surgery | Allopurinol | Monotherapy | ||||
|---|---|---|---|---|---|---|
| Calendar year | Cases | Person years | IR per 1000 person years | Cases | Person years | IR per 1000 person years |
| 1999– 2003 | – | – | – | 656 | 14690 | 44.6 |
| 2004– 2008 | – | – | – | 415 | 11255 | 36.9 |
| 2009– 2014 | 8 | 160 | 50.1 | 376 | 8240 | 45.6 |
- IR; incidence rate. Separate data on major surgery in the allopurinol group 1999– 2003 and 2004– 2009 are excluded due to few cases.
We also observed that patients who were diagnosed with IBD after the age of 40 years were at higher risk of the composite outcome if co-treated with allopurinol (for all calendar periods) (adjusted IRR, 2.04; 95% CI, 1.43–2.94) (Table 7).
| Composite Outcome | Allopurinol | Monotherapy | IRR in allopurinol vs. monotherapy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Person years | IR per 1000 person years | Cases | Person years | IR per 1000 person years | Crude | Adjusted | |||||
| IRR | 95%CI | IRR | 95%CI | |||||||||
| Overall result | 40 | 129 | 310.1 | 4745 | 24585 | 193.0 | 1.61 | 1.18 | 2.20 | 1.26 | 0.92 | 1.73 |
| Stratification by calendar year | ||||||||||||
| 1999–2003 | 9 | 19.6 | 459.9 | 1385 | 11150 | 124.2 | 3.70 | 1.92 | 7.13 | 4.88 | 2.53 | 9.45 |
| 2004–2008 | 10 | 30.8 | 325.2 | 1365 | 7973 | 171.2 | 1.90 | 1.02 | 3.54 | 2.19 | 1.17 | 4.09 |
| 2009–2014 | 21 | 78.2 | 268.4 | 1995 | 5462 | 365.2 | 0.73 | 0.48 | 1.30 | 0.80 | 0.52 | 1.23 |
| Stratification by IBD-subtype | ||||||||||||
| UC | 23 | 83 | 278.4 | 1937 | 12388 | 156.4 | 1.78 | 1.18 | 2.69 | 1.23 | 0.82 | 1.86 |
| CD | 17 | 46 | 370.1 | 2808 | 12198 | 230.2 | 1.61 | 1.00 | 2.59 | 1.25 | 0.78 | 2.02 |
| Stratification by sex | ||||||||||||
| Male | 30 | 82 | 367.6 | 2246 | 12985.4 | 173.0 | 2.13 | 1.48 | 3.05 | 1.06 | 0.58 | 1.92 |
| Female | 10 | 47 | 213.0 | 2499 | 11600.1 | 215.4 | 0.99 | 0.53 | 1.84 | 1.06 | 0.58 | 1.92 |
| Stratification by age at diagnosis | ||||||||||||
| <40 years | 10 | 48.1 | 208.0 | 3330 | 15537 | 214.32 | 0.97 | 0.52 | 1.81 | 0.58 | 0.31 | 1.07 |
| ≥40 years | 30 | 80.5 | 372.8 | 1415 | 9048 | 156.4 | 2.38 | 1.66 | 3.42 | 2.04 | 1.43 | 2.94 |
- Adjusted for IBD-subtype, sex, age at treatment initiation, and age at IBD diagnosis and calendar year. Composite outcome: IBD-related major surgery, IBD-related hospitalization, anti-TNFα, or death, IR; incidence rate, IRR; incidence rate ratio, UC; Ulcerative Colitis, CD; Crohn’s Disease, IBD; Inflammatory bowel disease CI; confidence interval
Discussion
In this nationwide population-based cohort study of 10,367 IBD-patients exposed to thiopurines, we did not find that co-exposure to allopurinol had an impact on the overall risk of the composite outcome (IBD-related hospitalization, IBD-related surgery, anti-TNFα therapy initiation and death). However, when stratifying by calendar year, due to the assumption that co-exposure to allopurinol before 2009 was unintentional, we observed a statistically significantly increased risk of the composite outcome before 2009, whereas a tendency towards a decreased risk of the composite outcome was observed in the period 2009–2014, when allopurinol was more systematically prescribed for IBD and thiopurine doses adjusted accordingly.
In our previous randomized trial, we found that allopurinol co-therapy was more efficient than monotherapy when comparing clinical steroid-free remission (IRR, 2.10; 95% CI, 1.07–4.11 after 6 months follow-up).8 In the randomized trial patients were naïve to thiopurine therapy and treatment was optimized guided by thiopurine metabolites, a method that had not yet been introduced in the early calendar periods of the present study. Other studies of various methodology have found beneficial effects of allopurinol co-therapy with treatment durations of 1–5 years, however, these studies did not compare the results with a monotherapy group.9, 19, 20
Allopurinol may be used for the treatment of gout or kidney stones and was first prescribed as a co-therapy to thiopurines for renal transplantations in 1993.21 The first study on allopurinol co-therapy for IBD was published in 2005,22 and to our knowledge, allopurinol co-therapy has been prescribed for IBD in Denmark since 2008. Allopurinol doses had been helpful in distinguishing the indication (100 mg vs 300 mg), but these data were not available. Still, most patients received allopurinol prior to thiopurines in the early calendar periods (in contrast to the later periods), which supports our assumption that allopurinol was prescribed for other indications than IBD. Due to the assumption that concomitant treatment with thiopurines and allopurinol before 2009 was unintentional, and to account for temporal changes in exposure and outcome (increasing thiopurine, allopurinol and anti-TNFα therapy use, Table 1 and Table 5) we stratified by era of medication initiation. We found no increased risk of the composite outcome in the era when co-therapy was more systematically prescribed (2009–2014) despite the fact that this era was best powered in terms of numbers to assess an association, and the increased risk in the early eras is therefore assumed to reflect unintentional prescription of allopurinol for other indications than IBD. Likewise, co-therapy treated patients >40 years of age were at higher risk of a composite outcome, potentially reflecting that allopurinol in this group was prescribed for comorbidities such as gout rather than for IBD.
When evaluating each outcome individually, we observed an increased risk of IBD-related hospitalization in allopurinol-treated patients. Hospitalization diagnoses were defined to reflect an increase in disease activity (Supplementary Table 1), however, diagnoses at hospitalization may be inaccurate and may also reflect adverse effects, need for diagnostics, or differences in hospitalization patterns. Previous studies show that co-therapy reverses some of the adverse effects induced by thiopurine monotherapy, but do not assess whether co-therapy results in an altered frequency of adverse effects.9,10 Also, previous studies report serious adverse events in unintentional concomitant thiopurine and allopurinol treatment, i.e. myelosuppression.23,24 We were not able to assess whether thiopurine doses were reduced when allopurinol was prescribed to prevent myelosuppression. The only prospective study that has investigated the impact of thiopurine monotherapy on hospitalization in CD patients found that hospitalizations were mainly caused by adverse effects (anaemia, abnormal liver tests and acute pancreatitis).25 These findings may explain our observations of an increased risk of hospitalization. IRs for hospitalization were also lower in the most recent calendar period among allopurinol co-exposed patients along with the reduction in the overall composite IRR for this period; however, due to the cohort size we were not able to perform meaningful calendar period stratified analyses to assess IRRs and the crude IRs should be interpreted with caution.
There are several strengths of this study. First, we identified an unselected, nationwide cohort of IBD-patients receiving thiopurine therapy from registers with documented high sensitivity and specificity for the IBD-diagnoses (90–97%). Second, strict definitions of medication exposure and outcomes were used, minimizing ascertainment bias. Third, data were analyzed by two separate epidemiologists to minimize the risk of analysis error. However, caution must be taken when data are interpreted given the cohort size and the observational design. Due to the limited cohort size, the number of outcomes was low, which limited the precision of our estimates, and we acknowledge that the study was marginally powered to adjust for five confounders and perform stratified analyses. Also, metabolite levels may have influenced outcomes, however, we did not have access to the data. The duration of follow-up was shorter in the allopurinol-exposed group compared with the monotherapy exposed group (245 PY for 217 persons in co-therapy vs 26,753 PY for 10,150 persons in monotherapy), which may have led to an overestimation of outcomes in the co-therapy group, as fewer outcomes are expected with longer duration of treatment.26,27 The relatively short follow-up time in the co-exposed group was due to methodological decisions of censoring at first outcome and to count PY only during the actual months of exposure. We find this to be the most appropriate approach. Contrarily, most patients were exposed to thiopurines prior to initiation of allopurinol and may have had prior effect of monotherapy, which may lead to an underestimation of outcomes. Prescription intervals below 12 months may have overestimated outcomes due to the risk of inclusion of incompliant patients, however, this definition was based on clinical practice in Denmark and was the same in both groups. Another potential limitation was the composition of the composite outcome. The associations of the individual outcomes – IBD-related hospitalization, IBD-related surgery, anti-TNFα therapy initiation and death – diverged when they were analyzed separately. This implies that opposite associations may outweigh each other when the outcomes are joined into a composite outcome. Lastly, the study lacks information regarding disease localization, disease behaviour and smoking status that would allow for more detailed disease severity estimates. To account for this, we evaluated disease severity assessing previous use of systemic steroid, surgery and hospitalization and did not observe a difference between the mono- and co-therapy groups.
Conclusion
In our nationwide population-based IBD cohort study, thiopurine-allopurinol co-exposed patients did not have an overall altered risk of the composite outcome IBD-related hospitalization, IBD-related surgery, anti-TNFα therapy initiation and death. Calendar period analyses suggested that intentional co-exposure in recent years might improve outcomes, potentially due to lower doses of both allopurinol and thiopurines. Clinically, this study implicates that concomitant allopurinol is as safe to use as thiopurine monotherapy with a tendency towards better efficacy (i.e. improved clinical outcomes) when used intentionally. This merits further investigation and could ideally be done in long-term follow-up of patients from randomized controlled trials.
Declaration of conflicting interest
None declared.
Ethics approval
The study was approved by the Danish Data Protection Agency. Ethical approval is not required for registry-based research in Denmark. The study adheres to The Strengthening the Reporting of Observational Studies in Epidemiology statement.
Funding
Louis-Hansen Foundation, Award ID: 18-2B-3487.
Informed consent
Informed consent is not required for registry based research in Denmark.