Change in incidence, characteristics and management of colorectal neuroendocrine tumours in the Netherlands in the last decade
Abstract
Background
Neuroendocrine tumours (NETs) are rare. However, a rising incidence has been reported over the past decades. For colorectal NETs, this is presumably caused by an increased awareness of colorectal diseases and colonoscopic procedures. This study aims to analyse the change in incidence of colorectal NETs, characteristics and management and evaluate the proportion of colorectal NETs detected in a national colorectal cancer (CRC) screening programme.
Methods
Histopathological reports on colorectal well-differentiated NETs detected between 2006 and 2016 were collected from the Dutch pathology database (PALGA) containing nationwide histo- and cytopathology reports of all pathology laboratories in the Netherlands.
Results
Colorectal NETs were detected in 1055 individuals. Increasing incidence rates were observed from 0.36 per 100,000 inhabitants in 2006 to 0.75 per 100,000 inhabitants in 2011 (p value < 0.001), remaining stable afterward. Most NETs were grade I (73.5%) and detected in the rectum (76.4%). The majority (88.2%) were detected by colonoscopy, and the final intervention depended significantly on primary location of the tumour; 94.6% of rectal NETs were endoscopically removed, whereas 61.0% of colonic NETs were removed by surgery. There was an increase in local excision both of rectal and colonic NETs over the years instead of radical resection. Screening for CRC started in 2014 and contributed by detecting 32% of the diagnosed colorectal NETs within the invited age group, of which 94.6% were detected at an early stage.
Conclusion
The incidence of reported colorectal NETs in the Netherlands doubled over the last decade. The Dutch CRC screening programme had a clear contribution to colorectal NETs incidence among its target population. A shift to more local management of detected lesions was observed over time.
Key summary
- Established knowledge on this subject
- Previous studies have reported a wide variety of incidence rates of neuroendocrine tumour (NET), presumably caused by underreporting, inconsistent nomenclature and changing classifications.
- The incidence of colorectal NETs has increased in the past decades.
- Screening for colorectal cancer incidentally detects colorectal NETs.
- Significant and/or new findings of this study
- Using accurate nationwide data and strict inclusion criteria of the most recent World Health Organisation classification (2017), we report transparent and well-defined incidence rates of colorectal NETs over the past decade.
- The incidence of colorectal NETs has doubled in the past decade, but has remained stable in recent years.
- A national colorectal cancer screening programme has a clear contribution in the detection of colorectal NETs, with a vast majority diagnosed at an early stage.
- Management of rectal and colonic NETs made a significant shift toward more local endoscopic excision techniques instead of surgical resection.
Introduction
Neuroendocrine tumours (NETs) are rare tumours, often detected as incidental findings. NETs can originate from almost any organ since they derive from neuroendocrine cells distributed throughout the human body, and the most common primary NET location is the gastrointestinal tract. Of all NETs, 10.9% to 25.2% are located in the colon or rectum, with reported incidences ranging from 0.33 to 1.65 per 100,000 inhabitants in different periods.1–3 This variation is presumably caused by underreporting, inconsistent nomenclature and several changes in classification throughout the past decades.3–7 The incidence of colorectal NETs seems to have increased over the past decades. This is caused by, among other things, a rising awareness of colorectal disease, increased endoscopy procedures and the implementation of colorectal cancer (CRC) screening programmes.8–13 As screening might result in early detection of colorectal NETs, the prognosis is likely to be improved.11, 14, 15 Along with improving endoscopic techniques, early detection might also result in a shift in management of colorectal NETs to relatively more endoscopic management instead of radical resection.
In the Netherlands practically all histopathological reports are collected in a national pathology database, enabling the conduction of an accurate study on nationwide data of colorectal NETs. Furthermore, a national CRC screening programme was initiated in 2014, and evaluation of colorectal NETs in the Netherlands could give better insight into its epidemiology. This study aims to analyse the change in incidence and the contribution of the population-based CRC screening programme to the incidence of colorectal NETs. Secondly, we will investigate the characteristics and management of colorectal NETs in the Netherlands in the past 10 years.
Method
Data collection
In this study we used the Dutch pathology database PALGA to search for histopathological reports of colorectal NETs during the period 2006–2016. PALGA has nationwide histo- and cytopathology reports of all pathology laboratories in the Netherlands since 1991 and hence covers the entire Dutch population (16.3 million in 2006, 17.0 million in 2016).16 Owing to encryption, all data were anonymous.
A population-based national CRC screening programme was launched in the Netherlands in 2014. By gradually inviting birth cohorts, it is step-wise enrolled to the whole target population of age 55–75 years until 2019, comprising 4.4 million individuals. From 2014 to 2016, this considered most birth cohorts between 1938 and 1957. Individuals biennially receive an immunochemical faecal occult blood test (FIT) and are scheduled for colonoscopy if testing positive.17
In PALGA, individuals who have participated in the CRC screening programme are marked since 2014. However, as a result of technical/start-up issues, numbers in 2014 may be underrated. The marking makes it possible to give insight into the contribution of the CRC screening programme to the incidence of NETs.
All records on colorectal well-differentiated NETs reported in the period from 2006 to 2016 were included in this analysis. Reports were excluded when the neuroendocrine neoplasia was described as high grade without the characteristics of a NET grade 3 according to the World Health Organisation (WHO) 2017 classification of NETs, and mixed adenocarcinoma neuroendocrine carcinoma and goblet cell carcinoids were excluded.18–20 We excluded appendicular NETs because the appendix is not within reach of colonoscopy (apart from the appendicular orifice), and we excluded ileal NETs because the terminal ileum is often not visualised in our screening programme colonoscopies, and if visualised, only the terminal ileum will be seen. Pathology reports that were ambiguous on tumour type and/or tumour grade were revised by a pathologist with expertise in neuroendocrine neoplasia.
Outcomes and analyses
Patient characteristics on sex and age at diagnosis were collected.
When provided by the PALGA excerpts, the following characteristics were extracted: year of diagnosis, primary tumour location, histologic grade, TNM stage, type of specimen (endoscopic, surgical, image-guided biopsy/resection or autopsy), final management (endoscopic local excision, transanal endoscopic microsurgery (TEM) or radical surgery) and whether the NET was detected by participation in the colorectal screening programme. With the data available, we reclassified the NETs according to the WHO 2017 criteria, including a new category of well-differentiated NETs grade III.21 Therefore, histological grade was registered as grade I, II or III as concluded in the pathology report, or, when given, estimated on the mitotical activity rate (mitoses/2 mm2 tumour) and/or ki-67 proliferation index (the percentage of cells positive for MIB-1 as assessed by immunohistochemistry). When the histological grade was estimated by mitotical activity or ki-67 proliferation index, grades I, II and III were respectively defined as less than 2, between 2 to 20 and more than 20 mitoses per mm2 or a ki-67 proliferation index of less than 3%, between 3% to 20% and more than 20%. When no data on proliferation were available and the NET was described as a “carcinoid”, the tumour was considered as a NET histological grade I/II. When data were missing on proliferation and the lesion was described as NET, it was considered a NET histological grade not otherwise specified (NOS). Reports without information on proliferation rate or mitotic rate, or without histological grade, were considered incomplete.
Stage of screen-detected NETs was based on the American Joint Committee on Cancer Cancer Staging Manual.22 An early-stage NET was defined as stage I or II. Primary NET location was defined as colon when located in the caecum, colon ascendens, colon transversum, colon descendens or sigmoid and defined as rectum when located in the rectosigmoid or rectum.
Crude overall and age-specific (five-year age groups) incidence rates were computed by weighing the incidence rates with data on the Dutch population size in the same years derived from Statistics Netherlands.23 To make the incidence rates globally comparable, direct standardisation was performed according to the WHO, European and United States (US) standard population and added as supplemental material.24–26 Pearson chi-squared or Fisher exact test and Student t -test were used to evaluate descriptive statistics between groups. Crude incidence rates were tested on a significant trend over time by modelling the number of tumours using a log-linear Poisson regression with the logarithm of the population size as the offset variable.27,28 All statistical analyses were performed in R version 3.4, and statistical significance was set at p < 0.05.
This study was approved by the scientific board of PALGA complying with the regulations for anonymised (epidemiological) studies on 10 January 2016, and considered to remain outside the restrictions of the Medical Research Involving Human Subjects Act (WMO) by the Medical Ethics Review Committee of the Netherlands Cancer Institute. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Results
The database search resulted in 1055 patients diagnosed with 1068 colorectal NETs in the period 2006–2016. Table 1 shows the baseline patient and NET characteristics. Of the patients detected with a NET, 554 (52.5%) were female and the median age at diagnosis was 61 years (interquartile range (IQR), 50–68 years) (Figure 1). The number of detected colorectal NETs varied between 58 and 118 per year. An increasing trend of the incidence rate was observed from 0.36 (95% confidence interval (CI): 0.27–0.46) per 100,000 inhabitants in 2006 to 0.75 (95% CI: 0.63–0.89) per 100,000 inhabitants in 2011 (p < 0.001), followed by a stable incidence rate between 2011 and 2016 (p = 0.69) (Figure 2(a)). Within the target group of screening (birth cohorts 1938–1957), incidence increased from 0.64 (95% CI: 0.43–0.94) per 100,000 inhabitants in 2006 to 2.0 (95% CI: 1.58–2.51) per 100,000 inhabitants in 2016 (Figure 2(b)). Again, only the incidence rates between 2006 and 2011 showed a statistically significantly increasing trend (p < 0.001), followed by stable incidence rates from 2011 to 2016 (p = 0.24), despite the initiation of the CRC screening programme in 2014. Incidence rates after standardisation showed relatively lower incidence rates when adjusted for the age distribution of the US and WHO standard population (Supplementary material). The specific primary NET location was available for 978 (92.7%) individuals, of whom 231 (23.6%) were diagnosed with NET in the colon and 747 (76.4%) in the rectum. The specific primary location of the detected NET was missing in 77 (7.3%) people. Of the reported colorectal NETs, 775 (74.3%) were (re)classified as grade I, 103 (9.9%) as grade II, and 11 (1.1%) as grade III NETs. The remaining 166 were in 160 (15.2%) cases (re)classified as carcinoid (grade I or II) and in six (0.9%) cases described as NET grade NOS. We observed a major improvement over time in completeness of pathology reports on histopathological parameters, facilitating correct classification according to the WHO classification. In total, 44.9% of the pathology reports were complete in 2006, whereas in 2016, 98.3% of the reports were complete.
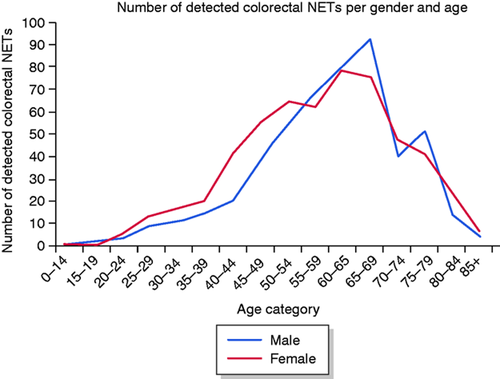
Colorectal neuroendocrine tumours (NETs) per age category and sex.
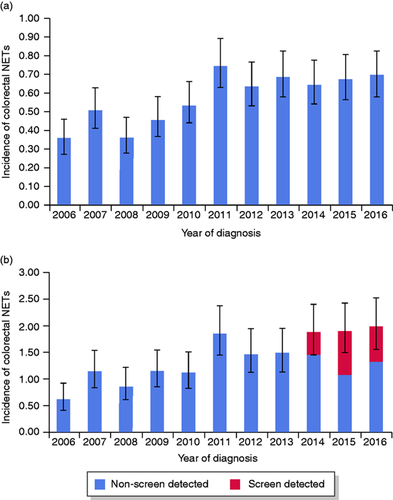
(a) Incidence of colorectal neuroendocrine tumours (NETs) in the total population per year of diagnosis per 100,000 inhabitants. (b) Incidence of colorectal NETs in the target age group for colorectal cancer screening (55–75 years*) per year of diagnosis per 100,000 inhabitants.
*Birth cohorts 1938–1957, of which four were not yet invited for screening because of the roll-out phase of the screening programme.
| Overall | Non–screen detecteda | Screen detecteda | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rectum | Colon | p value | Rectum | Colon | p value | |||||||
| Number of patients | 1055 | 693 | 220 | 54 | 11 | |||||||
| Median age (IQR) | 61 (50–68) | 58 (48–67) | 64 (54–74) | <0.001 | 65.5 (63–69) | 67 (61–74) | 0.92 | |||||
| n | % | n | % | n | % | n | % | n | % | |||
| Sex | ||||||||||||
| Male | 501 | 47.5% | 327 | 47.2% | 98 | 44.5% | 0.54 | 35 | 64.8% | 7 | 63.6% | 1 |
| Female | 554 | 52.5% | 366 | 52.8% | 122 | 55.5% | 19 | 35.2% | 4 | 36.4% | ||
| Grade | ||||||||||||
| I | 775 | 73.5% | 516 | 74.5% | 147 | 66.8% | <0.001 | 48 | 88.9% | 9 | 81.8% | 0.66 |
| II | 103 | 9.8% | 50 | 7.2% | 39 | 17.7% | 5 | 9.3% | 2 | 18.2% | ||
| III | 11 | 1.0% | 3 | 0.4% | 7 | 3.2% | – | – | – | – | ||
| I/IIb | 160 | 15.2% | 124 | 17.9% | 24 | 10.9% | – | – | – | – | ||
| I/II/IIIc | 6 | 0.6% | 0 | – | 3 | 1.4% | 1 | 1.9% | – | – | ||
| Histological diagnosis | ||||||||||||
| Endoscopy | 930 | 88.2% | 676 | 97.5% | 144 | 65.5% | <0.001 | 54 | 100.0% | 11 | 100.0% | 1 |
| Surgery | 114 | 10.8% | 15 | 2.2% | 69 | 31.4% | – | – | – | – | ||
| Autopsy | 7 | 0.7% | 2 | 0.3% | 5 | 2.3% | – | – | – | – | ||
| Radiological biopsy | 2 | 0.2% | – | – | 1 | 0.5% | – | – | – | – | ||
| Unknown | 2 | 0.2% | – | – | 1 | 0.5% | – | – | – | – | ||
| Therapy | ||||||||||||
| Endoscopic local excision | 813 | 77.1% | 634 | 91.5% | 78 | 35.5% | <0.001 | 52 | 96.3% | 7 | 63.6% | 0.006 |
| TEM | 22 | 2.1% | 21 | 3.0% | – | – | – | – | – | – | ||
| Radical resection | 212 | 20.1% | 36 | 5.2% | 137 | 62.3% | 2 | 3.7% | 4 | 36.4% | ||
| Unknown/NA | 8 | 0.8% | 2 | 0.3% | 5 | 2.3% | – | – | – | – | ||
- IQR: interquartile range; NA: not available; NET: neuroendocrine tumour; TEM: transanal endoscopic microsurgery.
- a Excludes 77 patients with a colorectal NET for which the specific primary tumour location was not available.
- b Carcinoid/Suggestive for Grade I or II.
- c NET with no data on histological grade.
Colonic vs rectal NETs
Of the 231 patients diagnosed with NET in the colon, 142 (61.5%) NETs were located in the caecum, 19 (8.2%) in the ascending colon, 12 (5.2%) in the transverse colon and 58 (25.1%) in the descending colon or sigmoid. The incidence of colonic NETs steadily increased from 0.092 per 100,000 inhabitants in 2006 to 0.16 per 100,000 inhabitants in 2016 (p = 0.002).
Of the 747 individuals diagnosed with rectal NETs, 19 (2.5%) NETs were diagnosed in the rectosigmoid and 728 (97.5%) in the rectum. The incidence rate of rectal NETs increased from 0.24 per 100,000 inhabitants in 2006 to 0.56 per 100,000 inhabitants in 2011 (p < 0.001), followed by a stable incidence rate between 2011 and 2016 (p = 0.72).
Screen-detected NETs
Through participation in the CRC screening programme, 68 lesions were detected in the period 2014–2016. This was equal to 19.9% of the total number of NETs detected in that period or 31.9% of the NETs detected in people within the target age group for screening (55–75 years) (Figure 2(b)). Of the individuals diagnosed with a screen-detected NET, 64.7% were male and median age at diagnosis was 65.5 years (IQR = 63–69). The primary location of the screen-detected NETs was available in 95.6%, showing a predominant localisation of the 54 (83.1%) primary NETs in the rectum (Table 1). Grade classification was possible in 98.5%, with 60 (89.6%) NETs classified as grade I and 7 (10.4%) as grade II. Stage classification was possible in 55 (80.9%) screen-detected NETs. Of those, 49 (89.1%) were classified as stage I, three (5.5%) as stage II and three (5.5%) as stage III.
Diagnosis and management
Per location of the primary tumour, the performed therapeutic interventions differed significantly, with local excision more often performed on rectal lesions (94.6%) and radical surgery more often performed on colonic lesions (61.0%) (p < 0.001). Throughout the last decade, local excisions increased both for rectal (87.2% in 2006 vs 95.1% in 2016) (Figure 3(a)) and colonic (6.7% in 2006 vs 51.9% in 2016) (Figure 3(b)) NETs.
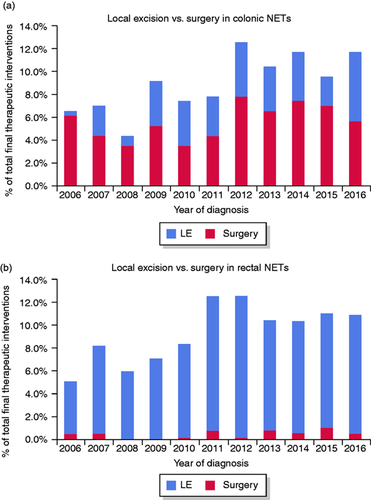
(a) Percentage of total detected neuroendocrine tumours (NETs) in the colon removed by local excision (LE) vs surgery per year of diagnosis. (b) Percentage of total detected NETs in the rectum removed by LE vs surgery per year of diagnosis.
LE includes transanal endoscopic microsurgery.
Screen-detected NETs were in 89.9% removed by endoscopic resection, in 1.4% by TEM and in 8.7% by radical surgery.
Discussion
The incidence of colorectal NETs in the Netherlands doubled from 2006 to 2011 and has remained stable since. The CRC screening programme detected more than 30% of all detected colorectal NETs in the screening target age group, predominantly at an early stage. Local excision both of colonic and rectal lesions has been performed significantly more often in the last decade.
Our study presents a reliable incidence rate because of a well-established nationwide pathology database. The rising incidence in the Netherlands of colorectal NETs between 2006 and 2011 was presumably caused by the growing awareness of colorectal disease and an increase in performed colonoscopies due to better access to health care.29,30 While the CRC screening programme was still in its roll-out period, we already observed a clear contribution of screening to the detection of colorectal NETs in the period 2014–2016. Our results suggest that the number of screen-detected colorectal NETs will increase until the year 2019, at which point the whole target group will have been invited. Also, screen-detected colorectal NETs in our study were for a vast majority (94.6%) detected at an early stage, known to significantly improve prognosis.10, 11, 14, 15 Despite the fact that the stage-distribution was based only on pathology reports, it was in line with previously conducted studies on NETs detected through a CRC screening programme.12,31 Four studies prior to ours reported on NETs detected by CRC screening; however, none quantified the absolute contribution of screening on colorectal NETs incidence.12,31–33
We are the first to focus on colorectal NETs described as in the latest WHO classification (2017), thereby excluding neuroendocrine carcinomas, mixed adeno-neuroendocrine carcinomas and goblet cell carcinoids. Our study presents transparent and well-defined results by using the strict inclusion criteria for colorectal NETs of this classification. A valid comparison with prior study results is restricted by the inclusion of neuroendocrine carcinoma, other conflicting in- or exclusion criteria and the presence or absence of an implemented CRC screening programme.
The obvious shift in the management of colorectal NETs through time to more local resection is presumably due to the detection of NETs at an earlier stage, but also suggests an ongoing optimisation of local interventions. In our study we did not evaluate the effectiveness of the chosen management because we did not include follow-up data.
The incompleteness of data on histological grade in a major portion of the older reports restricts a valid analysis of the change of grade over time. This warrants complete reporting by pathologists on NETs as proposed by international neuroendocrine tumour societies. Interestingly, the completeness in NET pathology reports increased over time, probably facilitated by the implementation of synoptic pathology reporting in the Netherlands. With synoptic reporting, an electronic reporting module is used with standardised reporting terms, multiple-choice answering including mandatory pathology parameters and automated generation of the conclusion, including tumour grade. Synoptic reporting has been shown to result in more-complete pathology reports.34
The strengths of our study are the reliable incidence rates and a well-established pathology database. Furthermore, we standardised the observed incidence rates using several (standard) populations to enable comparison by future studies on this rare tumour (Supplementary material).
Our study has some noteworthy limitations. The selected data from PALGA were manually extracted, and we were unable to distinguish between incidentally and symptomatically detected NETs. Also, the study has a retrospective design whereas the terminology and classification of neuroendocrine lesions has evolved during the analysed period of this study. If needed, NETs were reclassified according to the WHO 2017 NET classification based on available data in the pathology reports, without reassessing the pathology specimen. This could have resulted in misclassified lesions. In particular for the 11/1055 (1.0%) NETs that were classified as grade III, reviewing the pathology specimen might have been needed. However, alternate classification of this small sample size would not have altered our conclusion. Finally, stage classification was available only for screen-detected colorectal NETs, as the pathology reports of this population were relatively well described and sufficient for staging.
It is still unclear whether the rising number of detected colorectal NETs is the result of more prevalent disease or a higher detection rate of previously undetected lesions. Analyses should be performed using prospectively collected data from FIT-based screening programmes to validate our findings.
In conclusion, there has been a significant rise in incidence of colorectal NETs in the last decade. Within the target population of the Dutch CRC screening programme, almost one-third of the colorectal NETs were screen-detected. Management differed significantly among primary locations of the tumours and changed over time, with more local excisions in more recent years.
Acknowledgements
Author contributions include the following: Conception of the study: AIK, WHMV, METT and MEvL. Data collection and analysis: AIK, JGvdB and MEvL. Statistical analysis: AIK and MEvL. Manuscript writing: AIK, WHMV and MEvL. Critical revision of the manuscript: all authors.
Declaration of conflicting interests
None declared.
Ethics approval
This study was approved by the Scientific Board of PALGA complying with the regulations for anonymised (epidemiological) studies on 10 January 2016, and considered to remain outside the restrictions of the Medical Research Involving Human Subjects Act (WMO) by the Medical Ethics Review Committee of the Netherlands Cancer Institute. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed consent
Not applicable as anonymised data were used.