Seroprevalence of Brucellosis in Camels and Goats and Community Awareness Towards Its Zoonotic Risk in Selected Districts of Afar Region, Ethiopia
Abstract
Brucellosis is a highly infectious bacterial zoonotic disease that carries substantial economic and public health implications, especially within pastoral and agropastoral communities in Ethiopia. A cross-sectional study was employed from December 2022 to October 2023 to estimate the seroprevalence of brucellosis among camels and goats and to assess community awareness towards its zoonotic importance in selected districts of Afar, Ethiopia. A total of 528 serum samples were sequentially tested using the Rose Bengal plate test (RBPT) and the competitive enzyme-linked immunosorbent assay (c-ELISA). A questionnaire survey was administered to camel and goat owners to assess community awareness of zoonotic importance of brucellosis. Collected data were entered, coded, and analyzed using SPSS version 27 software. In the current study areas, the overall seroprevalence of camels’ and goats’ brucellosis, based on RBPT as well as c-ELISA, was found to be 3.03% and 2.27% with 95% CI; 0.48–5.53. Moreover, the seroprevalence of brucellosis among the species level was 3.75% (n = 9/240) in camels and 2.43% (n = 7/288) in goats in the study districts. The survey indicated that 85% of the pastoral community in the study areas is unaware of the zoonotic significance of camel and goat brucellosis. Moreover, almost 88% of pastoralists and agropastoralists in the study districts were found to handle abortions and retained placentas with their barehands. The calculated Kappa (κ) statistic was found to be 0.853, indicating an “almost perfect” level of agreement between the RBPT and ELISA serological tests. An increased seroprevalence of camel and goat brucellosis in pastoral communities, along with prevailing animal husbandry practices and raw food consumption habits, suggests that brucellosis could pose a significant public health threat in the study areas. This highlights the need for awareness creation to educate farmers about the disease and promote safer practices, ultimately helping to protect the community from brucellosis.
1. Introduction
Small ruminants are desirable livestock species since they grow more quickly, require less maintenance, have shorter production cycles, and are better adaptable to harsh climates than large ruminants [1]. Camels are a versatile animal species that ensure food security and fulfill the livelihood priorities of pastoral households in Ethiopia’s arid and semiarid areas. They provide pastoral communities with income, food supply, transportation services, and other social benefits such as prestige (social status), ceremonial uses insurance, and risk-buffering options [2]. The ability of camels to survive in harsh areas of the world, their endurance in prolonged drought, and above all, their high potential to convert the scanty resources of the desert into milk and meat make them more important to the pastoralists [1].
Despite Ethiopia having a vast population of small ruminants and camels, the country struggles to fully utilize this resource due to factors such as widespread infectious diseases and inadequate disease management strategies [3, 4]. Among these infectious diseases, brucellosis is a major issue, widely spread across all regions of the country [5], and hampers the productivity of small ruminants and camel populations. Brucellosis is the world’s most common bacterial zoonosis, highly contagious and of significant economic and public health importance. The World Health Organization ranks it as the second most significant zoonotic disease globally, with severe economic and public health impacts in sub-Saharan Africa, including Ethiopia [6, 7].
Brucellosis poses a significant economic and public health threat in pastoral and agropastoral communities. The close interaction with animals, consumption of raw milk, and lack of awareness about zoonotic diseases make it easier for the community to contract brucellosis from livestock [8]. Almost all pastoral and agropastoral communities consume raw milk, which increases the risk [9, 10]. Brucella species are facultative intracellular, Gram-negative, flagellated, immobile, oxidase-negative, catalase-negative, urease-positive, nonspore forming, noncapsulated, and partially acid-fast coccobacilli. They also lack endospores and native plasmids [11]. It is characterized by huge wastage in productivity in terms of abortions (late term), weak calves, stillbirths, and infertility accompanied by placentitis, epididymitis, and orchitis. Camel brucellosis is caused by Brucella abortus (B. abortus), B. melitensis, and B. ovis. Several studies revealed that B. abortus and B. melitensis are most frequently isolated from milk, aborted fetuses, and vaginal swabs of infected camels [12, 13]. Brucella species that occur in camels were linked and associated with those species affecting other animals [14, 15].
Raw milk consumption is common among pastoral communities, posing a significant risk of brucellosis transmission [16]. A study by Fekadu Gutema Wegi [17] reported a 10.5% seroprevalence of cattle brucellosis in Amibara Woreda, Afar Regional State. Similarly, Tschopp et al. [18] recorded seroprevalence rates of 9.0% in livestock and 48.3% in humans in the Afar region, highlighting the economic and public health issues linked to the disease, especially among vulnerable populations [19]. Additionally, due to ongoing drought conditions, camels and goats show a higher survival rate in the region’s changing climate compared to other livestock. Effective control and eradication of brucellosis in camels and goats require a thorough understanding of the disease’s epidemiology and serological patterns in specific geographic areas. Therefore, this study aims to estimate the seroprevalence of brucellosis in camels and goats in the study areas of the Afar region, Ethiopia.
2. Materials and Methods
2.1. Description of the Study Areas
Afar region is located between 39°34′ and 42°28′ east longitude and 8°49′ and 14°30′ north latitude [20]. The region shares common international boundaries with the state of Eritrea in the northeast and Djibouti in the east. The region has boundaries with the Regional States of Tigray in the northwest, Amhara in southwest, Oromia in south, and the Dire dawa federal administration in the southeast. Afar region was found in Great Rift Valley of East Africa. Total human population is estimated to be 1,493,409 Pastoralist Forum Ethiopia, [21]. There are four distinct seasons in the region, namely, Kerma (summer), Hagay (autumn), Gilele (winter), and Sugum (spring), and climatically characterized by an arid and semiarid climate with low and erratic rainfall. The annual temperature and rainfall in the region are 30°C–50°C and 200–600 mm, respectively. The altitude in the region ranges from 100 to 1000 m above sea level [22]. The production system of the region is pastoralism (90%) and agropastoralism (10%). The study was conducted in two woredas: Chifra (zone 1) and Telalak (zone 5) of Afar region as indicated in Figure 1.
2.2. Study Population
The study included camels, goats, and livestock owners in the study areas. Study animals were categorized as adult or old, unvaccinated, and healthy, regardless of sex or age.
2.3. Study Design
A cross-sectional study was employed for seroprevalence estimation of antibodies against Brucella infection from December 2022 to October 2023. Moreover, a questionnaire survey was conducted to assess the zoonotic risk of brucellosis among livestock owners during the study period.
2.3.1. Sampling Method and Sample Collection
A multistage sampling technique combined with the convenient sampling strategy was employed for sampling individual animal species to estimate the seroprevalence of camel and goat brucellosis. Accordingly, the peasant associations (PAs) were regarded as the primary units, the herds as the secondary units, and the individual animals as the tertiary units. Approximately, 6–10 mL of blood sample was collected from the jugular vein of each animal using plain vacutainer tubes, needle holders, and needles. The collected blood samples were allowed to clot at room temperature. Then, the serum was separated and decanted to screw-tight 1.5 mL Eppendorf tubes. Collected sera will be stored at −20°C until the laboratory tests are performed using the modified RBPT (mRBPT) and indirect enzyme-linked immunosorbent assay (I-ELISA).
2.3.2. Sample Size Determination for Seroprevalence Estimation
The minimum required sample size was calculated to be 96 small ruminants. However, to increase precision and minimize standard error, three-fold increases in the required sample size were obtained by calculation, and 288 small ruminants were considered from the study area. Then sampling was proportionally distributed based on the total small ruminant population in the study area. For the questionnaire survey, the sample size was calculated using the formula given by Arsham [27], N = 0.25/SE2, where N = sample size and SE (standard error) = 5%. Thus, the required sample size for the questionnaire survey was 100.
2.4. Questionnaire Administration
A semistructured questionnaire was prepared to document information on zoonotic brucellosis risk assessment as well as potential risk factors for brucellosis. Questionnaires were incorporated with demographic questions for participants. The questionnaire was translated into the local languages (Afar af), back-translated into English for translation consistency check, and pretested among households in the region. In this study, the owners of the sampled camels and goats, and interested individuals were interviewed using a semistructured questionnaire. The questionnaire focused on the knowledge, attitude, and practice (KAP) of the community about the zoonotic spread of brucellosis from camels and goats to humans.
2.5. Serological Laboratory Analysis
2.5.1. RBPT
The modified RBPT was used as a screening test to detect Brucella agglutinins in all collected sera, following the OIE [28] protocol. The test was conducted at the Bacterial Serology Unit, Animal Health Institute (AHI), Sebeta. Briefly, 30 μL of stained Rose Bengal antigen and 75 μL of serum were mixed on a card plate and observed for agglutination. Positive and negative controls were included. Reactions were graded as 0, +, ++, or +++, with any degree of agglutination (+ to +++) considered positive, and 0 recorded as negative.
2.5.2. I-ELISA
All RBPT-positive samples were confirmed using an I-ELISA at the National AHI, following the manufacturer’s protocol. The test detects anti-Brucella lipopolysaccharide antibodies. After bringing reagents and samples to room temperature, sera, controls, and diluents were added to 96-well plates. The plates were incubated, washed, and treated with conjugate, substrate, and stop solution. Optical density was measured at 450 nm using an ELISA reader to determine results.
2.5.3. Comparison of Serological Tests
2.6. Ethical Considerations and Consent to Participate
Ethical clearance for this study was obtained from Animal Health Institution by the Research Ethical and Review Committee with certificate Ref. No: RERC0004/12/2022. Before sample collection, the owners of the animals were informed about the objectives of the study, and verbal consent had been obtained to take blood samples from camels and goats, and this issue was included in the ethical clearance obtained.
2.7. Data Management and Analysis
The data gathered through the laboratory analysis and questionnaire survey were coded and stored in a Microsoft Excel spreadsheet and analyzed using STATA version 14.0 for Windows (Stata Corp. College Station, USA). Seroprevalence was computed by dividing the total number of camels and goats tested positive by confirmatory test I-ELISA by the total number of sheep and goat sera tested. Chi-square (X2) was used to assess the association between the demographic characteristics of the participant to knowledge and practice score. A CI of 95% and 5% cut-off value was set for significance. For all analyses, p < 0.05 was taken as statistically significant.
3. Results
3.1. Sociodemographic Characteristics of Study Participants
In the present study, a total of 528 study animals and 100 KAP respondents were recruited from the study districts to estimate the seroprevalence of camel and goats as well as to assess the community’s KAP towards brucellosis, respectively. The majority of the study respondents, 70% (n = 70) were males, and also 62% of the respondents were between 35 and 55 years. The demographic characteristics of the study population are presented in Table 1.
| Variables | Category | Sample size | Percentage |
|---|---|---|---|
| Gender | Male | 70 | 70 |
| Female | 30 | 30 | |
| Age | 18–35 | 18 | 18 |
| 36–55 | 62 | 62 | |
| 56–85 | 20 | 20 | |
| Woreda | Chifra | 50 | 50 |
| Telalak | 50 | 50 | |
| Marital status | Married | 91 | 91 |
| Unmarried | 9 | 9 | |
| Schooling stage | No education | 87 | 87 |
| Primary education | 5 | 5 | |
| Secondary education | 8 | 8 | |
| Livelihood | Pastoralist | 85 | 85 |
| Agropastoralist | 15 | 15 | |
| Total | 100 | ||
3.2. Seroprevalence of Camel and Goats Brucellosis
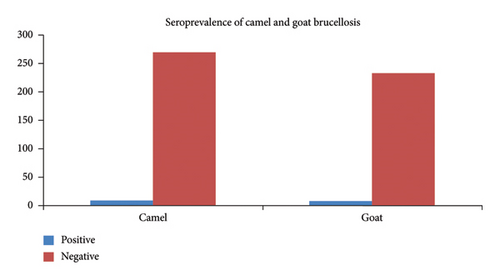
In the current study, out of 528 sera collected (240 camels and 288 goats) and screened using RBPT, only 16 (3.03%) samples were found to be positive for Brucella antibodies. Of these RBPT positive sera, 12 samples were also found to be positive by I-ELISA. Therefore, in the current study areas, the overall seroprevalence of camels’ and goats’ brucellosis, based on RBPT as well as I-ELISA, was found to be 3.03% and 2.27% with 95% CI; 0.48–5.53. Moreover, the seroprevalence of brucellosis among the species level was found to be 3.75% (n = 9/240) in camels and 2.43% (n = 7/288) in goats in the study districts. Among Brucella confirmed samples, 11 sera (5 of them from goats and 6 from camels) were detected from Chifra district and 5 positive samples (2 sera from goats and 3 sera from camels) were obtained from Telalak district. The seroprevalence of brucellosis among the species level was found 3.21% in camel and 2.92% in goats in the study districts of Afar region as indicated in Figure 2.
3.3. Diagnostic Serological Test Characteristics
The test characteristics of RBPL for the detection of Brucella infection in camels and goats were evaluated against ELISA results. In this study, out of 528 serum samples tested using RBPT and ELISA serological tests, 12 were identified as TPs. Moreover, four samples were FPs, none of the samples were FNs, and 512 were confirmed as TNs, as presented in Table 2. In this case, the disease prevalence (ELISA+/total sample size) and apparent prevalence (RBPT+/total sample size) of Brucella infection were found to be 2.27% (n = 12/528) and 3.03% (n = 16/528), respectively, in the study areas. In general, the diagnostic test performance parameters of RBPT, using ELISA as the gold standard, are presented in Table 2.
| Parameter | Formula | Calculation | Result (95% CI) |
|---|---|---|---|
| True positives (TP) | RBPT+ ∩ ELISA+ | 12 | 12 |
| True negatives (TN) | RBPT− ∩ ELISA− | 512 | 512 |
| False positives (FP) | RBPT+ ∩ ELISA− | 16 − 12 = 4 | 4 |
| False negatives (FN) | RBPT− ∩ ELISA+ | 0 (assumption) | 0 |
| Sensitivity (recall) | TP/(TP + FN) | 12/(12 + 0) | 100% (75%–100%) |
| Specificity | TN/(TN + FP) | 512/(512 + 4) | 99.22% (98%–100%) |
| Positive predictive value (PPV) | TP/(TP + FP) | 12/(12 + 4) | 75% (51%–90%) |
| Negative predictive value (NPV) | TN/(TN + FN) | 512/(512 + 0) | 100% (99%–100%) |
| Accuracy | (TP + TN)/total | (12 + 512)/528 | 99.24% |
| False positive rate | FP/(FP + TN) | 4/516 | 0.78% |
| False negative rate | FN/(FN + TP) | 0/12 | 0% |
| Disease prevalence | (ELISA+)/total | 12/528 | 2.3% |
| Apparent prevalence | (RBPT+)/total | 16/528 | 3.03 |
In this study, the calculated Kappa (κ) statistic value was found to be 0.853, indicating that an almost perfect level of agreement between the RBPT and ELISA serological tests, as reported by Carrouel et al. [29].
3.4. Community Awareness Towards Livestock Brucellosis
3.4.1. Knowledge of the Respondents About Brucellosis of Camel and Goat Farmers
According to the questionnaire survey result, the majority of respondents (85%) were not aware of the zoonotic potential risks associated with camel and goat brucellosis. In addition, almost 88% of study participants of pastoralists and agropastoralists in study districts assist camels and goats during parturition and in removing retained fetal membranes with barehands as shown in Table 3.
| Factors | Categories | Number of respondents | Percentage |
|---|---|---|---|
| Knowledge of the presence of zoonotic camels or goat disease | Yes | 15 | 15 |
| No | 85 | 85 | |
| Camel or goat raw milk is healthier and more nutritious than boiled | Agree | 91 | 91 |
| Disagree | 9 | 9 | |
| No risk of zoonotic disease delivering camels or goats with barehand | Agree | 88 | 88 |
| Disagree | 12 | 12 | |
| Only sick camels or goats could be a source of zoonotic disease | Agree | 85 | 85 |
| Disagree | 15 | 15 | |
| Milk-borne sicknesses are mortal | Agree | 85 | 85 |
| Disagree | 15 | 15 | |
| Priorities’ zoonotic camels or goats disease | Brucellosis | 7 | 7 |
| Tuberculosis | 3 | 3 | |
| Typhoid | 5 | 5 | |
| We don’t know | 85 | 85 | |
| Have you encountered reproductive disease events on camels or goats? | Agree | 93 | 93 |
| Disagree | 7 | 7 | |
3.4.2. Attitude of the Respondents Towards Brucellosis of Camel and Goat Farmers
Attitude analysis of this study respondents revealed that 87 (87%) need to know more information about the disease. More than half 73% did not believe that personnel working mostly with camels and goats exposed to the Brucella infection are at high risk of infection as indicated in Table 4.
| Factors | Categories | Number of respondents | Percentage |
|---|---|---|---|
| Do you need to know more information about the disease? | Yes | 87 | 87 |
| No | 13 | 13 | |
| Do you believe that you personnel working frequently with the camels and goats exposed to the Brucella infection are at high risk of infection? | Yes | 27 | 27 |
| No | 73 | 73 | |
| Do you believe that delivered camels and goats manage in separate rooms is important in disease prevention? | Yes | 38 | 38 |
| No | 62 | 62 | |
3.5. Practices of Respondents to Protect Against Zoonotic Camel or Goat Disease
Regarding the practice of the respondents, about 86% of the interviewed study participants slaughter their camels or goats at home while they display repeated clinical symptoms of abortion. Furthermore, this finding indicated that 93% of the participants handle aborted fetuses and placental membranes with barehands and, at the same time, provided/gave aborted fetuses to dogs as summarized in (Table 5).
| Factors | Categories | Number of respondents | Percentage |
|---|---|---|---|
| Do you slaughter camels or goats with repeated clinical symptoms of abortion at home? | Yes | 86 | 86 |
| No | 14 | 14 | |
| Do you assist camels or goats delivery with barehands? | Yes | 93 | 93 |
| No | 7 | 7 | |
| Do you throw away or dump aborted fetuses into the environment | Yes | 96 | 96 |
| No | 4 | 4 | |
| Do you mix your camels or goats with other herds when they become sick? | Yes | 86 | 86 |
| No | 14 | 14 | |
| Technique of inspection milk excellence | Boiling test | 11 | 11 |
| Organoleptic | 89 | 89 | |
3.6. Knowledge Score of the Study Participants
Analysis of the association between knowledge score and demographic characteristics of the respondents showed that educational level was found statistically significant association (X2 = 46.67; p value < 0.001) with 85 (85.0%), and 15(15.0%) of the participants had poor, and good knowledge scores, respectively. Moreover, gender, age, and marital status were found statistically significant associations with knowledge score as indicated in Table 6.
| Variable category | Good knowledge | Poor knowledge | X2 | p value |
|---|---|---|---|---|
| Gender | ||||
| Male | 6 | 64 | ||
| Female | 9 | 21 | 7.56 | 0.006∗ |
| Aged | ||||
| 18–35 | 11 | 7 | ||
| 36–55 | 3 | 60 | 36.06 | < 0.001∗ |
| 56–85 | 1 | 18 | ||
| Marital status | ||||
| Married | 9 | 82 | 20.70 | < 0.001∗ |
| Unmarried | 6 | 3 | ||
| Education level | ||||
| Uneducated | 5 | 82 | ||
| Elementary | 3 | 2 | 46.76 | < 0.001∗ |
| High school | 7 | 1 | ||
| Livelihood | ||||
| Pastoralist | 6 | 79 | 28.02 | < 0.001∗ |
| Agro-pastoralist | 9 | 6 | ||
| Study areas | ||||
| Chifra | 9 | 41 | ||
| Talalak | 6 | 42 | 0.70 | 0.40 |
- ∗The association value of variables with overall knowledge.
3.7. Practice Score of the Participants
The sociodemographic characteristics of the respondents and practice score of this study showed that practice score was significantly associated with age, marital status, and educational status. Besides this, 32%, 32%, and 32% were practicing in a good manner, respectively (Table 7).
| Variable category | Good practice | Poor practice | X2 | p value |
|---|---|---|---|---|
| Gender | ||||
| Male | 19 | 51 | ||
| Female | 13 | 17 | 2.53 | 0.089 |
| Aged | ||||
| 18–35 | 13 | 5 | ||
| 36–55 | 15 | 48 | 16.37 | < 0.001∗ |
| 56–85 | 4 | 15 | ||
| Marital status | ||||
| Married | 25 | 66 | 9.53 | 0.002∗ |
| Unmarried | 7 | 2 | ||
| Education level | ||||
| Uneducated | 24 | 63 | ||
| Elementary | 2 | 3 | 7.72 | 0.021∗ |
| High school | 6 | 2 | ||
| Livelihood | ||||
| Pastoralist | 24 | 61 | ||
| Agro-pastoralist | 8 | 7 | 3.61 | 0.06 |
| Study areas | ||||
| Chifra | 17 | 33 | ||
| Talalak | 15 | 35 | 0.18 | 0.668 |
- ∗The association values of variables with practice score.
4. Discussion
Brucellosis poses a significant economic and public health issue, especially in pastoral communities where livelihoods are deeply intertwined with livestock populations. In the current study areas, the overall seroprevalence of brucellosis in camels and goats, based on RBPT and I-ELISA, was found to be 3.03%. Furthermore, the seroprevalence among species was found to be 3.75% in camels and 2.43% in goats within the study districts. As previous studies indicate, the seroprevalence of brucellosis in camels and small ruminants across Ethiopia’s various agroecologies ranges from 0.73% to 12.2% and 1.2%–10.2% in the respective species. The findings of the current study regarding camel brucellosis align with previous reports, showing 3.1% in Yabello District of Borena Zone [30], 3.37% and 3.67% in Mehoni District of Southeastern Tigray [9, 31], and 3.0% in Southern lowland Ethiopia [32]. Additional reports indicate 2.43% in Jijiga and 3.1% in Babile districts of eastern Ethiopia [33] and 3.67% in Iraq [34]. These similarities may be due to comparable agroecological conditions and livestock management systems. However, the findings of the current study are lower than those reported in other regions and countries: 5.7% in Afar, Somali, and Borena [35], 7.6% in Awash Fentale and Amibara districts of Afar region [36], and 5.4% in four districts of Afar Regional State [37]. Higher prevalence rates have also been reported in Sudan (30.5%) [38], Egypt (7.61%) [39], and Jordan (19.4%) [40]. These variations in the seroprevalence of camel brucellosis may be attributed to differences in the management and husbandry practices, the virulence of the organism, the coverage and quality of veterinary services, awareness levels, animal susceptibility, and unrestricted movement among pastoralists. In contrast, the seroprevalence observed in the current study is higher than the rates reported in previous studies, including 0.4%–2.5% in Borena, Ethiopia [41], 2.43% in Jigjiga and Babile districts of the Somali Region, Ethiopia [33], 0.3%–1.9% in Somalia [42, 43], 1.8% and 1.5% in other areas of Ethiopia [44], and 1.4% in Saudi Arabia [45]. These variations in seroprevalence may be due to differences in sample size, lifestyle, diagnostic methodologies, the socioeconomic conditions of the study population, and agroecological factors.
The current study found a seroprevalence of goat brucellosis at 2.43% (n = 7/288). In contrast, previous studies reported higher seroprevalence rates: 13.6% in Tallalak, Afar region [46]; 13.7% in Chifra and Ewa districts, Afar region [47]. These differences could be due to variations in agroecological conditions, sampled animal composition, sensitivity and specificity of serological tests, management practices, sample sizes, breeds, flock sizes, and animal movement from brucellosis-prevalent areas. Conversely, the current study’s findings are higher than those reported by Bekele et al. [48] at 0.4%–2.5%, Gumi et al. [8] at 0.9%, and Tesfaye et al. [19] at 0.53%.
Awareness and understanding of brucellosis among high-risk groups are essential for influencing patients’ health-seeking behaviors and controlling its transmission among animals and humans in communities. In the present study, 85% of the pastoral community in the study area is unaware of the zoonotic significance of livestock brucellosis, meaning only 15% (n = 15/100) of respondents are knowledgeable about camel and goat brucellosis. The current result aligns with findings of 18% by Deka et al. [49] in peri-urban and rural areas of Assam and Bihar in India, as well as Bashahun [50] in the Jima zone, where 97% of respondents lacked awareness of the zoonotic importance of brucellosis. It also supports the systematic review and meta-analysis by Zhang et al. [7], which indicated the lowest knowledge level of brucellosis in Ethiopia (17.3%) among African countries. However, this result is significantly lower than the 79% reported by Obonyo and Gufu [51] in pastoral communities in Kenya; 70% by Arif et al. [52] in smallholder dairy livestock owners in Pakistan; and 59.9% by Cloete et al. [53] in communal cattle keeper groups in South Africa. Conversely, the current study’s findings surpass those of Kuma et al. [54] in the Jimma zone, Girma [55] in Debre-Birhan Town, and Gichamo et al. [56] in Southern Ethiopia, where knowledge levels were 0%, 2.2%, and 3.8%, respectively. These variations in knowledge across different regions and countries may result from factors such as access to formal education, prior exposure to bovine brucellosis, health education initiatives, extension services, and collaboration between animal and human health sectors. Other factors include societal willingness to participate in awareness campaigns, accountability of health extension workers, and the attention given by government and health professionals to provide health education [7].
The attitude analysis of the study respondents revealed that nearly 87% expressed a need to learn more about brucellosis, especially from animal health professionals. This finding aligns with Obonyo and Gufu [51] in Kenya, where 97% of respondents sought more information about the disease, preferring sources such as local FM radio stations (39%), religious leaders (25%), local community meetings (20%), and community health workers/animal health workers (16%). Differences in preferred information sources may result from the availability and reliability of these sources. In contrast, Lindahl et al. [57] found that only 63% of families wanted additional knowledge about brucellosis. Additionally, 27% of participants believed that individuals frequently working with Brucella-exposed camels and goats are more likely to contract the infection. However, 73% did not perceive a significant risk, as they primarily worked with animals already exposed to the disease.
Similar to the current study, Lindahl et al. [57] found that 14.7% of respondents thought people who worked with livestock that were exposed to the disease were highly likely to contract an infection.
In the current study, only 7% of respondents wore protective gloves, while 93% handled aborted fetuses and placental membranes with their barehands. Similarly, Wakene and Mamo [58] reported that 90.5% of respondents in the Yabello districts of Borena Zone, Oromia regional state, handled retained placentas and aborted fetuses with barehands, and Musallam et al. [59] found that only 6% of livestock owners used protective clothing for such tasks. Egyptian herders also reportedly never wore masks or protective clothing when handling placentas and aborted fetuses [60]. These behaviors likely contribute to the spread of camel and goat brucellosis, yet livestock owners continue them due to limited access to protective gear and inadequate understanding. Among the participants in the current study, 89% disposed of aborted fetuses in the environment, 4% buried them, and 7% gave them to dogs. Jilo [61] reported similar findings in the pastoral community of Borena, with 87.88% of respondents disposing of aborted fetuses in the environment. In contrast, 94% of dairy farmers in Tajikistan reportedly buried birth materials and aborted fetuses [57]. Brucella species can survive for 150–240 days in water, dung, and aborted fetuses [15] and for several months in humid environments (soil and manure) [62]. Disposing of aborted fetuses into the environment is a major risk factor for human brucellosis and facilitates the transmission and persistence of the pathogen. Therefore, it is crucial to address these risky practices through awareness programs for livestock owners and the entire community in the study area.
A descriptive analysis of factors affecting livestock owners’ knowledge and practice scores revealed that about 15% of respondents had good knowledge scores, while 80% had poor knowledge scores regarding the zoonotic risks of camel and goat brucellosis. This is consistent with reports from Tajikistan [57], northern Uganda [63], and Nigeria [64], which also found poor knowledge scores. Conversely, studies in Egypt [65] and Jordan [59] reported good knowledge scores among their participants. Furthermore, 32% of respondents had good practice scores related to camel and goat brucellosis, while 68% engaged in risky practices that could expose them to the disease. In South Africa, Cloete et al. [53] reported overall poor to average practice scores, along with high-risk behaviors, in the community. Similar findings were reported in Egypt [65], Tajikistan [57], Jordan [59], Nigeria [64], and northern Uganda [63], highlighting high-risk activities such as handling abortion and placental membranes without protection and consuming raw milk and its products.
5. Conclusion and Recommendations
This study confirmed the presence of camel and goat brucellosis in the study areas, with seroprevalence rates of 3.03% in camels and 2.27% in goats, as determined by RBPT and I-ELISA. The findings indicate a significant risk of Brucella infection, primarily due to raw milk consumption and shared milking practices among family members and neighbors. Moreover, the study revealed low awareness of brucellosis and high-risk behaviors, including handling livestock birth products, which contribute to zoonotic disease transmission. Addressing these risks requires community-based training to raise awareness of brucellosis and its health implications. Further epidemiological studies, including the isolation and identification of Brucella biotypes in camels and goats, are needed to better understand the role of livestock in zoonotic brucellosis.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Teshager Dubie contributed to the conception of the research idea, designing and data collection, data analysis, interpretation of data, and writing and editing of the manuscript.
Ahmed Seid contributed to the conception of the research idea, data collection, methodology, and writing and review of the manuscript.
Ashenafi Syoum contributed to the conception of the research idea, data collection, methodology, and writing and review of the manuscript.
Fanuel Bizuayehu Yihunie contributed to the conception of the research idea, data analysis, and supervision.
Funding
No funding was received for this manuscript.
Acknowledgments
The authors would like to express their gratitude to the Animal Health Institute for the laboratory space it provided for this study. Moreover, the authors would like to express our sincere gratitude to the study districts’ livestock owners and animal health staff for their cooperation and technical support during field sample collection.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




