Assessment of Margin Status in Feline Mastectomy: Predictive Factors and Agreement Between Intraoperative Cytology and Histopathology
Abstract
This study aimed to determine the agreement between margin status in feline mammary tumors’ surgery by comparing 3 margin assessment techniques and to determine the predictive value of prognostic clinical and histological parameters for margin status using intraoperative margin assessment techniques. During the intraoperative period, 69 surgical margins from 12 female cats undergoing unilateral radical mastectomy were assessed by imprint cytology (IC) and scrape cytology (SC), both intraoperative cytological techniques. The excised mastectomy specimens were then evaluated postoperatively by histopathology (HP). Cochran’s Q test was used to determine differences in positive margin detection for the three methods. The agreement between the three methods was assessed using the Fleiss kappa coefficient. A binary logistic regression model was used to assess whether clinical and histological prognostic parameters could predict intraoperative margin status. There was no significant difference in positive margins between the three methods (p = 0.174). The agreement between the three methods was poor (Fleiss kappa = −0.020; 95% CI −0.156 to 0.117). Lymphovascular invasion is a significant predictor of a positive intraoperative margin, with an OD of 18.652 (95% CI = 1.742–199.652). These findings suggest that IC and SC are viable methods for assessing the status of surgical margins during surgery for feline mammary tumors. Intraoperative cytology may be complementary to HP, particularly in cases of lymphovascular invasion.
1. Introduction
In oncological surgery, one of the main goals is the removal of all neoplastic cells and achieving a clean margin status to prevent local recurrence and prolong the patient’s life [1–3]. Accurate margin assessment is critical for minimizing morbidity and/or mortality [4]. Histopathology (HP) is the recommended protocol to determine appropriate excision; however, HP only evaluates a small fraction of surgical margins and its accuracy can be affected by several factors such as histologist expertise, methods of margin analysis, tissue shrinkage, artefactual distortions, tissue inking, tumor type, and effective communication between surgeons and pathologists [4, 5]. Simply identifying residual tumor cells within the resected tissue does not provide a reliable prediction of the risk of local recurrence after surgery [3]. To improve patient care, it is necessary to develop or improve techniques with superior recognition of residual tumor cells during the intraoperative period and within the surgical field [3, 4].
Real-time intraoperative assessment of clean surgical margins has been the focus of numerous studies in human medicine [2, 6–8]. However, few studies have been performed in veterinary medicine [4]. Imprint cytology (IC) [4, 7, 9, 10] and scrape cytology (SC) [8, 11] are simple intraoperative cytology techniques, which are inexpensive and easy to implement. Both have high accuracy rates [9, 11, 12], excellent preservation of cellular details, and the possibility of identifying focal, microscopically undetectable neoplastic lesions in large tissue fragments [7] that could be helpful in feline mastectomies. IC and SC have a high accuracy rate of differentiation between benign and malignant tumors [11]. When comparing the 2 techniques, SC allows obtaining a larger number of cells in smears [11]. SC applied to the surgical field may have an advantage over other techniques such as the shaved margin technique, since SC does not remove tissues, which is useful when the operative field is close to the vital structures. According to D’Halluin [7], IC is a quick technique and has a good sensitivity (88.6%), specificity (92.2%), and correlation with histology (91.5%) and prevented 11.75% of secondary re-excision procedures for positive margins.
Feline mammary tumors (FMTs) can be malignant in 80%–90% of clinical cases [3, 13–15]. Surgery is the main treatment recommended [13, 14] and incomplete surgical resection or positive margins are common [16]. Prognostic parameters for FMTs such as the number and size of tumors, cutaneous ulceration, lymphovascular invasion, lymph node invasion, and tumor grade are related to overall survival [16] and may affect surgical planning. A clean margin status is associated with longer disease-free intervals, and overall survival, so proper surgical management of FMT favorably impacts the outcome [13, 15, 16]. For these reasons, despite proper surgical planning [14], intraoperative margin assessment methods such as IC and SC may help to detect the presence of neoplastic cells in surgical margins and allow their correction during the surgical procedure.
With this study, we aim to determine the agreement between margin status with IC, SC, and, HP in unilateral radical mastectomy for FMT and evaluate the effect of prognostic parameters on surgical margin status. We hypothesized that intraoperative methods and HP will have an equal positive margin detection rate in FMT surgery. We also hypothesized that prognostic factors may be predictors for positive intraoperative and HP margins.
2. Materials and Methods
Twelve client-owned female cats with surgical indications for mastectomy were recruited. The study was carried out over a consecutive 16-month period (February 1, 2022–May 31, 2023) and took place at the veterinary teaching hospital of the Faculty of Veterinary Medicine, Lusófona University, in Lisbon, Portugal. Ethical approval was obtained from the Comissão de Ética e Bem-Estar Animal (CEBEA) from the Faculty of Veterinary Medicine of Lusófona University (17-2022). Written informed consent was obtained from all owners. Female cats undergoing surgery were previously evaluated by an oncologist and performed oncological staging according to the 2016 AAHA Oncology Guidelines for Dogs and Cats [17].
All surgeries were performed by 3 experienced surgeons (JM, PC, or LM). Exclusion criteria included animals with detectable tumors, other than mammary tumors adjacent to the mammary chain or close or within the delineated margins of the surgical field.
To determine the required sample size for the study, a priori power calculation was performed using the G ∗Power 3.1.9.7 statistical package program. With a specified alpha threshold of 0.05, an OR of 2.2%, and 80% power, a minimum of 12 animals (68 samples) were necessary to test the study hypothesis. Eleven unilateral radical mastectomies and 1 partial cranial unilateral mastectomy were included.
2.1. Surgical Technique
Cats were placed in dorsal recumbency. A skin incision was made around the four left or right mammary glands, leaving a margin of at least 2 cm around the tumor(s) [18]. The deep muscular fascia of the tumor(s) was excised to remove a minimum of 2 cm of macroscopically healthy tissue around the tumor (s) [18]. Metzenbaum scissors were used to undermine the tissue in a cranial to caudal direction [18].
Axillary lymph node excision was performed, and the inguinal lymph node was removed together with the most caudal mammary gland [18]. The cranial and caudal epigastric vessels were double ligated with 2-metric absorbable glyconate monofilament (Monosyn; B. Braun Surgical S.A., Spain). Forceps were applied distal to the ligatures and the vessels were transected between the forceps and the ligatures [18].
After sampling from the surgical field, washing was performed with warm lactated Ringer’s solution (Lactated RingerVet; B. Braun Medical Inc., Germany). Gloves, surgical drapes, and surgical instruments were changed.
The subcutaneous tissue was closed in a simple continuous pattern with a 2-metric absorbable glyconate monofilament (Monosyn; B. Braun Surgical S.A., Spain). The skin was closed with a 2-metric absorbable glyconate monofilament (Monosyn; B. Braun Surgical S.A., Spain) in a simple continuous intradermal pattern or with an interrupted suture pattern using nylon (Dafilon; B. Braun Surgical S.A., Spain).
2.2. Sample Collection
Since mastectomy specimens and operative field surfaces measure more than 5 cm, so to standardize and simplify intraoperative sample collection, both surfaces were divided into the cranial surface (first and second mammary gland) and the caudal surface (third and fourth mammary glands) (Figure 1). Samples from the cranial and caudal aspects of the mammary gland were collected from medial, lateral, and deep margins, resulting in 6 glass slides to cover the entire mammary gland surface (IC) and 6 glass slides to cover the surgical field (SC) (Figure 2).
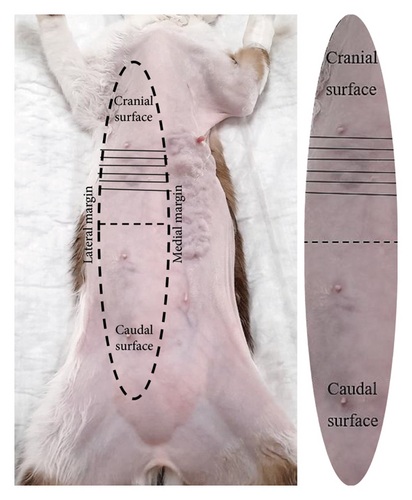
2.3. IC
All imprints were identified and prepared from each ex vivo mastectomy specimen after surgical excision. Each identified glass slide was gently pressed against each freshly cut mastectomy specimen margins of interest (e.g. cranial surface: lateral, medial, and deep and caudal surface: lateral, medial, and deep).
2.4. SC
All imprints were identified and prepared from the surgical field margins with a gentle # 24 scalpel blade scraping to each surface of interest (e.g. cranial surface: lateral, medial, and deep and caudal surface: lateral, medial, and deep) and extension on a glass slide.
All glass slides from IC and SC methods were allowed to air dry and stained manually with a rapid aqueous-based Romanowsky stain (Hemacolor, Merck KGaA, Darmstadt, Germany). Cytology samples were read by an experienced clinical pathologist (JF) after the surgical procedure, who was unaware of the HP margin status. Also, indications of the precise site of the lesion were described as follows: cranial and caudal aspects of lateral, medial, or deep regions.
2.5. HP
The excised mastectomy specimens were identified with sutures [19] in 4 directions: Cranial-1 suture, Caudal-2 sutures, Lateral-3 sutures, and Medial-4 sutures. After 10–25 min of air drying at room temperature and after suturing and collecting samples for IC, mastectomy specimens were immersed in 10% neutral buffered formalin at a ratio of 1:10 tissue/formalin volumetric ratio and submitted for routine HP.
The samples of the study were trimmed and read by two anatomic pathologists (PF and JC). To confirm the diagnosis and quantify the histologic tumor-free margin of the areas of interest (e.g. cranial surface: lateral, medial, and deep and caudal surface: lateral, medial, and deep), complete bread loafing sections were trimmed (Figure 3). All trimmed samples were processed for routine HP at the Laboratório de Análises Clinicas e Histológicas Veterinárias in Universidade Lusófona. FMTs were evaluated, and morphological diagnosis was performed following the most recently validated scheme [20]. Histological grading was performed following both Ellston and Ellis (NHG) [21] and Mills et al.’s grading systems [22]. The axillary and superficial inguinal lymph nodes were evaluated for the presence of metastases.
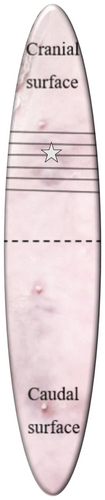
 ).
).2.6. Evaluation of Margin Status
All individual margins (n = 69) were evaluated in the areas of interest by IC, SC, and complete bread loafing sections’ HP and determined as positive or negative for tumor cells.
On cytologic evaluation, the IC and SC margin smears were scanned at 10x objective to address the cellularity, presence of clusters, and background features and at 40x and 100x objectives to evaluate cellular and nuclear details. The smears were defined as positive for malignant cells if at least one cluster of epithelial cells showed more than three cytological criteria of malignancy and negative if the slides only contained blood, fat droplets, adipocytes, muscle fibers, inflammation, or monomorphic epithelial cells with no or mild features of malignancy (Figure 4). We used the cytological features of malignancy that are mentioned in most cytological research and veterinary cytology textbooks: pleomorphism; anisocytosis and macrocytosis; variable nuclear size and shape; large, prominent, bizarre or multiple nucleoli; nuclear molding; and alteration of chromatin pattern [23–26].
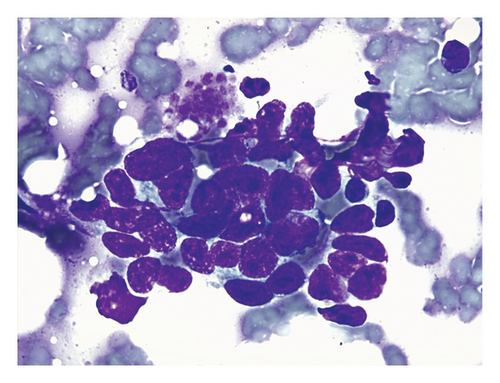
On bread loafing sections’ HP, the margin was defined as positive or incomplete if neoplastic cells were present in the surgical margin (0 mm of tumor-free margin). The margin was defined as negative if no neoplastic cells contacted with the surgical margin. Inside the negative margin category, margins were classified as narrow (0–2 mm to the surgical margin) and clean (> 2 mm to the surgical margin) [13].
2.7. Statistical Methods
Statistical analysis was performed using SPSS software (IBM SPSS Statistics for Windows, Version 28.0. Armonk, New York: IBM Corp.). Cochran’s Q test was used to test for differences in positive margin detection rates among the 3 methods (IC, SC, and HP). Fleiss kappa value was calculated to test the agreement among the IC, SC, and HP. We used a binary logistic regression model to evaluate if the prognostic parameters were able to predict the status of intraoperative and HP margins.
Results were considered statistically significant when p < 0.05.
3. Results
In this study, 69 margins from 12 mastectomy specimens were included. Mastectomy specimens were from 12 female cats with mean ± standard deviation body weight and age of 3.9 ± 0.9 kg and 12 ± 3.3 years, respectively. This study included 11 domestic short-haired cats and 1 Persian cat. From these, 5 were intact females, and 7 were spayed females. Regarding tumor location, 11 cats had a bilateral presentation and 1 unilateral. Five cats were treated with left radical mastectomy, 6 right radical mastectomies, and 1 left partial mastectomy (who had a partial mastectomy years before). Information summarized in Table 1 shows that cats included in the study presented different tumor grading. Regarding the number of tumors present in each mastectomy specimen, 1/12 (8.3%) had 1, 4/12 (33.3%) had 2–5 cm, and 7/12 (58.3%) had more than 5. The mean ± standard deviation for the largest tumor size in each mastectomy specimen was 2.8 ± 1.8 cm. Cutaneous ulceration was present in 4/12 (33%) cats and lymphovascular invasion was exhibited in 2/12 (16.7%), both demonstrated positive margins in intraoperative cytology (IC and/or SC). In 6/12 (50%) cats, lymph nodes were positive on HP. According to TNM stage (14), 1/12 (8.3%) cats were Stage I, 1/12(8.3%) were Stage II, 6/12 (50%) were Stage III, and 3/12 (25%) were Stage IV. Finally, during the study period (16 months), 3 cats were euthanized with complications related to mammary tumors.
| Cat | No tumors | Largest tumor size (cm) | Cutaneous ulceration | L. invasion | Lymph node | HP classification [20] | HP grading ([21], NHG) | HP grading [22] | TNM stage | Method | Follow-up | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC | SC | HP | |||||||||||
| 1 | 2–5 | 3.5 | + | — | +si | Comedocarcinoma | Grade III | Grade III | Stage III | — | — | — | Died |
| 2 | 2–5 | 1.5 | — | — | +a | Tubulopapillary carcinoma | Grade II | Grade III | Stage III | — | — | — | Alive |
| 3 | > 5 | 6.5 | — | — | − | Tubulopapillary carcinoma | Grade I | Grade I | Stage III | — | — | — | Alive |
| 4 | 1 | 1.5 | — | — | − | Tubulopapillary carcinoma | Grade I | Grade I | Stage I | — | — | — | Alive |
| 5 | > 5 | 2.5 | — | — | +si | Tubulopapillary carcinoma | Grade II | Grade III | Stage III | — | + | — | Died |
| 6 | > 5 | 0.5 | + | — | +si |
|
Grade III | Grade III | Stage IV | — | — | — | Alive |
| 7 | > 5 | 5 | + | + | +si | Solid carcinoma | Grade II and III | Grade III | Stage IV | + | + | — | Died |
| 8 | > 5 | 5 | + | + | − |
|
|
|
Stage III | — | + | — | Alive |
| 9 | 2–5 | 2.5 | — | — | +si | Tubulopapillary carcinoma | Grade III | Grade III | Stage IV | — | — | — | Alive |
| 10 | 2–5 | 4.5 | — | — | − | M. fibroadenomatosis | — | — | — | — | — | — | Alive |
| 11 | > 5 | 3 | — | — | +si |
|
Grade II | Grade III | Stage III | — | — | — | Alive |
| 12 | > 5 | 1.5 | — | — | − |
|
Grade II | Grade II | Stage II | — | — | — | Alive |
- Note: +, positive; −, negative.
- Abbreviations: HP, histopathology; IC, imprint cytology; L., lymphovascular; M., mammary; No, number; SC, scrape cytology.
- aAxillary.
- siSuperficial inguinal.
Information summarized in Table 2 shows that from the 69 included margins, an HP status of clean margin (> 2 mm) was found in 58/69 margins (84%). Narrow margins (0–2 mm) were found in 11/69 margins (16%), and there were no incomplete margins. Among the narrow margins, 1/11 (9%) was cranial lateral, and 10/11 (91%) were deep margins (cranial or caudal).
| Margin assessment method | Area of interest | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cranial (n = 36) | Caudal (n = 33) | |||||||||||
| Lateral (n = 12) | Medial (n = 12) | Deep (n = 12) | Lateral (n = 11) | Medial (n = 11) | Deep (n = 11) | |||||||
| HP status |
|
|
|
|
|
|
|
|
|
|
|
|
| IC (positive status) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| SC (positive status) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
- Note: Narrow (0–2 mm); clean (> 2 mm).
- Abbreviations: HP, histopathology; IC, imprint cytology; SC, scrape cytology.
Regarding intraoperative cytology, IC had a positive status margin in one HP clean margin and SC was positive in one HP narrow margin and 2 HP clean margins. Also, no patient presented more than one positive margin on intraoperative cytology (IC and SC).
With Cochran’s Q test, positive margins’ detection rates did not differ between methods (p = 0.174), but agreement between the 3 methods was poor (Fleiss kappa = −0.020 [95% CI: −0.156 to 0.117]).
Since no positive margins were detected in HP, a binary logistic regression model to predict the margin status with clinical and histological prognostic parameters was only created for intraoperative cytology.
The prognostic parameters met the binary logistic regression assumptions. Prognostic parameters were not predictors of intraoperative margins, except lymphovascular invasion (χ2 [1] = 23,564; p < 0.08, R2 Nagelkerke = 0.269). Histopathologic lymphovascular invasion was a significant predictor for positive intraoperative margin (IC and SC) (OR = 18, 652; 95% CI = 1742–199, 652).
4. Discussion
Despite the large size of mastectomy specimens in cats and the postoperative processing of samples, it was possible to demonstrate the feasibility of IC and SC to access surgical margins during the intraoperative period in FMT surgery. IC and SC are cost-effective methods [9] when compared to other intraoperative methods [27–29] and only require the availability of a clinical pathologist [4, 7], and despite producing a small delay in surgery, they can be communicated to the surgeon in a short period [9, 30].
Results from this study show no significant differences in positive margin detection between the 3 methods, but the agreement between the 3 methods was poor. In a canine mast cell tumors and soft tissue sarcomas study [4], shaved margin from the patient wound bed, IC, and HP applied for margin assessment also showed poor agreement. The poor agreement between the 3 methods may be explained by the differences between the 3 methods in obtaining a sample. Although HP allows to confirm the tumor presence and margin status (incomplete, narrow, and clean), HP only evaluated a small fraction of surgical margins [4], IC collected cells by apposition from the mastectomy specimen, and SC collected cells by scraping off the operating field. It was also possible to verify that the glass slides prepared with SC showed a higher number of cells when compared to IC which could justify differences in the number of positives between intraoperative methods. In intraoperative cytology (IC and SC), it is important to consider that negative results may not always represent the examined margin due to potential sample collection failures. According to Bray [3], maintaining an appropriate boundary over the entire circumference of the tumor during dissection may pose a challenge for the surgeon. This skill may be related to the surgeon’s experience. Surgeons with less experience may become less confident, resulting in smaller tumor margins once surgery extends deeper [3]. Despite in our study, surgeries were performed by experienced surgeons, and the majority of narrow margins (10/11) and 50% of positive intraoperative cytology were observed in the deep plane of mastectomy. Prognostic parameters can have an impact on the overall survival. The number and size of tumors can affect the surgical margin status in FMT, as it can increase positive margins related to more difficult surgical margin planning.
Despite the existence of studies showing the importance of prognostic parameters for FMTs such as number and size of tumors, cutaneous ulceration, lymphovascular invasion, lymph node invasion, and tumor grade on overall survival, the authors did not find any studies in veterinary medicine, particularly in cats, that address the predictive value of prognostic parameters in predicting the presence of incomplete surgical margins. Since the presence of incomplete surgical margins is associated with oncological disease recurrence and a decrease in overall survival, this aspect remains understudied in the feline context [16]. Our results indicate that, with the application of intraoperative cytology methods, all prognostic parameters, except for lymphovascular invasion, are not correlated with an increased likelihood of incomplete intraoperative margins. Our report shows a strong correlation between lymphovascular invasion and incomplete intraoperative margins, with an 18.652-fold increase in risk. However, it is important to note that this correlation is based on a short series of 12 clinical cases.
The OR for prognostic parameters, namely, lymphovascular invasion, was not assessed for HP, since 69/69 margins (100%) had a negative result (clean or narrow). The positive intraoperative margins, 3/4 corresponded to two animals with lymphovascular invasion, even though, on HP examination, these margins were classified as negative. Although HP successfully identified lymphovascular invasion, it did not detect its presence within the surgical margins. A possible explanation for this may be associated with the limitation of HP, that is, the impossibility of analyzing the totality of the margins of the mammary gland, and the fact that, in intraoperative techniques, sample collection is carried out from the entire extension of the margins. Migration of individual clusters of tumor cells beyond the tumor boundary is recognized in human and dog cancers and their occurrence is a feature of higher-grade tumors and is also important to tumor recurrence [3, 16]. This fact can be observed in our work since intraoperative positive margins were from TNM Stages III and IV animals and Grades II or III carcinoma and may be related to the possible detection of lymphovascular emboli or intraorgan metastatic dissemination.
The development of complementary methods to HP for margin assessment, such as intraoperative cytology methods, is important to improve patient care. A histological margin devoid of tumor cells is regarded as the most reliable predictor for local tumor control. However, the pathologist’s ability to precisely delineate an adequate resection margin is hindered by the inherent limitations in histological analysis, including individual tissue elasticity, tissue distortion, contraction, tissue fixation, and the complexities of histological processing and interpretation [3]. HP only evaluates approximately 0.1%–0.01% of the total circumferential surgical margin [3]. The pathologist must select regions of interest on macroscopic data, which is a source of sampling error [7]. Also, to reduce the incidence of this type of error, an effective exchange of information between surgeons/oncologists and pathologists is necessary [19].
To the authors’ knowledge, this is the first time that SC has been described in veterinary medicine to classify the status of surgical margins. SC has a great advantage over the shaved margin technique, since contrary to the shaved margin technique, when SC is performed in the surgical field, it can be performed over vital structures at the edges of the resection wound bed. Due to this limitation, in a study by Milovancev [4], the authors did not collect samples by shaved margin technique in approximately 5% of margins. Another advantage of SC over shaved margin is related to the fact that SC has a laboratory service requirement equal to IC, which is much lower than shaved margins that require the availability of a pathology service to prepare and read frozen sections [4]. SC is less time-consuming, inexpensive, and lacks freezing artifacts when compared to the shaved margin technique [31]. Also, to the author’s knowledge, this is the first report that describes the predictive effect of prognostic factors of FMT on intraoperative surgical margin status.
An advantage of this study is related to the fact that the three methods were applied only to feline mammary neoplasms and not to different types of tumors and animal species, thus reducing the variability of the results and improving their reliability. Despite IC being particularly well suited for breast carcinoma in humans since the identification of malignant epithelial cells is readily performed [7], the same investigation on IC and SC utility to define intraoperative clean status margins in FMT is not described in the veterinary literature. In our study, complete bread loafing sections’ HP was applied in mastectomy specimens. This technique has the advantage of increasing the percentage of marginal tissue examined [14, 19].
In this study, 5 females were intact and 7 females were sterilized at the time of mastectomy. However, the relationship between reproductive status and mammary tumor development was not investigated. According to one study [32], the development of mammary tumors is reduced in 91% of feline females sterilized before 6 months of age.
The limitations of this work are related to differences between intraoperative cytology methods and HP, as cytology techniques lack the contextual tissue architecture present in histopathological assessments [4]. When intraoperative cytology techniques are compared, IC is related to fewer cells on glass than SC, which is related to poor exfoliation of neoplastic cells [4]. Another limitation is related to the lack of inking of the critical tissue margins since identification was obtained with margin tissue sutures. Inking of critical tissue margins by the surgeon is recommended by most pathologists to prevent false positive and false negative margin status classifications related to tissue distortion during fixation [3]. Another limitation is related to the fact that each animal provided 6 margins, except one that provided 3 margins for each method. However, no animal exhibited more than one positive margin, which excludes the possibility of contamination between margins in intraoperative methods. The cat’s outcome was only evaluated during the study period (16 months), and two animals with positive intraoperative margin status died. Finally, the different TNM stages present in this study may have influenced the results, and probably in a larger sample, grouping samples by tumor grade or TNM stage could yield different positive margin rates for the techniques. It will be important that future studies evaluate the correlation of IC and SC with local recurrence rates and overall survival in FMT, given that only a small period was evaluated in our study.
5. Conclusions
IC and SC are feasible methods for assessing surgical margins during the intraoperative period in FMT surgery. The margin ratings obtained in IC, SC, and HP are comparable. In cases of lymphovascular invasion, intraoperative methods may serve as valuable complements to HP. Additional studies are warranted to understand the clinical relevance of IC and SC as complementary techniques to HP in the prognosis of FMT.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was funded by the Cooperativa de formação e animação cultural CRL (COFAC), Universidade Lusófona, Lisboa, Portugal.
Open Research
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.




