Anti-MDR Escherichia coli Activity, Phenolic and Flavonoid Content, and Antioxidant Potential of Azadirachta indica A. Juss Ethanolic Leaf Extract: An HR-LCMS-Based Profiling Study
Abstract
The rise of multidrug-resistant (MDR) Escherichia coli (E. coli) poses a critical challenge due to limited treatment options. This study evaluates the anti-MDR E. coli activity, phenolic and flavonoid content, antioxidant potential, and bioactive compounds in ethanolic leaf extracts of Azadirachta indica A. Juss. Among 115 MDR E. coli isolates, 61.7% showed susceptibility to the extract, with inhibition zones of 8–15 mm. The extract exhibited high phenolic (53.6 ± 0.444 mg GAE/g) and flavonoid (84.3 ± 0.889 mg QE/g) content, which may account for its strong antioxidant activity based on total antioxidant capacity (ascorbic acid IC50: 9.0 ± 0.255 μg/mL), although it showed only moderate free radical scavenging ability (DPPH IC50: 406.69 ± 1.64 μg/mL). High-resolution liquid chromatography–mass spectrometry (HR-LCMS) analysis identified 153 and 107 metabolites in negative and positive ESI modes, respectively, with several displaying antibacterial properties. The presence of alkaloids, phosphoramidates, and phenolic compounds supports its antimicrobial and antioxidant potential.
1. Background
Escherichia coli is among the most common pathogens affecting humans and animals, causing a wide spectrum of diseases [1, 2]. Additionally, E. coli serves as a significant reservoir of genes coding for antimicrobial drug resistance, making it a valuable indicator of resistance within bacterial communities [3, 4]. Recent studies have reported high resistance rates among E. coli isolates to commonly used antibiotics such as erythromycin, amoxicillin, and tetracycline [5–7]. Antimicrobial resistance (AMR) represents a serious and growing global threat to human, animal, and environmental health. This challenge is exacerbated by the emergence, spread, and persistence of MDR bacteria, often referred to as “superbugs” [8]. The global prevalence of MDR E. coli strains is increasing, primarily due to the dissemination of mobile genetic elements such as plasmids. In Europe, the rise of multidrug-resistant E. coli strains has also become a significant concern [9]. Over the past two decades, the prevalence of acquired MDR infections has risen sharply, primarily due to the production of β-lactamases. These enzymes confer resistance to critical antibiotic classes, such as third-generation cephalosporins and carbapenems, severely limiting therapeutic options [10]. Treating infections caused by MDR strains has become increasingly challenging, resulting in prolonged hospital stays, higher healthcare costs, and elevated risks of morbidity and mortality [11, 12].
AMR is among the leading global threats to public health and development [13, 14]. In 2019, bacterial AMR caused an estimated 1.27 million deaths and contributed to 4.95 million more [14]. By 2021, AMR was responsible for 4.71 million deaths. By 2050, AMR-related deaths could reach 8.22 million annually, with 1.91 million directly attributable to resistant bacterial infections—a 69.6% increase from 2022 [15].
In developing countries like Nepal, where the irrational use of antibiotics is widespread, the AMR problem is escalating rapidly [16]. The therapeutic management of E. coli infections is increasingly threatened by the rise of AMR [9]. MDR E. coli strains exhibit high levels of resistance to most commonly used antimicrobials, posing significant challenges to effective treatment [17]. Furthermore, in developing countries, synthetic drugs are often expensive, insufficient for effective disease treatment, and frequently associated with adulteration and adverse side effects. In this context, natural products derived from plants present promising alternatives for combating AMR, providing safer, cost-effective, and sustainable solutions [18].
Medicinal plants have been used for disease treatment since ancient times [19] and remain a primary method of healthcare even in the modern era [20]. Of the 7000 recognized medicinal plant species worldwide, more than 900 valuable species are found in Nepal, reflecting the country’s rich biodiversity [21]. The knowledge of medicinal plant use is deeply embedded in the traditions and culture of Nepalese people, particularly those living in rural areas [22].
One of the most commonly used medicinal plants is the neem tree (Azadirachta indica), which belongs to the Meliaceae family [23]. Neem trees are found in Nepal’s Terai and foothills, which represent tropical and subtropical regions, respectively [24]. This plant is widely recognized for its medicinal properties and is used to treat a variety of ailments [25]. Various parts of the neem plant—including the leaves, bark, seeds, and flowers—possess medicinal properties. However, leaf extracts are considered more effective due to their rich content of bioactive compounds such as nimbin, quercetin, nimbolide, and other phenolics and flavonoids (FLV), which contribute to their strong antimicrobial, antioxidant, and anti-inflammatory activities [26, 27]. Neem tree leaves, found in various tropical regions, contain varying levels of polyphenolic compounds and FLV due to their wide geographical distribution [28].
Many solvents like water, methanol, ethanol, acetone, and their mixtures can be used to prepare plant extracts. Eighty percent ethanol is commonly used for neem extract due to its effectiveness in extracting both polar and moderately nonpolar compounds which contribute to neem’s bioactivity [29].
The marked increase in infections caused by MDR E. coli in recent years is a significant concern [17, 30, 31], as therapeutic options for these pathogens remain limited. Several studies have demonstrated that neem leaf extracts can inhibit the growth of MDR E. coli strains, suggesting its potential as an alternative or complementary antimicrobial agent [32, 33]. Despite neem’s extensive traditional use, scientific validation of its efficacy against MDR E. coli is still insufficient, particularly regarding the phenolic and FLV content and the bioactive components responsible for its antimicrobial properties. Therefore, this study is aimed at evaluating the in vitro anti-MDR E. coli activity, quantifying the total phenolic content (TPC) and total flavonoid content (TFC), assessing antioxidant potential, and performing high-resolution liquid chromatography-mass spectrometry (HR-LCMS) profiling of bioactive compounds in the ethanolic leaf extracts of the Nepalese medicinal plant A. indica A. Juss.
2. Materials and Methods
A laboratory-based cross-sectional study was conducted involving both patients and healthy individuals. Clinical samples, including pus, sputum, stool, and urine, were collected from patients visiting Capital Reference Laboratory, Bharatpur, Chitwan, Nepal. In addition, stool samples were obtained from healthy college students at Manmohan Memorial Institute of Health Sciences, Soalteemode, Kathmandu, Nepal, who had no history of antibiotic use in the preceding 6 months.
All collected samples were evaluated according to the criteria recommended by the American Society for Microbiology [34]. Only those meeting the inclusion criteria were selected for further microbiological processing and analysis; samples not meeting these standards were excluded. The overall procedure of the study is indicated in Figure 1.
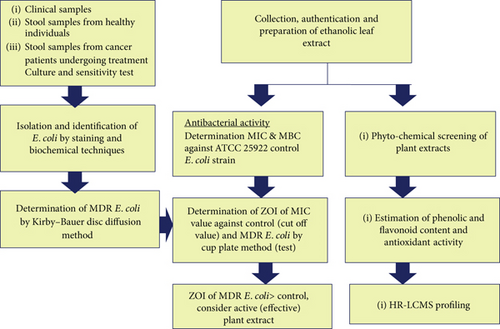
2.1. Isolation and Identification of E. coli
Various samples were inoculated by applying standard procedure onto various culture media. E. coli was identified after 24 h of incubation at 37°C aerobically by examining colony morphology, gram staining, and other various biochemical tests [35].
2.2. Identification of MDR E. coli
The antibiotic sensitivity testing of identified E. coli was performed by the Kirby–Bauer disk diffusion method on Mueller–Hinton agar (MHA) using standard methods recommended by CLSI guidelines (2017) [36]. The antibiotics tested were amoxicillin (25 μg), ceftazidime (30 μg), cefotaxime (30 μg), cefixime (5 μg), cefepime (30 μg), imipenem (10 μg), meropenem (10 μg), tetracycline (30 μg), gentamicin (30 μg), amikacin (30 μg), ciprofloxacin (5 μg), levofloxacin (5 μg), and aztreonam (30 μg). MDR E. coli were categorized as per the definition given by Magiorakos et al. [37].
2.3. Collection and Identification of Plants
The leaves of A. indica A. Juss were collected in May 2024 from Madi Municipality, Chitwan District, Nepal, located at latitude 27.454206° N, longitude 84.3231867° E (decimal degrees). In DMS format, the coordinates are latitude 27°27′15.142″N, longitude: 84°19′23.472″E. The collection was carried out during the mature leaf stage, ensuring the highest concentration of bioactive compounds. The plant material was identified at the National Herbarium and Plant Laboratories, Godawari, Lalitpur, Nepal, in June 2024. A voucher specimen (Code Number 03-2081/2/20) was deposited at the herbarium for future reference. The collection was conducted in accordance with local regulations, and necessary permits were obtained.
2.4. Preparation of Extracts
The fresh leaves of A. indica A. Juss were thoroughly washed and cut into small pieces using a sterile knife. They were spread out and shade-dried for 2–4 weeks with periodic turning and made into powder. The maceration technique was then applied using 100 g of dried A. indica leaf powder and 500 mL of 80% ethanol, with occasional shaking over 7 days, with slight modifications as described by Seriana et al. [38]. The extract was filtered through muslin cloth followed by Whatman No. 1 filter paper. The filtrate was concentrated using a rotary evaporator under reduced pressure. The concentrated extract was stored at 4°C, protected from light and humidity, for further analysis.
2.5. Extractive Value
The concentrated extract was subsequently analyzed for phytochemical screening, antibacterial properties, TPC and TFC quantification, antioxidant activity, and HR-LCMS profiling.
2.6. Antibacterial Activity
The minimum inhibitory concentration (MIC) of the ethanolic leaf extract was determined against the reference strain E. coli ATCC 25922 and used as a functional cutoff to assess the susceptibility of MDR E. coli isolates. The corresponding zone of inhibition (ZOI) at this MIC served as a comparative benchmark. Isolates showing ZOI values greater than this cutoff were considered susceptible. In this MIC-based interpretive approach, the reference strain effectively served as an internal standard. It focused on plant extract efficacy relative to a defined MIC threshold [39].
2.6.1. Determination of MIC and Minimum Bactericidal Concentration (MBC)
A twofold serial dilution of the leaf extract was prepared in nutrient broth to achieve final concentrations of 80, 40, 20, 10, 5, 2.5, and 1.25 mg/mL, respectively. A standardized bacterial culture (100 μL) of E. coli ATCC 25922 was inoculated into each dilution and incubated at 37°C for 24 h. Positive and negative controls were set up using ATCC 25922 with broth and broth alone, respectively. The lowest concentration at which no turbidity was observed was recorded as the MIC of the extract.
To determine the MBC, the contents of the tubes showing the MIC, as well as those with concentrations both greater and less than the MIC, were spread onto separate MHA plates. These plates were incubated at 37°C for 24 h. The lowest concentration at which no bacterial growth was observed on the agar plate was recorded as the MBC [40].
2.6.2. Determination of Anti-MDR E. coli Activity
The agar well diffusion method was used for in vitro anti-MDR E. coli activity of the extracts. The fresh broth cultured inoculums of MDR E. coli isolates compared with McFarland Standard Tube No. 0.5 were spread uniformly on the dry surface of sterile MHA plates to make lawn culture. Three wells (test, positive, and negative control), each of 8-mm diameter, were bored in the inoculated plates using a sterile cork borer. Fifty microliters of the MIC of 80% ethanolic extract against ATCC E. coli strain was loaded into the respective wells along with positive control and negative control (ATCC 25922 strain of E. coli and 80% ethanol, respectively). Loaded plates were left for 15 min to allow absorption and diffusion of extract and incubated for 24 h at 37°C. The ZOI was measured and compared with controls to interpret the result as effective or ineffective.
2.7. Preliminary Phytochemical Screening of A. indica A. Juss Extract
Qualitative phytochemical screening was performed following the standard methods described by Harborne (1998) and Sofowara (1993) to detect various phytochemicals such as reducing sugars (RS), anthraquinones, tannins (TAN), alkaloids (ALK), resins, and glycosides. The results were interpreted based on the color reactions observed during qualitative tests [41, 42]. Different test solutions were prepared for the detection of specific phytochemical groups, including ALK, glycosides, TAN, saponins (SAP), FLV, terpenoids (TER), and carbohydrates. The reagents used included Dragendorff’s reagent and Mayer’s reagent for ALK, 10% ferric chloride solution for TAN, lead acetate solution for FLV, and Molisch’s reagent for carbohydrates.
2.8. Quantitative Estimation of Phytochemicals
2.8.1. Estimation of Total Polyphenolic Content
The TPC of the ethanolic extract was estimated using the Folin–Ciocalteu reagent, following the method described by Bhatt and Parajuli [43] with necessary modifications. Gallic acid was used as the standard.
2.8.1.1. Preparation of Standard Solutions
A stock solution of gallic acid (1 mg/mL or 1000 ppm) was prepared by dissolving 50 mg of gallic acid in 50 mL of methanol. Serial dilutions were made to prepare various concentrations of gallic acid: 20, 40, 60, 80, and 100 μg/mL (ppm). For each concentration, 1 mL of the gallic acid solution was transferred to a 15-mL test tube.
2.8.1.2. Reaction Procedure
To the gallic acid solution, 5 mL of Folin–Ciocalteu reagent (10%) and 4 mL of 7% sodium carbonate (Na2CO3) were added, making a total volume of 10 mL. The mixture was shaken well and incubated for 30 min at 40°C in a water bath. The absorbance was measured at 760 nm against a blank containing all reagents except gallic acid. A calibration curve was generated using the average absorbance values obtained for different concentrations of gallic acid.
2.8.1.3. Preparation of Extract Solutions
Stock solutions of the ethanolic extract were prepared by dissolving 50 mg of extract in 50 mL of methanol (1 mg/mL). For analysis, 1 mL of the stock solution was taken, and the same procedure as for gallic acid (addition of Folin–Ciocalteu reagent and Na2CO3 followed by incubation) was followed. Absorbance was measured at 760 nm against a blank. The concentration of total phenolic compounds in the extract was calculated and expressed as milligrams of gallic acid equivalents (GAE) per 100 g of sample (mg GAE/100 g). All measurements were performed in triplicate to ensure accuracy.
2.8.2. Determination of FLV Content
The TFC of the extracts was estimated using the aluminum chloride (AlCl3) colorimetric assay, as described by Bhatt and Parajuli [43], with slight modifications. Quercetin was used as the standard.
2.8.2.1. Preparation of Standard Solutions
A stock solution of quercetin (1 mg/mL or 1000 ppm) was prepared by dissolving 50 mg of quercetin in 50 mL of methanol. Serial dilutions were made to prepare various concentrations of quercetin: 20, 40, 60, 80, and 100 μg/mL (ppm). For each concentration, 1 mL of the quercetin solution was transferred into a 15-mL test tube.
2.8.2.2. Reaction Procedure
At time zero, 0.15 mL of 5% sodium nitrite (NaNO2) was added to the test tube. The solution was vortexed and left to stand for 5 min. Then, 0.15 mL of 10% AlCl3 was added, and the solution was vortexed again. At 6 min, 1 mL of 1 M sodium hydroxide (NaOH) was added to the mixture. The total volume of the solution was immediately adjusted to 5 mL by adding double-distilled water, followed by vortexing. The absorbance of the resulting pink-colored mixture was measured at 510 nm against a blank containing all reagents except quercetin. A calibration curve was plotted using the average absorbance values obtained for the different concentrations of quercetin.
2.8.2.3. Preparation of Extract Solutions
Stock solutions of the ethanolic extract were prepared by dissolving 50 mg of extract in 50 mL of methanol (1 mg/mL). For analysis, 1 mL of the stock solution was taken, and the procedure described above was followed. Absorbance values for each concentration of the extract were recorded. The TFC of the samples was calculated and expressed as milligrams of quercetin equivalents (QE) per 100 g of sample (mg QE/100 g). All measurements were performed in triplicate to ensure accuracy.
2.8.3. Determination of Antioxidant Activity
The antioxidant activity of the extracts was evaluated using the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay, following the method described by Khatun et al. [44], with slight modifications.
The percentage scavenging was plotted against the concentration, and the regression equation was used to calculate the IC50 value, representing the micromolar concentration required to inhibit DPPH radical formation by 50%. The IC50 value indicates antioxidant potency; a lower IC50 value corresponds to higher antioxidant activity.
2.9. HR-LCMS Analysis
The HR-LCMS analysis of ethanolic leaf extract of A. indica A. Juss was conducted at the Sophisticated Analytical Instrument Facility (SAIF), IIT Mumbai, India. The analysis was performed using a Q-Exactive Plus Orbitrap mass spectrometer coupled with a Thermo Vanquish UHPLC system. The system operated in positive and negative electrospray ionization (ESI) modes. Samples (1–10 μg/mL) were dissolved in HPLC-grade methanol, filtered through a 0.22-μm PTFE syringe filter, and transferred to autosampler vials. Chromatographic separation was performed using a Thermo Accucore C18 column (2.1 × 100 mm, 2.6 μm). The mobile phase consisted of the following: Solvent A: water with 0.1% formic acid and Solvent B: acetonitrile with 0.1% formic acid. The gradient was as follows: 0–1 min: 5% B, 1–10 min: linear increase to 95% B, 10–12 min: 95% B, 12–13 min: return to 5% B, and 13–15 min: equilibration at 5% B. The flow rate was 0.3 mL/min, with a 5-μL injection and a column temperature of 40°C. The mass spectrometer was operated with the following settings: spray voltage: +3.5 kV, capillary temperature: 320°C, resolution: full MS at 70,000, MS/MS at 17,500, scan range: 105–1100 m/z (full MS), 200–2000 m/z (MS/MS), NCE: 30 for HCD MS/MS. Raw data were processed using Thermo Xcalibur and Compound Discoverer 3.2. Compounds were tentatively identified based on accurate mass (within ±5 ppm), retention time, and MS/MS fragmentation patterns. Identification was performed by matching the data against mzCloud and ChemSpider.
2.10. Ethical Consideration
Ethical approval for the study was obtained from the Nepal Health Research Council (NHRC), Ramshahpath, Kathmandu, Nepal (Approval Number 74-2024). Informed written consent was collected from all participants after thoroughly explaining the objectives and procedures of the study.
3. Data Analysis
Each isolate was assigned a unique identification code to ensure traceability. Experimental data, including MIC, MBC, and ZOI measurements, were recorded manually and later entered into a structured database. Statistical analysis was conducted using IBM SPSS Statistics Version 20. Descriptive statistics, such as means and standard deviations, were used to summarize the ZOI values across three groups: clinical patients, cancer patients, and healthy individuals. Group-wise antibacterial activity was further evaluated based on the percentage of sensitive and resistant isolates.
A one-way ANOVA was applied to determine whether differences in mean ZOI between groups were statistically significant. Additionally, a chi-square test was performed to assess differences in sensitivity rates among the three sample sources. A p value < 0.05 was considered statistically significant in all analyses.
4. Results and Discussion
4.1. Distribution of MDR E. coli
Out of 346 samples taken from three different sources for culture and sensitivity testing, 70.8% showed growth of E. coli. Among the 245 isolated E. coli bacteria, 115 (46.9%) were found to be MDR (Table 1). Our findings align with similar results reported in other studies [45–49]. However, the observed prevalence of MDR E. coli is lower than previously reported by Parajuli et al. [50], Taneja et al. [51], and Baral et al. [17]. These variations may be attributed to several factors, including differences in the study periods, geographic regions, sample types, sources, and the antibiotics used during susceptibility testing.
| Subjects | Number of samples for c/s test | Positive growth for E. coli, n (%) | Distribution of MDR E. coli, n (%) |
|---|---|---|---|
| Patients | 117 | 90 (76.9) | 40 (44.4) |
| Normal individuals | 129 | 98 (75.9) | 40 (40.8) |
| Cancer individual | 100 | 57 (57) | 35 (61.4) |
| Total | 346 | 245 (70.8) | 115 (46.9) |
4.2. Extractive Value of Extract
The yield obtained from A. indica A. Juss leaves was 18.33%. The percent yields of the ethanol extracts, along with their respective extraction times, are summarized in Table 2. The yield of A. indica A. Juss leaf extracts can vary depending on the extraction method, solvent used, and conditions such as time and temperature. Most findings [52–55] report a yield value in the range of approximately 16%–20% for ethanol extraction of leaves. Slight variations in yield can be attributed to differences in factors such as leaf maturity, drying methods, and regional growth conditions. A higher yield value was noted in a study by Siddiqui et al. [56], which reported an ethanol extract yield of 22% for A. indica leaves using Soxhlet extraction with 95% ethanol over 8 h. In comparison, Khan et al. [57] reported a significantly lower yield of 9.8% for aqueous extracts of A. indica leaves. Similarly, Patel et al. [58] documented an ethanol extract yield of 11.5% using a shorter extraction time of 6 h at moderate temperatures (40°C). These differences highlight the impact of extraction techniques and conditions on the yield of bioactive compounds from A. indica leaves.
| Plant name (parts used) | Solvent | Extraction time | Extractive value (%) |
|---|---|---|---|
| A. indica A. Juss (leaves) | 80% ethanol | 7 days | 18.33 |
4.3. Antibacterial Activity of Ethanolic Extracts
4.3.1. MIC, MBC, and ZOI of Extract Against Reference Strain
The MIC of the A. indica A. Juss extract was found to effectively inhibit the growth of the ATCC 25922 E. coli strain at a concentration of 20 mg/mL, while complete inhibition (no growth) was observed at 40 mg/mL (MBC). The mean ZOI for the MIC value of the extract was 8.8 ± 0.05 mm in diameter against the ATCC reference strain. Detailed results are summarized in Table 3.
| Leaf extract | MIC (mg/mL) | MBC (mg/mL) | Mean ZOI (mm) |
|---|---|---|---|
| A. indica A. Juss | 20 | 40 | 8.8 ± 0.05 |
Our findings are consistent with previous studies. Timilsena et al. reported similar MIC and MBC values for neem ethanolic leaf extract against E. coli ATCC 25922, with a slightly higher ZOI of 10.83 ± 1 mm [52]. Another study found MIC values of 64 μg/mL for methanolic neem extract against E. coli ATCC 25922, along with a higher ZOI of 9 mm [59]. Additionally, Mehta et al. reported a MIC of 37.4 mg/mL for ethanolic neem leaf extract against E. coli ATCC 25922 [60], which is slightly higher than our reported MIC. The higher concentration of the MIC and MBC of ethanolic neem leaf extract against E. coli was reported as 100 and 112.5 mg/mL, respectively, by Ali et al. [61]. Lower MIC (64 μg/mL) was reported by Altayb et al. from methanolic leaf extract against E. coli (ATCC 25922) [59]. These variations can be attributed to differences in extraction methods, solvent types, the specific strains of E. coli tested, and geographical variations in plant samples.
4.3.2. Anti-MDR E. coli Activity of Ethanolic Extracts
Among the 115 multidrug-resistant E. coli isolates obtained from clinical samples, cancer patients, and healthy individuals, 71 (61.7%) were found to be sensitive to the ethanolic leaf extract of A. indica A. Juss. The ZOIs ranged from 8 to 15 mm, with a mean ZOI of 9.35 ± 1.14 mm. Although isolates from cancer patients exhibited the highest apparent sensitivity rate (74.3%), the difference in sensitivity rates among the groups was not statistically significant (p = 0.077). However, a statistically significant difference was observed in the mean ZOI values between the groups (p = 0.003), indicating variation in extract efficacy based on the source of the isolates. A summary of the findings is presented in Table 4. The antibacterial activity of A. indica ethanolic leaf extract may be attributed to the presence of phytochemicals such as FLV and TER, which have been reported to disrupt bacterial membranes, inhibit key enzymes, and interfere with efflux pumps and biofilm formation [62, 63].
| Sample sources | Sensitive n (%) | Resistant n (%) | Total isolates (n) | Mean ZOI ± SD (mm) | p value |
|---|---|---|---|---|---|
| Clinical patients | 21 (52.5%) | 19 (47.5%) | 40 | 8.70 ± 0.86 | |
| Cancer patients | 26 (74.3%) | 9 (25.7%) | 35 | 9.84 ± 1.35 | 0.077a |
| Healthy individuals | 24 (60.0%) | 16 (40.0%) | 40 | 9.51 ± 1.21 | 0.003b |
| Total | 71 (61.7%) | 44 (38.3%) | 115 | 9.35 ± 1.14 |
- aChi-square test.
- bOne-way ANOVA.
A study by Timilsina et al. (2021) assessed the antimicrobial potential of three Nepalese medicinal plants against MDR E. coli isolated from the stool samples of healthy individuals. The ethanolic extract of A. indica showed a 68.51% effectiveness rate [52]. Similarly, a study by Hikaambo et al. evaluated the antibacterial activity of ethanolic neem leaf extracts against E. coli, where the aqueous extract at 50 mg/mL exhibited a ZOI of 10.67 ± 0.58 mm, while the ethanolic extract showed an 8.7 ± 0.58 mm ZOI at the same concentration [64]. These results align closely with our observed ZOI range of 8–15 mm. In contrast, research conducted by Thapa et al. found that none of the MDR E. coli strains were sensitive to the methanolic extract of neem leaves [65]. However, a study from India by Monali et al. reported that A. indica exhibited a ZOI in the range of 12–19 mm against MDR gram-negative pathogens [66].
4.4. Preliminary Phytochemical Screening of A. indica A. Juss Leaf Extracts
The ethanol extracts of A. indica A. Juss contained TAN, ALK, SAP, FLV, and RS but not TER, cardiac glycosides (CAG), and anthraquinone glycosides (ANG) (Table 5). These bioactive compounds have been widely recognized for their antimicrobial properties. TAN and FLV are known to possess antibacterial activity by disrupting bacterial membranes and inhibiting enzymes essential for microbial survival [67]. ALK also exhibit antimicrobial effects through mechanisms such as DNA intercalation and enzyme inhibition [68]. The presence of SAP, which have been reported to have antimicrobial and anti-inflammatory properties, further supports the potential of A. indica as a bioactive agent [69].
| Sample | Phytochemical compounds | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Extract of A. indica A. Juss (leaves) | TAN | ALK | SAP | FLV | RS | ANG | CAG | TER | PRO | CAR |
| + | + | + | + | + | − | − | − | − | + | |
- Note: + present, − absent.
- Abbreviations: CAR, carbohydrates; PRO, proteins.
4.5. Quantitative Estimation and Antioxidant Activity of Phytochemicals
The ethanolic extract of neem leaves demonstrated a TPC of 53.6 ± 0.444 mg GAE/g extract and a TFC of 84.3 ± 0.889 mg QE/g extract, indicating a significant presence of phytochemicals. The extract exhibited strong antioxidant activity with an ascorbic acid IC50 of 9.0 ± 0.255 μg/mL but moderate free radical scavenging capacity, as evidenced by a DPPH IC50 of 406.69 ± 1.64 μg/mL (Table 6). These findings suggest that A. indica leaves are a valuable source of bioactive compounds, particularly polyphenols and FLV, which contribute to their antioxidant and antimicrobial properties. Its strong antioxidant potential is likely due to the high content of phenolics and FLV, which act through free radical scavenging, metal ion chelation, and inhibition of lipid peroxidation [70, 71].
| Sample | Phytochemical compounds | Antioxidant activities | ||
|---|---|---|---|---|
| Ethanolic extract of A. indica A. Juss (leaves) | Total phenol content (mg GAE/g extract) | Total flavonoid content (mg QE/g extract) | Ascorbic acid IC50 (μg/mL) | DPPH IC50 (μg/mL) |
| 53.6 ± 0.444 | 84.3 ± 0.889 | 9.0 ± 0.255 | 406.69 ± 1.64 | |
Higher values of TPC (70 mg GAE/g extract) and TFC (119 mg QE/g extract) were reported by Kumar et al. in neem leaves from the Bundelkhand region of India [72]. Similarly, Mehta et al. recorded even higher values, with mean ± SD TPC of 68 ± 0.46 mg GAE/g extract and TFC of 114 ± 2.7 mg QE/g extract [60]. These variations in phytochemical content may be attributed to differences in environmental conditions, extraction methodologies, and solvent systems used across studies.
Phenolic and FLV compounds play a crucial role in scavenging free radicals and protecting cells from oxidative damage [73, 74]. The strong antioxidant activity of the extract, as indicated by its ascorbic acid IC50 of 9.0 ± 0.255 μg/mL, further supports the high presence of these phytochemicals. However, its moderate free radical scavenging activity (DPPH IC50 of 406.69 ± 1.64 μg/mL) suggests that specific antioxidant mechanisms may differ among these compounds. The variation in antioxidant potential between assays may be due to differences in the reaction mechanisms of ascorbic acid and DPPH radicals with the phytochemicals present in A. indica [75].
Notably, Hossain et al. reported significantly higher antioxidant activity in neem roots, with a TPC of 238.81 ± 0.98 mg GAE/g extract and a DPPH IC50 value of 13.81 ± 0.06 μg/mL, suggesting that different plant parts may contain varying levels of bioactive compounds [76]. This discrepancy highlights the need for further research into the distribution of phenolic and FLV compounds in different parts of the neem tree. Several studies have reported the antioxidant potential of A. indica extracts. Sharma et al. demonstrated that neem leaves contain high levels of polyphenols and FLV, which contribute significantly to their antioxidant activity [77]. Similarly, Priyadarsini emphasized the role of FLV in reducing oxidative stress and their potential application in disease prevention [78]. These variations underscore the influence of factors such as extraction techniques, solvent polarity, and regional conditions on the phytochemical yields and antioxidant activities of A. indica extracts.
The interplay between antibacterial and antioxidant properties is especially relevant in MDR pathogens. Antioxidants may sensitize bacteria by disrupting redox balance, making them more vulnerable to antimicrobial agents [79]. Additionally, the antioxidant properties may reduce infection-related oxidative stress in host tissues, providing a dual therapeutic effect [80].
4.6. Identification of Bioactive Metabolites by HR-LCMS
The analysis of bioactive metabolites in the stored extract using HR-LCMS identified 13 major compounds (Table 7) in the positive ionization mode and 19 major compounds (Table 8) in the negative ionization mode. Representative chromatograms for both positive and negative ionization modes are presented in Figure 2. ALK were found to be the most abundant and dominant class, followed by phosphoramidates and organophosphorus compounds and phenolic and aromatic compounds. The diverse range of bioactive compounds in the leaf extract of A. indica explains its broad therapeutic potential, including antimicrobial, antioxidant, and other pharmacological activities.
| S.N. | Compounds | Molecular formula | Delta mass (ppm) | Mass | RT |
|---|---|---|---|---|---|
| 1 | 4,5,7-Trimethyl-3-thioxooctahydro-6H-imidazo[4,5-e][1,2,4]triazin-6-one | C7H13N5OS | 1.72 | 215.0845 | 1.25 |
| 2 | Diethyl N-{4-[(1E)-3-(2-hydroxyethyl)-3-methyl-1-triazen-1-yl]benzoyl}glutamate | C19H28N4O6 | 3.99 | 408.2025 | 2.14 |
| 3 | Diethyl 2-pyridinylphosphoramidate | C9H15N2O3P | −1.71 | 230.0816 | 9.60 |
| 4 | S-[(2Z)-2-Amino-2-{[3-(dimethylamino)propyl]imino}ethyl] dihydrogen phosphorothioate | C7H18N3O3PS | −4.2 | 255.0796 | 9.99 |
| 5 | 4-(2-Phosphinoethyl)morpholine | C6H14NOP | −1.97 | 147.081 | 10.23 |
| 6 | Tetraisopropyl methylenediphosphonate | C13H30O6P2 | 1.03 | 344.1521 | 10.44 |
| 7 | 3-(Benzyloxy)-17-methyl-7,8-didehydro-4,5-epoxymorphinan-6-yl myristate | C38H51NO4 | −3.63 | 585.3797 | 15.57 |
| 8 | 1-(2-Methylphenyl)-3-[4-(octyloxy)phenyl]thiourea | C22H30N2OS | −1.02 | 370.2075 | 16.01 |
| 9 | 9-Dodecyn-1-yl 2-thiophenecarboxylate | C17H24O2S | −3.85 | 292.1486 | 19.42 |
| 10 | 1-Cyclohexyl-1H-1,2,3,4-tetrazole-5-thiol | C7H12N4S | −3 | 184.0777 | 19.92 |
| 11 | 1,1′-(Butylphosphorothioyl)dipiperidine | C14H29N2PS | 0.65 | 288.1791 | 23.10 |
| 12 | 4-Fluoro-beta-nitropropenylbenzene | C9H8FNO2 | 2.89 | 181.0544 | 24.83 |
| 13 | 13,22-Dihydroxy-8,14-dimethoxy-4,10,12,16-tetramethyl-3,19,20-trioxo-2-azabicyclo[16.3.1]docosa 1(21), 4,6, 10,18(22)-pentaen-9-yl carbamate | C28H38N2O9 | 2.36 | 546.259 | 25.00 |
| S.N. | Compounds | Molecular formula | Delta mass (ppm) | Mass | RT |
|---|---|---|---|---|---|
| 1 | 2-(Trimethylsilyl)-1,4-benzoquinone | C9H12O2Si | −4.43 | 180.0599 | 1.25 |
| 2 | NP-009092 | C19H22O3 | −3.45 | 298.1559 | 2.14 |
| 3 | 2,2′-(Nitrosoimino)bis(N′,N′-dimethylacetohydrazide) | C8H18N6O3 | −4.51 | 246.1429 | 7.37 |
| 4 | 9-(3-Amino-3-deoxy-2-S-ethyl-2-thiopentofuranosyl)-9H-purin-6-amine | C12H18N6O2S | 1.98 | 310.1218 | 9.60 |
| 5 | N-[(Z)-2-(4-Nitrophenyl)-1-(6-oxo-2-thioxo-2,3,6,7-tetrahydro-1H-purin-8-yl)vinyl]benzamide | C20H14N6O4S | −3.42 | 434.0782 | 9.99 |
| 6 | Ethyl (4-{acetyl[6-(methylsulfanyl)-9H-purin-9-yl]amino}butyl)carbamate | C15H22N6O3S | −1.24 | 366.147 | 10.25 |
| 7 | 1-(Acetamidomethyl)-1,5-anhydro-2-O-benzoyl-3-(benzoylamino)-4,6-O-benzylidene-3-deoxyhexitol | C30H30N2O7 | 4.32 | 530.2076 | 10.74 |
| 8 | 4-Boronobenzoic acid | C7H7BO4 | 3.07 | 166.0443 | 12.38 |
| 9 | 5-Tert-Butyl-2-methyl-[1,3,2]dioxaphosphinane 2-sulfide | C9H19O2PS | 0.23 | 208.0687 | 14.44 |
| 10 | Ethyl 2,2-bis(diphenylmethyl)-3-oxobutanoate | C32H30O3 | −1.98 | 462.2186 | 14.78 |
| 11 | Diethyl 2-[(4-methoxy-2-nitroanilino)methylene]malonate | C15H18N2O7 | −2.34 | 338.1106 | 17.67 |
| 12 | Quinolinic acid | C7H5NO4 | −0.42 | 167.0218 | 19.21 |
| 13 | 9-(3-Amino-3-deoxy-2-S-ethyl-2-thiopentofuranosyl)-9H-purin-6-amine | C12H18N6O2S | 1.98 | 310.1218 | 20.23 |
| 14 | 3,6-Dihydro-3,6-ethanocyclohepta(cd)(1)benzofuran-10,10,11,11-tetracarbonitrile | C18H8N4O | −0.32 | 296.0697 | 22.12 |
| 15 | NP-015864 | C13H20N6O6S | −1.58 | 388.1159 | 22.98 |
| 16 | Cambinol | C21H16N2O2S | 3.55 | 360.0945 | 23.80 |
| 17 | Folitixorin | C20H23N7O6 | 3.59 | 457.1726 | 24.00 |
| 18 | Ethyl trifluoroacetate | C4H5F3O2 | 3.47 | 142.0247 | 26.12 |
| 19 | Furazan-3,4-diamine, N,N′-dimethyl-N,N′-dinitro- | C4H6N6O5 | −1.78 | 218.0396 | 28.04 |
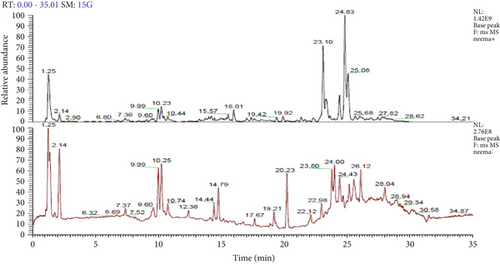
4.6.1. HR-LCMS-MS Zoomed Spectrum of Different Compounds
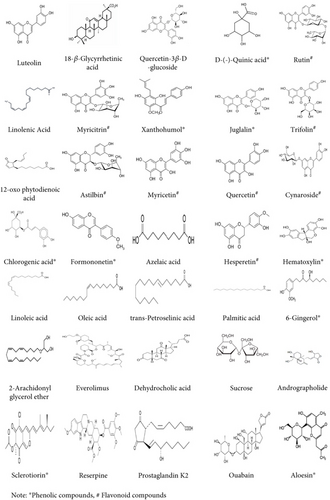
HR-LCMS, coupled with the mzCloud database, was used to analyze the chemical composition of neem extract. The analysis identified 153 metabolites in the negative ESI mode and 107 metabolites in the positive ESI mode. Based on literature, 29 metabolites in the negative ESI mode and 7 metabolites in the positive ESI mode exhibited antibacterial properties. These findings, detailed in Tables 9 and 10, underscore the significant potential of neem leaf–derived metabolites as antibacterial agents. The molecular structures of selected antimicrobial metabolites identified are shown in Figure 3.
| S.N. | Compounds | Molecular formula | Molecular mass | Antibacterial activity |
|---|---|---|---|---|
| 1 | Chlorogenic acida | C16H18O9 | 354.0951 | Chen et al., 2022 [81] |
| 2 | Andrographolide | C20H30O5 | 350.2093 | Zhang et al., 2020 [82] |
| 3 | Sclerotiorina | C21H23Cl O5 | 390.1234 | Lucas et al., 2007 [83] |
| 4 | Reserpine | C33H40N2O9 | 608.2734 | Negi et al., 2014 [84] |
| 5 | Prostaglandin K2 | C20H30O5 | 350.2093 | Ikeh et al., 2018 [85] |
| 6 | Ouabain | C29H44O12 | 584.2833 | Kumari et al., 2019 [86] |
| 7 | Aloesina | C19H22O9 | 394.1264 | Hiruy et al., 2021 [87] |
- aPhenolic compounds.
| S.N. | Compounds | Molecular formula | Molecular mass | Antibacterial activity |
|---|---|---|---|---|
| 1 | Luteolinb | C15H10O6 | 286.047 | XI et al., 2022 [88] |
| 2 | 18-β-Glycyrrhetinic acid | C30H46O4 | 470.339 | Zhao et al., 2023 [89] |
| 3 | Quercetin-3β-D-glucosideb | C21H20O12 | 464.095 | Wang et al., 2018 [90] |
| 4 | D-(-)-Quinic acida | C7H12O6 | 192.063 | Bai et al., 2018 [91] |
| 5 | Rutinb | C27H30O16 | 610.153 | Miklasińska-Majdanik et al., 2023 [92] |
| 6 | Linolenic acid | C18H30O2 | 278.224 | Kusumah et al., 2020 [93] |
| 7 | Myricitrinb | C21H20O12 | 464.095 | He et al., 2021 [94] |
| 8 | Xanthohumola | C21H22O5 | 354.146 | Stomporal et al., 2016 [95] |
| 9 | Juglalina | C20H18O10 | 418.090 | Wan et al., 2023 [96] |
| 10 | Trifolinb | C21H20O11 | 448.100 | Li et al., 2002 [97] |
| 11 | 12-Oxo phytodienoic acid | C18H28O3 | 292.203 | Bisio et al., 2014 [98] |
| 12 | Astilbinb | C21H22O11 | 450.116 | Moulari et al., 2006 [99] |
| 13 | Myricetinb | C15H10O8 | 318.037 | Su et al., 2021 [100] |
| 14 | Quercetinb | C15H10O7 | 302.042 | Nguyen et al., 2022 [101] |
| 15 | Cynarosideb | C21H20O11 | 448.100 | Bouyahya et al., 2023 [102] |
| 16 | Chlorogenic acida | C16H18O9 | 354.095 | Chen et al., 2022 [81] |
| 17 | Formononetina | C16H12O4 | 268.073 | Dutra et al., 2021 [103] |
| 18 | Azelaic acid | C9H16O4 | 188.1049 | Holland and Bojar, 1993 [104]. |
| 19 | Hesperetinb | C16H14O6 | 302.079 | Choi et al., 2022 [105] |
| 20 | Hematoxylina | C16H14O6 | 302.079 | Pratt et al., 1959 [106] |
| 21 | Linoleic acid | C18H32O2 | 280.240 | Dilika et al., 2000 [107] |
| 22 | Oleic acid | C18H34O2 | 282.255 | Stenz et al., 2008 [108] |
| 23 | trans-Petroselinic acid | C18H34O2 | 282.255 | Lee et al., 2022 [109] |
| 24 | Palmitic acid | C16H32O2 | 256.240 | Casillas-Vargas et al., 2021 [110] |
| 25 | 6-Gingerola | C17H26O4 | 294.183 | Park et al., 2008 [111] |
| 26 | 2-Arachidonyl glycerol ether | C23H40O3 | 364.297 | Chouinard et al., 2023 [112] |
| 27 | Everolimus | C53H83NO14 | 957.581 | Ashley et al., 2020 [113] |
| 28 | Dehydrocholic acid | C24H34O5 | 402.240 | Bellini et al., 1979 [114] |
| 29 | Sucrose | C12H22O11 | 342.116 | Zhao et al., 2015 [115] |
- aPhenolic compounds.
- bFlavonoid compounds.
5. Conclusions
This study demonstrates that A. indica ethanolic leaf extract possesses significant antibacterial activity against MDR E. coli, along with strong antioxidant potential and high phenolic and FLV content. HR-LCMS analysis confirmed the presence of diverse bioactive phytochemicals that are likely responsible for these effects. The findings support the translational potential of neem leaf extract as a natural antimicrobial agent, especially in the context of rising antibiotic resistance. It may be useful in developing topical, oral, or supportive therapies in clinical settings. Furthermore, HR-LCMS profiling offers promising lead compounds for future drug development. Given its low cost and widespread availability, neem could be particularly impactful in resource-limited regions where conventional antibiotics are either ineffective or inaccessible.
Further in vivo studies, pharmacokinetic evaluations, and toxicological assessments are recommended to validate the clinical safety and efficacy of neem-based formulations. Exploration of nanoformulations, combination therapies, or standardized extracts could enhance its therapeutic application. Integrating neem into antimicrobial strategies may offer an affordable and sustainable solution to combat MDR pathogens, particularly in developing healthcare systems.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research was supported by external funding from the University Grants Commission (UGC), Nepal, under the Faculty Research Grant (FRG-79/80-HS-02).
Acknowledgments
We sincerely thank the National Herbarium and Plant Laboratories, Kathmandu, for their assistance in plant identification. We also extend our gratitude to Capital Reference Laboratory, Chitwan, for their support in sample collection and analysis. Additionally, we are grateful to the University Grants Commission, Sanothimi, Bhaktapur, for providing financial support through the faculty grant for this research.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




