Exploring the Therapeutic Potential of Podophyllum hexandrum Root Extract: Chemical Composition, Antimicrobial Efficacy, and Antioxidant and Anticancer Activities
Abstract
Medicinal plants have been used for centuries as therapeutic compounds to address various health issues. Their rich phytochemical composition offers diverse bioactive substances with potential health benefits. This study aimed to explore the therapeutic potential of Podophyllum hexandrum root extract by investigating its chemical composition and antimicrobial, antioxidant, and anticancer properties. The phytochemical profiling of P. hexandrum root was conducted using GC-MS analysis, which identified 26 compounds in the extract. The ethanolic root extract displayed strong inhibitory effects in the well diffusion assay against all tested microbes, with minimum inhibitory concentration (MIC) values ranging from 64 to 256 μg/mL. Candida albicans exhibited the lowest MIC value of 64 μg/mL. The antioxidant activity of the extracts was compared to standard antioxidant, revealing a dose-dependent response with a notable radical scavenging activity of 59.23% at 100 μg/mL. Furthermore, the extract demonstrated strong cytotoxic effects against the human cancer cell line HT-29, with IC50 values of 38.20 and 32.5 μg/mL for 24 and 48 h. Overall, this study emphasizes the remarkable antibacterial, antioxidant, and anticancer properties of P. hexandrum root extract.
1. Introduction
Medicinal plants have long been recognized for their potential to complement or even substitute modern medical treatments. The use of plants for therapeutic purposes is rooted in traditional medicine and has garnered increasing attention from both the scientific and medical communities [1, 2]. There is a pressing requirement to assess the therapeutic capabilities of herbal remedies to encourage their use. However, efforts to promote traditional medicine have been relatively limited, with much work remaining to be done in this regard [3]. One of the primary obstacles in global healthcare is the requirement for innovative, efficacious, and cost-effective pharmaceuticals to address microbial illnesses, particularly in developing nations. Certain species of Staphylococcus and Streptococcus bacteria are known to cause respiratory and skin infections. Additionally, Pseudomonas, members of Enterobacteriaceae, and Candida albicans can lead gastrointestinal urogenital illnesses and wound infections. These microorganisms often exhibit resistance to conventional antibiotics [4]. Therefore, it is crucial to assess the therapeutic efficacy of herbal medicines further [5]. The buildup of free radicals within the body can result in oxidative stress. Antioxidants are compounds capable of counteracting free radicals, binding metal ions, preventing lipid peroxidation, and exhibiting reducing characteristics. They are vital in safeguarding the human body against oxidative stress [6]. Oxidative stress is linked to various illnesses and circumstances, such as cancer, neurological ailments, cardiovascular disorders, Alzheimer’s disease, and the aging process [7]. Plant-derived antioxidants offer several advantages, such as lower toxicity, greater effectiveness, and cost-efficiency, which has sparked a growing interest in natural plant-based antioxidants [8]. Secondary metabolites like phenolics and flavonoids found in plants are known for their potent ability to scavenge free radicals, making them valuable as antioxidants [9]. Numerous antioxidants derived from natural and synthetic sources have been suggested as potential therapeutic agents for various human ailments [10]. The incidence of various types of cancer significantly increases with age in human populations, particularly from the fourth to the eighth decade of life. Cancer kills nearly 6 million people globally, making it the top cause of mortality in men and women [11]. Approximately 60% of anticancer drugs originate from plant sources, such as taxol from Taxus brevifolia and camptothecin from Cuscuta reflexa [12]. Ideally, anticancer drugs should have minimal side effects, trigger apoptosis, and display selective cytotoxicity toward cancer cells, making them the preferred therapeutic option [13]. P. hexandrum, commonly called the Himalayan Mayapple, is a perennial plant native to the Himalayas. This herb has a history of traditional use in treating conditions like condylomata or venereal warts. Additionally, research has indicated its effectiveness in combating various cancer types, including lung, brain, bladder, breast, and non-Hodgkin’s lymphoma. P. hexandrum is a rich source of podophyllotoxin, a lignan compound extensively used in the development of anticancer drugs. However, the species is endangered due to overharvesting and habitat loss, highlighting the need for sustainable conservation efforts alongside its therapeutic exploration. The study focused on examining the phytochemical composition and antimicrobial and anticancer properties of P. hexandrum root extract [14, 15]. While previous studies have explored the antimicrobial, antioxidant, and antitumor activities of P. hexandrum in isolation, a comprehensive evaluation of these properties together remains unexplored. This study integrates these activities to provide a holistic understanding of the plant’s therapeutic potential, particularly focusing on its unique chemical profile from the Kashmir Himalayas. By investigating the interconnected roles of these activities, we aim to uncover synergistic effects and validate P. hexandrum as a multifunctional therapeutic agent for addressing modern health challenges.
The findings from this study could provide significant insights into the therapeutic capabilities of P. hexandrum and potentially lead to the development of new treatments for various infectious diseases and cancers.
2. Collection of Plant Material
The fresh plant material of P. hexandrum Royle (Figure 1) was collected in June, 2022, from Daksum Village located in the Anantnag District of Jammu and Kashmir. The site is geographically positioned at Longitude 33°N 75°E, Latitude 33°36′43″ N, approximately 95 km from the capital city, Srinagar (Figure 2).To ensure the accuracy of plant identification, experts in the Centre for Plant Taxonomy at Kashmir University were consulted for biological authentication. A reference sample has been placed in the KASH-Herbarium with voucher number 3069.


2.1. Preparation of Extract
2.2. Phytochemical Analysis
The P. hexandrum root extract was subjected to determine the presence of different phytochemical constituents (phenolic flavonoids, tannins alkaloids, steroids, saponins, and terpenoid compounds) using standard procedures [16].
2.3. Phenol Detection
1 mL of the extract was added to a test tube, followed by 1-2 drops of FeCl3. The presence of phenols was indicated by a color change in the solution after a few minutes.
2.4. Alkaloid Detection
The presence of alkaloids was determined by mixing 1 mL of the extract with 2-3 drops of Wagner reagent. A yellow coloration in the mixture served as evidence of alkaloids.
2.5. Flavonoid Detection
2 mL of the extract was mixed with small pieces of magnesium ribbon, and 5% concentrated HCl was added drop by drop. After 2 min, the formation of a pink-scarlet color confirmed the presence of flavonoids.
2.6. Terpenoid Detection
A test for terpenoids involved dissolving 1 mL of the plant extract in 1 mL of chloroform. Two drops of concentrated sulfuric acid were added, and the mixture was shaken. A yellow color appearing in the lower layer indicated terpenoids.
2.7. Saponin Detection
Saponins were identified by diluting 1 mL of the extract with 5 mL of distilled water in a test tube. The mixture was shaken vigorously for 15 min. The appearance of a stable foam layer on the surface indicated the presence of saponins.
2.8. Tannin Detection
2 mL of the plant was treated with a few drops of 10% ferric chloride solution. The development of a blue-green coloration confirmed the presence of tannins.
2.9. Steroid Detection
The presence of steroids was assessed by dissolving 1 mL of the extract in 1 mL of chloroform. To this solution, 1 mL of acetic anhydride and two drops of concentrated sulfuric acid were carefully added along the sides of the test tube. A red coloration in the upper layer indicated steroids.
2.10. GC-MS Analysis
The analysis of the compounds found in the ethanolic root extract of P. hexandrum was conducted using GC-MS, following a procedure previously outlined [17]. Prior to analysis, the extract was prepared in 100% ethanol. GC-MS Shimadzu QP 2010 analyzer was used to perform GC-MS. The sample was introduced into the instrument using the split mode with split ratio of 1: 2. Helium was used as carrier gas. Initially, the column oven was set to 80°C. The temperature was then gradually increased by 3°C per minute until it reached 200°C. After this, the temperature was raised to 260°C at a rate of 10°C per minute and maintained at that level for 5 min. Overall run time was 54 min. Identification of the biologically active compounds of the extract was achieved by examining their retention times and fragmentation patterns. This was done by comparing the obtained data to the NIST17.lib and WILEY8.LIB.
2.11. Antimicrobial Activity
The antimicrobial potential of the plant extract was assessed using well diffusion method [18]. Microbial strains, Candia albicans ATCC 10231, Escherichia coli ATCC 11229, Pseudomonas aeruginosa ATCC 15442, Staphylococcus aureus ATCC 25923, and Enterococcus faecalis ATCC 29212, were cultured and then evenly spread on the entire surface of MHA. Wells of 6 mm diameter were made on the agar plates and were saturated with 100 μL of extract from each stock solution of the extract prepared (5, 7.5, 10, 12.5, and 15 mg/mL). The plates were left to incubate at 37°C for 24 h. The diameter of the inhibitory zone around each well was measured. As positive controls, standard antibiotics ampilox, ciprofloxacin (30 μg), and fluconazole were employed. DMSO served as a negative control. The antimicrobial assay was conducted in triplicates.
2.12. Determination of Minimum Inhibitory Concentration (MIC)
The assay was carried out using a 96-well plate with flat-bottom wells, following the procedure established by Gabrielson et al. [19]. The first 10 wells of the plate were filled with varying concentrations of P. hexandrum root extract, ranging from 512 to 1 μg/mL. Once the wells were filled with the extract, 5 μL of 12-hour-old microbial cultures was added to each well. The plates were incubated at 37°C and left undisturbed for 16–18 h. Following the incubation, 10 μL of (5 mg/mL) MTT solution was introduced into each well. MTT is a yellow dye that living cells convert into a purple formazan product. After a 2 h incubation with MTT, 100 μL of DMSO was added to dissolve the formazan product. The visual change in color from yellow to purple was observed, and the MIC, which corresponds to the lowest concentration of the root extract that completely halted bacterial growth, was determined. This experiment was conducted in triplicate.
2.13. Determination of In Vitro Antioxidant Activity
2.14. ABTS + Scavenging Assay
The evaluation of P. hexandrum ethanolic extract’s ability to neutralize ABTS + radicals was conducted, following previously adapted method [20, 21] with slight modifications. An ABTS radical cation (ABTS+) was prepared by combining 7 mM ABTS with 2.45 mM potassium persulfate in equal proportions and then allowing it to sit in a dark room at ambient temperature for a period of 12–16 h. The resultant ABTS + solution was then diluted using methanol until it achieves an absorbance of 0.700, at 734 nm. Different concentrations of the plant extract ranging from 20 to 100 μg/mL were introduced to the diluted ABTS + solution. Each combination was stirred for half an hour, after which their absorbance was noted. A solvent blank was processed for every assay. The percentage inhibition of 734 nm absorbance was computed using the formula: ABTS + neutralizing effect (%) = ((AB − AA)/AB) × 100. Here, “AB” is the absorbance of the ABTS radical mixed with methanol, while “AA” is the absorbance of the ABTS radical combined with the sample extract or standard. Ascorbic acid was utilized as the standard compound in this analysis. The experiment was conducted in triplicate.
2.15. Cell Culture
The HT-29 cell line was obtained from the National Centre for Cell Science (NCCS), Pune, India. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Sigma-Aldrich, United States, Catalog: D1152), supplemented with 10% fetal bovine serum (FBS) (Gibco, United States, Catalog: 10270106), 100 U/mL penicillin, and 100 μg/mL streptomycin. The culture was maintained in a humidified incubator at 37°C with 5% CO2 to ensure optimal growth conditions. To propagate the cells, trypsinization was performed using 1X trypsin-EDTA (Gibco, United States, Catalog: 25200-056) when they reached 70%–80% confluency. The detached HT-29 cells were then seeded into a 96-well plate at an appropriate density and allowed to adhere for 21–24 h before treatment.
2.16. Cell Viability Assay
2.17. Statistical Analysis
The data are presented as mean ± S.D. from three independent experiments. Statistical analysis was performed using 2-way ANOVA followed by Tukey’s multiple comparison test to determine significance between the control and treated groups and Sidak’s multiple comparison for antioxidant assay.
3. Results and Discussion
The percentage of plant extract obtained using ethanol as a solvent was assessed, and the outcomes are presented in Table 1.
| Species | Mass of the dried root (g) | Mass of the extract (g) yield (%) |
|---|---|---|
| P. hexandrum | 20 | 2.2 (11%) |
The phytochemical evaluation of root extract of P. hexandrum confirmed the presence of various phytocompounds (Table 2). The findings highlight the rich diversity of phytochemicals present in the extract.
| Chemical test | Observation |
|---|---|
| Phenolic compound | (+) |
| Alkaloids | (+) |
| Flavonoids | (+) |
| Terpenoids | (+) |
| Saponins | (+) |
| Tannins | (+) |
| Steroids | (+) |
- Note: (+) result indicates the presence of the respective compound.
The GC-MS chromatogram of the ethanolic root extract of P. hexandrum recorded a total of 29 peaks corresponding to the bioactive compounds. The phytoconstituents that were identified are listed in Table 3. The chromatogram of the P. hexandrum root extract clearly revealed the presence of various phytocompounds, some of which have previously been linked to antimicrobial, antioxidant, and anticancer activities.
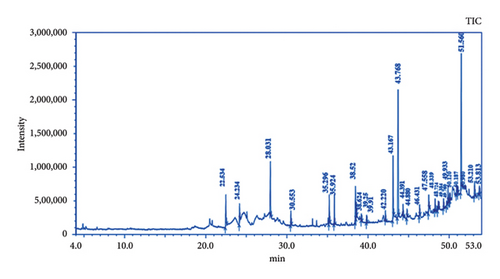
| Peak# | R. time | Area (%) | Name |
|---|---|---|---|
| 1 | 22.534 | 4.13 | 4-Hydroxybenzene ethanol |
| 2 | 24.234 | 2.14 | Apocynin |
| 3 | 28.031 | 6.66 | α-Benzylphenethylamine |
| 4 | 30.553 | 1.80 | (E)-4-(3-Hydroxyprop-1-en-1-yl)-2-methoxyphenol |
| 5 | 35.296 | 3.42 | n-Hexadecanoic acid |
| 6 | 35.924 | 3.11 | Benzenepropanoic acid, 3,5-bis (1,1-dimethylethyl)-4-hydroxy-, methyl ester |
| 7 | 38.527 | 5.45 | 9,12-Octadecadienoic acid (Z,Z)- |
| 8 | 38.624 | 2.11 | 9,12,15-Octadecatrienoic acid, (Z,Z,Z)- |
| 9 | 39.255 | 0.69 | Phenol, 4,4′-(1-methylethylidene) bis- |
| 10 | 39.910 | 1.04 | Octadecamethyl-cyclononasiloxane |
| 11 | 42.220 | 0.96 | Cyclodecasiloxane, eicosamethyl |
| 12 | 43.167 | 8.08 | Trioxsalen |
| 13 | 43.768 | 22.69 | 1,3,5-Triphenylcyclohexane |
| 14 | 44.391 | 1.05 | Eicosamethyl-cyclodecasiloxane |
| 15 | 44.880 | 0.92 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester |
| 16 | 46.431 | 1.20 | Tetracosamethyl-cyclododecasiloxane |
| 17 | 47.558 | 1.88 | n-Propyl 9,12-octadecadienoate |
| 18 | 48.339 | 1.39 | Tetracosamethyl-cyclododecasiloxane |
| 19 | 48.724 | 1.00 | 7H-Furo [3′,2′:4,5] furo [2,3-c] xanthen-7-one, 3a,12c-dihydro-8-hydroxy-6,10,11-trimethoxy-, (3aR,12cS)- |
| 20 | 49.384 | 1.09 | 21H-Biline-1,3,19 (2H)-trione,17-ethyl-23,24-dihydro-2,2,7,8,12,13,18-heptamethyl- |
| 21 | 49.760 | 0.69 | Neotigogenin |
| 22 | 49.933 | 1.76 | Dibenzo [c,E]cycloheptene, 2,3,4,7-tetrameth |
| 23 | 50.120 | 0.81 | Tetracosamethyl-cyclododecasiloxane |
| 24 | 50.187 | 0.85 | 5-(3,4,5-Trimethoxyphenyl)-2H-naphtho [2,3-d] [1,3] dioxolec-chloro-n-[2-(6,7-dimethoxy-isoq)] |
| 25 | 50.980 | 1.79 | 1-Chloro-N-[2-[(6,7-dimethoxyisoquinolin-1-yl)methyl]-4,5-dimethoxyphenyl]methanesulfonamide |
| 26 | 51.133 | 0.75 | (.+−.)-5,12b-Dihydro-9,10-dimethoxy-1,3-dioxolo [4,5-g]isoindolo [1,2-a] isoquinolin-8 (6H)-one |
| 27 | 51.560 | 18.70 | 2,2′-Benzylidenebis (3-methylbenzofuran) |
| 28 | 53.210 | 2.2 | 511H-Cyclopenta [a]phenanthrene-15-carboxylic acid, 12.13,16,17-tetrahydro-3-methoxy-13-methyl-17-oxo-, methyl ester, (S)- |
| 29 | 53.813 | 1.58 | 3,4-Bis [(trimethylsilyl)oxy] dihydro-2 (3H)-furanone |
| 100.00 | |||
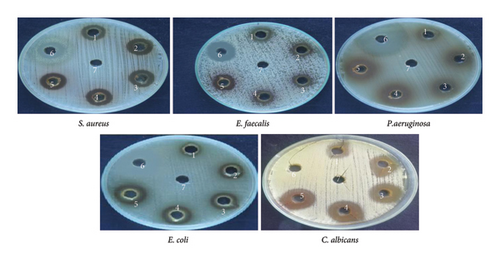
Among these constituents, tyrosol has been shown to possess antimicrobial, anticancer, and antioxidant activities [23, 24]. Trioxsalen, found in the ethanolic extract at a concentration of 8.08%, is a psoralen derivative and belongs to the furanocoumarin group, originating from various plants, primarily Psoralea corylifolia. Similar to other psoralens, it triggers photosensitization of the skin. This compound is utilized in the phototherapy treatment of conditions like vitiligo and eczema. In its photoactivated state, it induces the formation of bonds within DNA strands, ultimately leading to cellular apoptosis. Trioxsalen can also be linked to dyes for confocal microscopy, enabling the examination of DNA damage in research. It has also been investigated for its potential use in generating antisense oligonucleotides that can selectively cross-link with mutant mRNA sequences [25, 26]. 9,12-Octadecadienoic acid (Z,Z)-, n-hexadecenoic, and 9,12-octadecadienoic acid methyl ester acid have been reported to have antimicrobial activity [27]. 2,2′-Benzylidenebis(3-methylbenzofuran), a predominant compound detected in the ethanolic extract of P. hexandrum, has also been identified in the seeds of the Hunteria umbellata plant [28]. This compound showed considerable potential in forming lead targets for combating diabetes through molecular docking. 11H-Cyclopenta[a]phenanthrene-15-carboxylic acid has been reported to have antitumor activities. 3,4-Bis[(trimethylsilyl)oxy]dihydro-2(3H)-furanone is a polyphenol, a lignan, an angiogenesis inhibitor, and an antiproliferative and antiasthmatic agent [29–31]. The exploration of the antibacterial properties of various phytochemicals has been undertaken to assess their potential utility in combatting infectious diseases [32]. The emergence of many drug-resistant human pathogens has spurred efforts to seek out new antimicrobial agents from natural sources [33]. Plants, in particular, are recognized for their ability to produce specific compounds with natural toxicity toward microorganisms, particularly those resistant to multiple drugs [34]. In the current investigation, the extracts derived from the investigated plant displayed different degrees of inhibitory potential against the tested microbes, as indicated in Figures 4 and 5. The results were quantified in terms of the clear zones representing the diameter of the growth inhibition area. The findings revealed the susceptibility of the tested microbes to ethanolic extract as indicated by the low MIC values against the microbes. The ethanolic crude extract of P. hexandrum exhibited the highest activity against C. albicans (27 ± 0.40 mm), followed by E. coli (19 mm ± 0.5), S. aureus (18 ± 0.27 mm), E. faecalis (16 ± 0.20 mm), and P. aeruginosa (15 ± 0.75 mm). However, the standard drug fluconazole did not show any effectiveness against C. albicans. A similar study on the ethanolic leaf extract of P. hexandrum reported strong antimicrobial potential against B. subtilis, S. aureus, E coli, and P. aeruginosa [35]. Another research conducted by Ahmad and Salam revealed that aqueous and methanolic rhizome extracts of P. hexandrum exhibited strong antimicrobial properties against B. megaterium and P. aeruginosa, resulting in inhibition zones ranging from 9 to 14 mm. Additionally, the extracts demonstrated inhibitory effects on fungi, specifically A. flavus and F. solani, with inhibition zones ranging from 9 to 18 mm [36]. Our results align with previous findings from various studies. In our research, the ethanolic extract displayed significant (p < 0.05) activity against all tested microorganisms, with MIC values ranging from 64 to 256 μg/mL. C. albicans showed the lowest MIC value of 64 μg/mL, followed by E. coli (128 μg/mL), S. aureus (128 μg/mL), E. faecalis (128 μg/mL), and P. aeruginosa (256 μg/mL). It is often reported in existing literature that the antibacterial characteristics found in higher plants are frequently attributed to secondary metabolites, such as phenolic, alkaloids, flavonoids, tannins, and other compounds [37]. Plant extracts exhibit antimicrobial activity through various mechanisms targeting essential microbial structures and functions. Bioactive compounds such as phenolics, flavonoids, and terpenoids disrupt microbial cell walls and membranes, increasing permeability and leading to cell lysis. These compounds also inhibit nucleic acid synthesis, protein translation, and enzyme activities crucial for microbial growth and survival. Additionally, they generate reactive oxygen species (ROS), causing oxidative stress that damages microbial DNA, proteins, and lipids. Some plant-derived compounds further interfere with biofilm formation and quorum sensing, weakening bacterial communication and resistance strategies. This multitarget approach makes plant extracts effective against diverse pathogens and promising alternatives in combating antibiotic resistance [38–40].

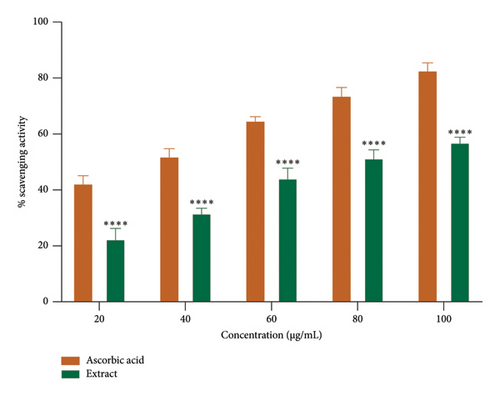
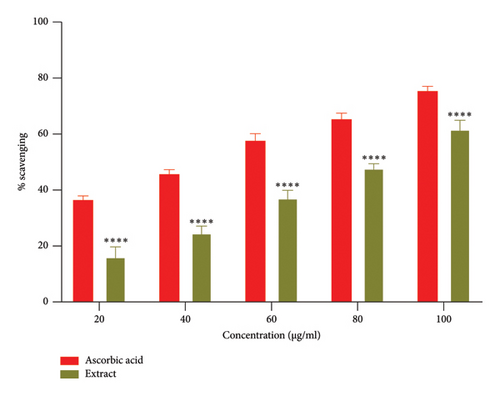
Free radicals are implicated in various diseases, including cancer and cardiovascular and neurodegenerative disorders. The ability of antioxidants to scavenge these radicals plays a crucial role in managing these conditions [41]. The DPPH and ABTS + assays are the most commonly employed methods for assessing the antioxidant potential of extracts from plants, fungi, or algae [42, 43]. The ethanolic root extract of P. hexandrum demonstrated concentration-dependent radical scavenging activity (Figures 6(a) and 6(b)). The highest DPPH scavenging activity was 59.23% at 100 μg/mL, with an IC50 value of 75.50 μg/mL. Similarly, for ABTS+, it was 61.81% at 100 μg/mL with IC50 value of 81.72 μg/mL. The results from this study are especially significant (p < 0.0001) when compared to previous research on P. hexandrum. In a previous study, the methanolic and ethanolic seed extracts of P. hexandrum, tested at a concentration of 700 μg/mL, showed DPPH scavenging activities of 86 ± 1.5% and 78 ± 1.2%, respectively [41]. Our results suggest that extract derived from the roots of P. hexandrum has the potential to protect against or prevent oxidative damage to fats, DNA, and proteins caused by ROS.
The research for innovative cancer treatments that do not cause side effects is rooted in ancient wisdom, particularly the use of traditional or ethnomedicinal plants. Many of these plants, such as those from the Berberidaceae family, including the Podophyllum genus, have long been recognized for their antiviral, anti-inflammatory, antidiabetic, antimicrobial, antioxidant, and anticancer effects [44, 45]. The current research observed the potent antiproliferative effects of an ethanolic root extract of P. hexandrum species native to the Kashmir Himalayas, India, on HT-29 cells. The findings of the MTT assay demonstrated that the ethanolic extract significantly (p < 0.0001) inhibited the proliferation of HT-29 cancer cells, with an IC50 of 38.7 μg/mL for 24 h and 32.6 μg/mL for 48 h. The MTT assay demonstrated that the ethanolic extract exhibited a dose-dependent inhibition of HT-29 cancer cell growth (Figures 7 and 8). These results indicate a promising potential for the ethanolic extract of P. hexandrum roots as an anticolon cancer agent.
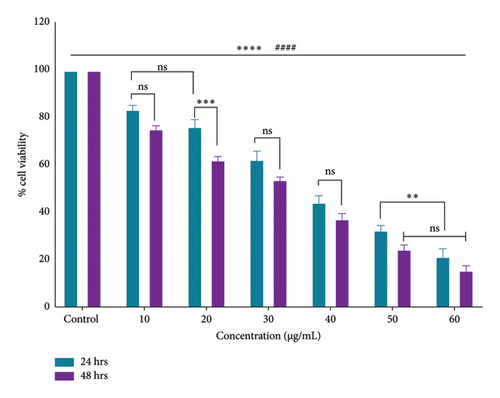

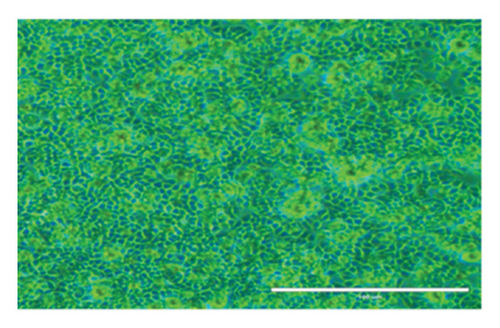
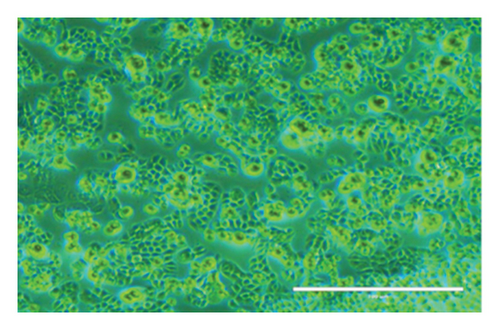
After 24 h, the cell morphological changes occur, indicating a potential cytotoxic effect. This effect becomes more pronounced at 48 h, suggesting a time-dependent response to the treatment. In related studies, Zarger et al. [44] showed that seed extracts from P. hexandrum have strong inhibitory effects on MCF and HeLa cancer cell lines. The methanolic extract showed the highest inhibition, with 78% for MCF and 52% for HeLa, followed by the ethanolic extract, which showed 63% inhibition for MCF and 52% for HeLa at 100 μg/mL. Separately, Kitaeva et al. [46] found that extracts from the roots and rhizomes of P. peltatum exhibited cytotoxic effects on HeLa cells, reducing their viability by 52% to 60%. The anticolon cancer potential of other medicinal herbs used historically in the Kashmir Himalayas has also been studied. Extracts from Rhodiola imbricata suppressed the proliferation of HT-29 cancer cells, with acetone and methanol extracts at 200 μg/mL achieving an 84% reduction in cell growth [47]. Additionally, protease inhibitors from Lavatera cashmeriana seeds showed significant anticancer activity against Colo 205 and HCT-116 colon cancer cell lines, with IC50 values of 60 and 43 μg/mL, respectively [48]. In contrast to these plants, our findings showed a 50% inhibition of HT-29 cells at 38.20 μg/mL for 24 h and 32.5 μg/mL for 48 h, indicating that the ethanolic root extract of P. hexandrum has promising potential for combating colon cancer. It is important to note that while podophyllotoxin and its derivatives are well-known bioactive compounds in P. hexandrum responsible for its antitumor activity [15], these compounds were not detected in our GC-MS analysis, potentially due to geographical and environmental variations, seasonal differences, or extraction and analytical limitations [49–52]. However, the cytotoxic effects observed in our study suggest the presence of other bioactive compounds that may contribute to the anticancer activity of the extract. Despite the absence of podophyllotoxin, the potential toxicity of P. hexandrum extracts remains a critical consideration, as previous studies have shown that podophyllotoxin and related lignans can cause severe side effects, including myelosuppression, gastrointestinal toxicity, and hepatotoxicity, due to their mechanism of action involving microtubule disruption and topoisomerase II inhibition [53]. The observed cytotoxicity underscores the need for careful evaluation of the extract’s safety profile. Future research should focus on identifying the specific bioactive compounds responsible for the cytotoxic effects, conducting toxicological evaluations to ensure safety for human consumption, and exploring advanced drug delivery systems (e.g., nanoparticles and liposomes) and combination therapies to enhance selective targeting of cancer cells and minimize off-target effects. Further studies are also needed to confirm the anticancer properties of P. hexandrum and to explore the mechanisms behind its effects.
4. Conclusion
In conclusion, the present study underscores the immense therapeutic potential of P. hexandrum root extract, enriched with diverse bioactive compounds. Its significant antimicrobial, antioxidant, and anticancer properties align with the historical use of medicinal plants in addressing complex health challenges. The observed antimicrobial activity against a broad spectrum of microbes, including C. albicans followed by E. coli S. aureus, E. faecalis, and P. aeruginosa coupled with potent antioxidant capabilities, highlights the extract’s ability to mitigate oxidative stress and combat pathogenic organisms. Moreover, the pronounced cytotoxic effects on HT-29 cancer cells point to its promise as a natural anticancer agent. However, translating these findings into clinical applications requires further exploration, including detailed mechanistic studies, in vivo validations, and toxicity profiling. This study contributes to the ongoing efforts to harness plant-derived compounds as sustainable and effective solutions for modern healthcare challenges.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
M.A.S. wrote the original draft, conducted the investigation, proposed the methodology, performed formal analysis, reviewed and edited the manuscript, curated the data, provided resources and software, managed the project, and conceptualized the study. A.N. and B.K.N. contributed to writing the original draft, supervised, validated the study, and reviewed and edited the manuscript. Z.O. and M.Z.S. contributed to writing, reviewing, editing, data interpretation, and software analysis. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was obtained for this research.
Acknowledgments
The authors express gratitude to Biomedical Engineering Department, Sathyabama Institute of Science and Technology, Chennai, as well as the University of Madras, Guindy Campus. These institutions provided the laboratory facilities.
Open Research
Data Availability Statement
All relevant data for this research are provided in the manuscript.




